Abstract
Introduction
Point-of-care (POC) musculoskeletal ultrasound (MSKUS) is increasingly used by hemophilia providers to guide management, however, pathologic tissue differentiation with US is uncertain. We sought to determine the extent to which POC MSKUS can identify and discriminate pathologic soft tissue changes in hemophilic arthropathy.
Materials and Methods
36 adult patients with hemophilia A/B were prospectively enrolled. POC MSKUS was performed on arthropathic joints (16 knees, 10 ankles, and 10 elbows) using standard views by a MSKUS-trained and certified hematologist, who recorded abnormal intra-articular soft tissue accumulation. Within three days, magnetic resonance imaging (MRI) was performed using conventional and multi-echo ultrashort echo time (UTE) sequences. Soft tissue identification (synovial proliferation with or without hemosiderin, fat, and/or blood products) was performed by a musculoskeletal radiologist. Findings obtained with both imaging modalities were compared and correlated in a blinded fashion.
Results
There was perfect agreement between the modalities on the presence of abnormal soft tissue (34/36 cases). However, MSKUS was unable to discriminate between coagulated blood, synovium, intra- or extra-synovial fat tissue, or hemosiderin deposits due to wide variation in echogenicity.
Conclusion
MSKUS is valuable for POC imaging to determine the presence of soft tissue accumulation in discrete areas. However, due to limitations in MSKUS in discriminating the nature of pathological soft tissues and detecting hemosiderin, MRI will be required if such discrimination is clinically important.
Keywords: Arthropathy, Hemophilia, Ultrasound, Magnet Resonance Imaging, Musculoskeletal
Introduction
Joint arthropathy is a common clinical manifestation of hemophilia1–3. Contributing factors to hemophilic joint damage are thought to include recurrent hemarthroses, synovial inflammation, and soft tissue hypertrophy, ultimately leading to osteochondral deformities and destruction4–7. Use of imaging in hemophilia continues to progress. Although MRI has long been considered the “gold standard,” recent advances in technology, accessibility, and training have made ultrasound an attractive alternative. In fact, it is now evident that musculoskeletal ultrasound (MSKUS) has a number of benefits compared with MRI in that MSKUS is faster, more economical, and without the need of sedation for claustrophobic patients or children. MSKUS does not require intravenous contrast to distinguish synovial proliferation from fluid8–10 and can be used to assess synovial vascularity in arthritic conditions11, including hemophilic arthropathy3, 5, 12, 13.
One of the most important aspects of MSKUS is that point-of-care (POC) imaging is now possible. MSKUS has been shown to be reliable and helpful for management in a wide spectrum of musculoskeletal pathology spanning multiple disciplines14–16 and been introduced into hemophilia clinics to assist providers with in-office management of hemophilic arthropathy3, 12, 17, 18. In particular, MSKUS has been found critical to identify tissue abnormalities contributing to pain in patients with hemophilia12 and to rapidly and accurately determine if hemarthrosis is present3, 17, 18. MSKUS has also been proposed to quantify tissue abnormalities in semi-quantitative scoring algorithms2, 13, 19, 20.
MSKUS use is growing rapidly in routine management of hemophilia and it is evident that validation of pathological tissue annotation is necessary, particularly for the development of scaling systems to assess overall joint health status, either by semi-quantitative algorithms, or, quantitatively by applying direct tissue measurements. Towards this goal, standardization must occur and consensus regarding ultrasound definitions of pathology must be reached. While it is accepted that US can readily distinguish between fluid and soft tissue3, 13, 21, the correct assignment and differentiation between individual tissue types such as synovium (with and without hemosiderin deposits) and fat remain less certain. For instance, synovial hypertrophy is typically hypoechoic relative to subdermal fat, but at times also can be isoechoic or hyperechoic22. In addition, if and to what extent altered echogenicity in synovium of hemophilic joints can be ascribed to hemosiderin deposition is unclear and currently debated23, 24. Altogether, this may confound the distinction of different pathological tissues and their discrimination from other surrounding structures. The purpose of this study was to elucidate the role and limitations of MSKUS for the detection and discrimination of soft tissue findings in hemophilic arthropathy, to and to inform the development of ultrasound joint assessment tools as well as diagnostic and therapeutic management decisions. Comparison was made with MRI as a reference standard.
Materials and Methods
Patient population and data extracted
Adult patients with hemophilia A or B of all severities, age 21 years and older (n=36), and who were obtaining US examinations of arthropathic joints during routine clinic visits at (blinded for review) over a four-month period, provided written informed consent to undergo consecutive MRI of the same joint within 3 days. In total, 16 knees, 10 ankles, and 10 elbow joints were imaged, which together represent the three most commonly affected joints in hemophilic arthropathy. Joints were defined as arthropathic by the responsible hematologist prior to imaging, when patients identified them as previous target joints (frequent bleeding) and/or when notable deformities and function deficits were present on physical exam. Additionally, Hemophilia Joint Health Scores (HJHS;25) and radiographic Pettersson scores26 were collected at the time of inclusion to provide objective proof of arthropathy. The degree of arthropathy was also determined by MRI scoring as recommended by the International Prophylaxis Study Group (IPSG;27). The study protocol, data acquisition and patient confidentiality safeguards were approved by the Human Research Protection Program at the University of California San Diego.
Imaging
All US studies were performed by a hematologist ([blinded for review] with 5 years of MSKUS experience) who was formally trained and certified in MSKUS through the American Registry for Diagnostic Medical Sonography (ARDMS). A 6-15 MHz linear transducer was employed for imaging (LOGIQS8, GE Healthcare Technologies, Milwaukee, WI). MSKUS examinations were performed using standard imaging planes for each joint area19. Sonopalpation was used as appropriate.
MR imaging was performed on a clinical 3T scanner (Signa HDx, GE Healthcare Technologies, Milwaukee, WI) and an 8-channel knee coil using the following 2D sequences: sagittal fast spin-echo (FSE) T1-weighted (650/10 ms; echo-train length of 4; 4 mm slice thickness; 0.5 mm interslice gap; 384 × 320 matrix; 14 cm field of view; and 1 signal average), sagittal FSE T2-weighted with fat suppression (4,000/65 ms; echo-train length of 12; 4 mm slice thickness; 0.3 mm interslice gap; 384 × 288 matrix; 14 cm field of view; and 2 signal averages), coronal FSE T1-weighted (650/10 ms; echo-train length of 4; 4-mm slice thickness, 0.5-mm interslice gap, 384 × 320 matrix, 14-cm field of view, and 2 signal averages), coronal FSE T2-weighted with fat suppression (5,000/65 ms, echo-train length of 16, 4-mm slice thickness, 0.5-mm interslice gap, 384 × 320 matrix, 14-cm field of view, and 1 signal average), axial FSE T1-weighted (650/10 ms; echo-train length of 4; 4-mm slice thickness; 0.5-mm interslice gap; 320 × 288 matrix; 14-cm field of view; and 1 signal averages), and axial FSE intermediate-weighted with fat suppression (3200/40 ms; echo-train length of 9; 4-mm slice thickness; 0.5-mm interslice gap; 320 × 288 matrix; 14-cm field of view; and 1 signal averages). In addition, sagittal three-dimensional (3D) ultrashort echo time (UTE) images were acquired with a cones readout trajectory at four different echo times (TR/TEs, 15 ms / 0.03, 2.8-3, 5.6-6, and 8.4-9ms; flip angle = 11°; 4 mm slice thickness; 256 × 256 matrix, 14-cm field of view, time ~6 minutes)28. Intravenous contrast was administered for select cases when clinically indicated.
Joint Assessment, Image Interpretation and Data Analysis
On MSKUS, the presence of soft tissue was recorded when non-compressible abnormal intra-articular material was detected22. The echogenicity of the non-compressible material was compared relative to the adjacent soft tissue and was noted as hypoechoic, hyperechoic, or mixed. Intra-articular fluid was also noted when the material was entirely compressible.
A fellowship-trained, musculoskeletal radiologist ([blinded for review] with 6 years of experience) was blinded to the MSKUS results and independently recorded the presence or absence of intra-articular joint fluid, soft tissue, or blood products after evaluation of all images in the MRI protocol. Prior MR imaging exams were also evaluated, when available. Fluid and tissue discrimination was performed in a standard manner as follows: fluid demonstrates increased signal on all fluid sensitive sequences and comparable signal on T1-weighted images relative to muscle, most typically hypointense when the fluid is bland; fat demonstrates hyperintense signal on T1-weighted images relative to muscle with hypointense signal on all sequences after a spectral fat suppression preparatory pulse; hemosiderin demonstrates progressive loss of signal with increasing TEs on the multi-echo UTE sequence (with special consideration of chemical shift artifacts of the second kind for voxels containing both fat and water on out-of-phase TEs29). It is recognized that conventional MRI sequences cannot reliably distinguish between fluids of various degrees of complexity (such as saline versus blood)30. However, when blood is coagulated or a blood-fluid level is appreciated, a hemarthrosis can be diagnosed and blood clots are distinguished based on retraction and degradation products as previously described31–34. Regarding synovial proliferation, it is recognized that synovium without hemosiderin may demonstrate a variety of signal intensities depending on precise composition, at times approaching that of fluid on conventional fluid-sensitive clinical sequences35–37. However, synovial proliferation in hemophilia38, 39 often contains fibroblasts and fibrotic tissue and in these cases should be less intense than fluid on fluid-sensitive sequences.
Statistical analyses
We assessed the agreement between US and MRI for the detection of soft tissue of any type. Fisher’s exact test was performed to compare MRI-based synovial proliferation with and without hemosiderin versus ultrasound echogenicity. Descriptive statistics were applied to joint scoring using radiographic, clinical and MRI scales.
Results
Patient and Joint Characteristics
In total, 36 patients (mean age 44 years, standard deviation [SDE] 15.7 years, range 21-70 years]) were recruited (10 ankles, 16 knees, 10 elbows) and imaged with both MRI and MSKUS. All 36 joints were affected by hemophilic arthropathy, evidenced by either a positive radiographic Pettersson score (mean 8.0, SDE 4.5, range 0-12), and/or clinical HJHS (mean 4.7, SDE 3.7, range 0-11) and/or IPSG MRI scores (mean 10.9, SDE 4.6, range 0-22).
Soft Tissue Assessment with MSKUS compared to MRI
Both MSKUS and MRI detected the presence or absence of abnormal soft tissue expansion in 34 or 2 of the 36 patients, respectively, with complete agreement between the two imaging modalities in all cases. MRI was used for tissue delineation and determined that synovial proliferation with or without hemosiderin was present in 26 and 6 cases, respectively, whereas fat tissue expansion was present in 6 cases. Blood clots were seen in 3 cases. Using MRI as a comparator, MSKUS was unable to discriminate soft tissue types (synovial proliferation with or without hemosiderin, fatty tissue expansion and blot clot) based on echogenicity. None of the abnormal soft tissue types was found to have specific features on MSKUS. Echogenicity of fatty tissue expansion was deemed predominantly hypoechoic in 4 cases, and hyperechoic in 2 cases. Of the 3 blood clots, 2 were predominantly hypo-echoic and one was of mixed echogenicity. Hemosiderin-laden synovium could be predominantly hypo- or hyperechoic, or appear with mixed echogenicity, similar to non-hemosiderin-laden synovial proliferation (Table 1). Although there was a propensity of hypoechogenic synovium in the presence of hemosiderin (18/26 cases; 69%) compared to synovial proliferation without hemosiderin (2/6; 33%), this difference was not significant (p=0.13; Table 1).
Table 1.
Predominant echogenicity of synovial proliferation on ultrasound in the presence or absence of hemosiderin documented by MRI
| MRI appearance | Predominant Echogenicity on Ultrasound | |||
|---|---|---|---|---|
|
| ||||
| Hyper | Hypo | Mixed | Total | |
| Hemosiderin absent | 3 | 2 | 1 | 6 |
| Hemosiderin present | 3 | 18 | 5 | 26 |
| Total | 6 | 20 | 6 | 32 |
Fisher’s exact test, p = 0.126; MRI, Magnetic Resonance Imaging
Key findings are illustrated by the figures. Hemosiderin-laden synovial proliferation had no unifying sonographic characteristics. Echogenicity of hemosiderin varied widely from predominantly hypo- to hyperechoic patterns. Figure 1 demonstrates three distinct ultrasound appearances of MRI-proven hemosiderin-laden synovial proliferation, including a nearly anechoic pattern (Figure 1A), a mixed but predominantly hypoechoic pattern (Figure 1D), and a hyperechoic pattern (Figure 1G). Moreover, synovial proliferation, even without confounding hemosiderin depositions, also had no unifying echogenic features, and could present either with a nearly anechoic pattern (Figure 2A) or a hypoechoic pattern (Figure 2D), again resembling certain echogenicity patterns of hemosiderin-laden synovial proliferation (Figures 1A, 1D; Figures 2A, 2D). Moreover, it appeared that hemosiderin was not always distributed equally in proliferating synovium, sometimes with zones of highly focal depositions abutted by enhancing less hemosiderin-laden synovitis as shown by MRI in Figures 3B, 3C, 3E, and 3F. The example shown in Figure 3 demonstrates that joints with advanced hemophilic arthropathy and abundant synovial proliferation can exhibit complex patterns of hemosiderin distribution and synovial inflammation which, while discernable on MRI, cannot be discriminated by ultrasound. The corresponding ultrasound images were highly heterogenous, not permitting a distinction of the different synovial properties shown on MRI based on echogenicity. Ultrasound images included regions that were predominantly anechoic or hypoechoic and coarsely granular in some areas (Figure 3A, 3D). The various ultrasound patterns were not associated with distinct MRI appearances of hemosiderin-laden synovium or areas of enhancing synovitis.
Figure 1. Various echotextures of hemosiderin-laden synovial proliferation.
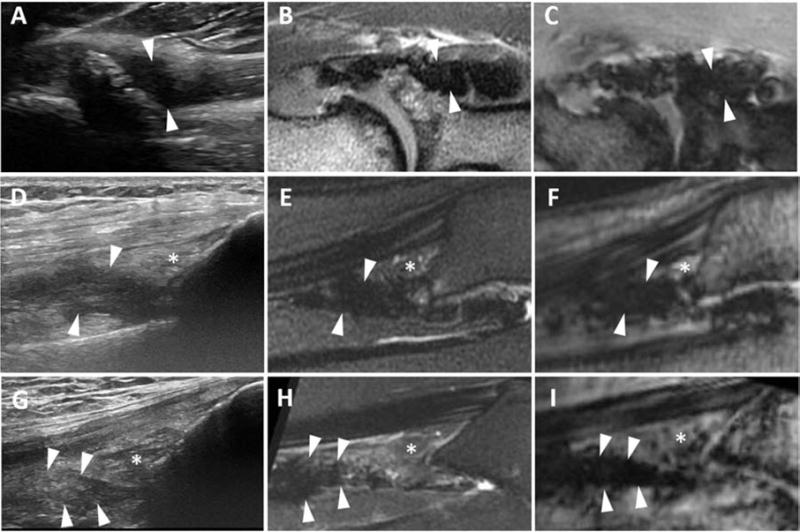
The annular recess in the elbow of a 39-year old (A-C) and suprapatellar recesses in the knees of a 23-year-old (D-F) and a 38-year-old (G-I) patient. (A, D and G) Longitudinal US images show thick, non-compressible tissue with increasing coarseness and heterogeneous echotexture (arrowheads) that differs between patients. (B,C,E,F,H,I) Sagittal T2-weighted and UTE gradient MR images (TE 6.0 ms) show hypointense tissue with blooming, consistent with hemosiderin-laden synovium. * = suprapatellar fat pad. US, ultrasound; MR, magnetic resonance; UTE, ultrashort time-to-echo.
Figure 2. Various echotextures of synovial proliferation without hemosiderin.
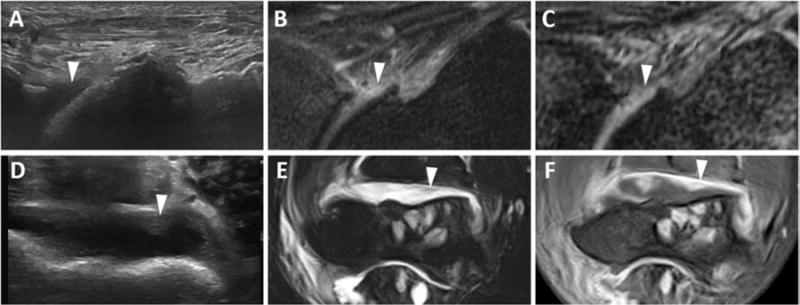
Anterior recess of the tibiotalar joint in a 57-year-old man (A-C) and the anterior recess of the elbow in a 42-year-old man (D-F). (A) Longitudinal US image shows hypoechoic, non-compressible tissue (arrowhead). (B) Sagittal T2-weighted fat-suppressed MR image shows hyperintense synovial proliferation (arrowhead). (C) UTE gradient MR image (TE 10.8 ms) shows lack of blooming, indicative of lack of hemosiderin (arrowhead). (D) Axial US image shows iso-echoic, non-compressible tissue (arrowhead). (E) Axial T2-weighted MR fat-suppressed image shows predominantly hyperintense synovial proliferation without blooming artifact (arrowhead). (F) Axial T1-weighted fat-suppressed MR image post-intravenous contrast confirms thick, enhancing synovium without hemosiderin. US, ultrasound; MR, magnetic resonance; UTE, ultrashort time-to-echo.
Figure 3. Various echotextures of synovial proliferation containing hemosiderin.
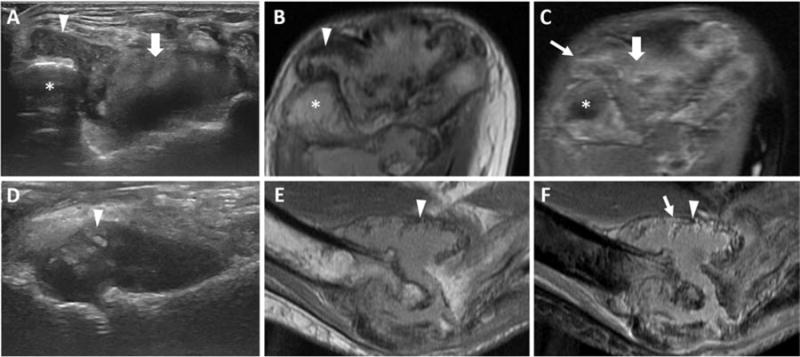
Axial posterior view (A-C) and long axis anterior view (D-F) of the elbow joint in a 44-year-old man (distal humerus labeled with an asterix, *). (A) Axial US image shows non-compressible tissue in the posterolateral recess (arrowhead) with a granular, hypoechoic pattern and the posterior recess (thick arrow) with a hyperechoic pattern. (B) Axial intermediate-weighted (TR/TE 1712 ms/ TE 23 ms) fat-suppressed image shows hemosiderin laden-synovial proliferation in the posterolateral recess (arrowhead). (C) Axial T1-weighted fat-suppressed, post-intravenous contrast MR image shows enhancing synovium in the posterolateral (thin arrow) and posterior (thick arrow) recesses. (D) Longitudinal US image shows mixed, predominantly hyperechoic, non-compressible tissue directly at the level of the joint line (arrowhead). (E) Sagittal T1-weighted MR image shows hypointense hemosiderin-laden synovium (arrowhead). (F) Sagittal T1-weighted fat-suppressed post-intravenous contrast MR image shows areas of avid enhancement (thin arrow) adjacent to hypointense areas (arrowhead), consistent with synovium containing various amounts of hemosiderin. US, ultrasound; MR, magnetic resonance.
Abnormal intraarticular soft tissue composition can be highly complex, change in character over time, and represent ectopic tissue other than synovial proliferation as demonstrated in one elbow studied with sequential MRI 6 years apart. Figures 4A and 4D demonstrate two adjacent images from the ultrasound examination that was performed concurrently with the latest elbow MRI. The ultrasound demonstrates non-compressible soft tissue in the annular recess of a severely arthropathic elbow, where usually synovial hypertrophy would be suspected. However, ultrasound revealed a multi-structured tissue mass with areas of distinctly different echogenicities that were not consistent with the usually more uniform appearance of synovium. This soft tissue mass was shown to be predominantly hypointense on MRI (Figures 4B and 4E), consistent with hemosiderin-laden synovial proliferation. However, there were internal regions which were hyperintense, suspected to be an area of active bleeding. Of note, the soft tissue mass had no distinctive features compared to previously shown synovial ultrasound appearances mentioned above. Upon review of a comparison MRI exam 6 years prior, it was noted that the hemosiderin-laden synovial proliferation was previously homogenously hypointense (Figures 4C and 4F), without regions of different intensities. This case illustrates that hemosiderin-laden synovium remains biologically active, can occasionally demonstrate frank intra-synovial bleeding thus creating a complicated appearance on both US and MRI images and further highlighting the limitations of pathologic tissue discrimination with ultrasound.
Figure 4. Various echotextures of hemosiderin-laden synovial proliferation.
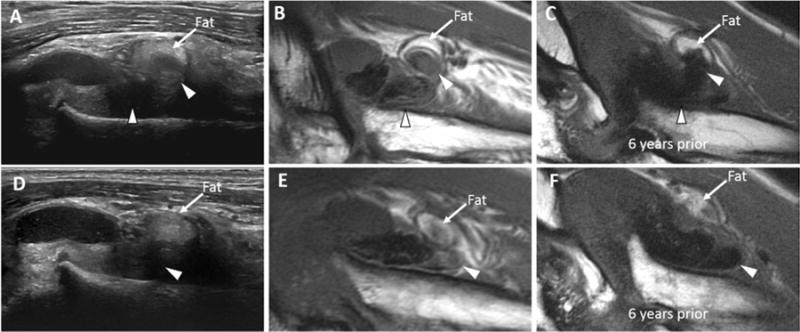
Anterior aspect of the radiocapitellar compartment of the elbow in a 48-year-old man (A, B, D, E) and 6 years prior (C and F). (A and D) Long axis US image shows mixed echogenicity in the annular recess, with predominantly non-compressible tissue (arrowheads). (B and E) Sagittal T1-weighted MR images performed on the same day as the US show hemosiderin laden-synovial proliferation with mixed internal signal intensity, suggestive of more recent blood products (arrowheads). (C and F) Sagittal T1-weighted MR images six years prior to presentation show the homogenously hypointense hemosiderin-laden synovium (arrowheads). To facilitate comparison between all images, a curvilinear region of intra-articular fat is present anteriorly and labeled, acting as an internal anatomic landmark. US, ultrasound; MR, magnetic resonance.
Moreover, coagulating blood or blood clot was indistinguishable from synovial proliferation with or without hemosiderin. Figure 5 demonstrates suprapatellar expansion in an asymptomatic patient. The area was mildly compressible, with a predominantly hypoechoic sonographic appearance, whereas the soft tissue echogenicity was found to be nearly identical to previously demonstrated sonographic patterns of synovial proliferation with (Figure 1D) or without (Figure 2D) hemosiderin. However, MRI demonstrated the presence of a blood clot rather than synovial proliferation. Moreover, findings in this patient also demonstrate that fat pad echogenicity can be heterogeneous in hemophilic joints. The prefemoral fat pad, usually visualized as a hyperechoic area abutting the suprapatellar recess in normal joints, demonstrated heterogeneous echogenicity (Figure 5A), blending into the area of coagulating blood. The T2-weighted fat-suppressed MR images demonstrated increased signal at the periphery of the inferior aspect, which may represent edema (Figure 5C). This observation highlights that pathological fat pads or blood clots can be easily confused with synovial hypertrophy usually assumed to be the cause of suprapatellar soft tissue expansion. Figure 6 further illustrates various appearances of intraarticular fatty tissue, bearing the potential to be mistaken for synovial hypertrophy. Figure 6A and 6B show small floating fronds of hyperechoic tissue in fluid which displaced upon sonopalpation, only to return to the same position after release of pressure. MRI confirmed these fronds to represent fat and not synovial tissue, consistent with secondary lipoma arborescens (intra-synovial fat metaplasia). In another patient, a tongue-like, hyperechoic soft tissue structure was surrounded by fluid (Figure 6I), which extended in and out of plane upon repeated compression with the ultrasound probe. MRI showed this tissue to represent a thickened projection of expanded extra-synovial pre-femoral fat pad (Figure 6K-L), whereas on ultrasound this finding may have been recorded as synovial proliferation due to its typical location.
Figure 5. Soft tissue accumulation (blood clot).
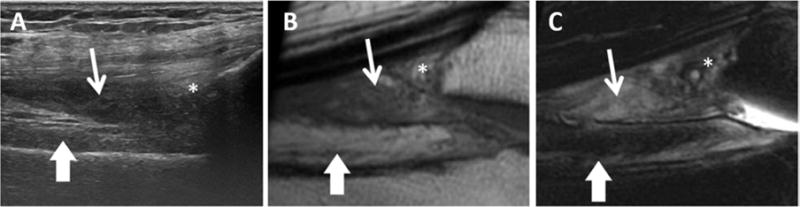
(A) Long-axis US image shows hypoechoic tissue that was partially compressible on real time imaging in the suprapatellar recess (thin arrow). (B) Sagittal T1-weighted and (C) T2-weighted fat-suppressed MR images shows heterogeneously hyperintense signal in the suprapatellar recess on both sequences, consistent with coagulated subacute blood products. Prefemoral (thick arrows) and suprapatellar (asterisks) fat pads are marked. Note that the prefemoral fat pad is more hyperintense proximally compared with distally. US, ultrasound; MR, magnetic resonance.
Figure 6. Appearance of fat in joint recesses of 3 patients.
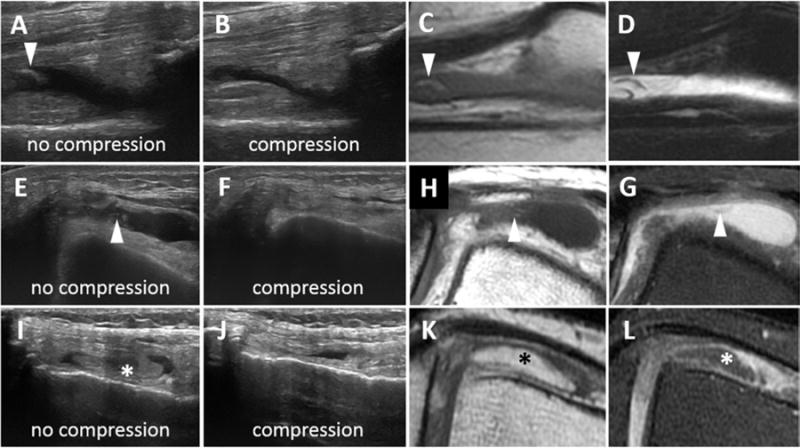
(A and B) Suprapatellar recess: Longitudinal US shows a frond of hyperechoic material surrounded by fluid that was compressible on real time imaging (arrowheads). Sagittal T1-weighted (B) and T2-weighted fat-suppressed (C) MR images elucidates the soft tissue frond as fat metaplasia. (E and F) Transverse US images show compressible, anechoic fluid (arrow) with few fronds of hyperechoic material (arrowhead), consistent with an effusion and solid material. (H) Axial T1-weighted and (G) T2-weighted fat-suppressed MR images confirm the effusion (arrow) and demonstrate that the solid material is fat (arrowheads). (I and J) Transverse US images show a thickened tongue of tissue (*) which displaces out of plane on compression. (K) T1-weighted and (L) intermediate-weighted fat-suppressed MR images demonstrated that this tissue was an inferior projection of the extra-synovial, pre-femoral fat pad (*). US, ultrasound; MR, magnetic resonance.
Discussion
Non-radiologists across medical specialties increasingly use ultrasound in clinics and at the bedside for rapid imaging-guided diagnosis and interventions to enable efficient and immediate personalized care3, 12, 17, 18. In hemophilia patients, who have established arthropathy, it is not clinically possible to reliably distinguish between an acute bleeding event and a flare in inflammation – circumstances that call for very different interventions. Thus, there is a need to develop and validate ultrasound scales to provide an assessment of overall joint health outcomes longitudinally19, 20, similar to what has been developed with MRI27, 40, 41. Pathological tissue recognition with MSKUS during the examination of hemophilic joints requires validation in order to answer pertinent diagnostic questions relevant to hemophilia care and/or to develop ultrasound scales. Current interpretation algorithms for tissue discrimination are derived from studies in rheumatoid arthritis or osteoarthritis, and infer that the pathobiology of hemophilic arthropathy is comparable to these other arthritic conditions. However, this may not be true given numerous confounders inherent to the different etiology of hemophilic arthropathy. Examples of such confounders are bleeding with hemosiderin accumulation or extensive vascular remodeling with leaky vessels5, 12, 42, both unique to hemophilic arthropathy and not encountered in the other conditions. It is therefore important to perform validation studies to define the ability and limitations of MSKUS to answer specific questions relevant to hemophilic arthropathy, and/or define to what extent MSKUS can discriminate between different pathological soft tissue types or states.
Of note, and to provide a perspective, the validation process for the use of MSKUS in rheumatoid arthritis is still ongoing43, and only recently, after a decade of progress, was MRI deemed valid44. The Outcome Measures in Rheumatology (OMERACT) group, an international initiative focused on standardization in rheumatology, has set forth clear guidelines for validation that first involve a consensus definition of pathology visible by a certain imaging modality, preferentially supported by cross-reference to another validated imaging modality, and, secondarily, determination of the reliability of pathology recognition and discrimination. Others have begun the validation process of US in hemophilic arthropathy20, 45, but predominantly in children and youth in early stages of the disease. Therefore, MSKUS tissue validation studies in hemophilic arthropathy, especially for findings in more advanced stages and adults, remain an unmet clinical need, and are urgently required to advance diagnostic recognition patterns in the POC setting.
Our study, involving direct comparison of MSKUS with MRI, begins to fill this gap, delineating to what extent MSKUS can recognize the presence of abnormal soft tissue and discriminate between various soft tissue abnormalities. This was accomplished by comparing imaging findings on MSKUS with the accepted “gold standard” of MRI, with inclusion of both routine clinical sequences and sensitive 3D multi-echo UTE sequences. Although not yet available on most clinical MRI machines, the multi-echo UTE sequence (with echo times ranging from 0.03-9ms) is analogous to gradient echo sequences and facilitates optimal detection of various amounts of hemosiderin, including a low burden (which is less apparent on images using an echo time of 0.03 ms but more apparent at longer echo times) to a very high burden (visible on all images, including with an echo time of 0.03 ms).
We demonstrated that MSKUS reliably detected expanded or heterotopic soft tissue, but that it was not able to discriminate between soft tissue types such as coagulated blood, synovial proliferation, fat pad expansion or intra-synovial fatty tissue. Interestingly, our observations also suggest that hemophilic joint bleeding is not limited to intracavitary hemarthrosis, but can also manifest as soft tissue or synovial hemorrhages, as illustrated by the case presented in Figure 4. These hemorrhages cannot be detected by ultrasound, nor easily by MRI. This finding was unexpected and highlights that our understanding of the pathobiology of hemophilic joint bleeding and imaging modalities for detection are incomplete. We found that intra-articular tissues in hemophilic joints can lose their usual ultrasound discriminating features, including morphology, echogenicity, and expected location, in pathological states. For instance, extra-synovial fat pads which are typically smooth and hyperechoic may become irregular, hypoechoic, and form extensions into joint recesses, potentially resembling hypertrophic synovium. We also observed that lipoma arborescens, which signifies fatty synovial metaplasia46, 47, can be easily misinterpreted as synovial proliferation on MSKUS when present in smaller amounts. Extra-synovial fat pad extension into the adjacent medial and lateral recesses has not been previously described, to the best of our knowledge, although the authors have seen this phenomenon in patients without hemophilia. The frequencies and significance of this finding remain to be elucidated. We have also shown that the usual hyperechoic fat pad appearance may change on ultrasound, in analogy to what was previously described with MRI in other arthritic conditions48, 49. While echogenic features of pathologic fat pad alterations have not yet been described systematically, observations from this study suggest that alterations are present and complicate the distinction of fat expansion or displacement from hypertrophic synovium.
Importantly, MSKUS was unable to detect hemosiderin deposits in our study. There were no distinct echogenic features that permitted the determination of whether or not synovium was hemosiderin-laden. This is in contrast to previous findings20, 24, describing hemosiderin-laden synovium as relatively hypoechoic, an observation considered controversial by other experts in the field23. Towards that end, it has to be noted that the hemosiderin-laden synovium tended to appear hypoechoic in a substantial number of cases in our study, but the difference to non-hemosiderin laden synovial proliferation was not statistically significant. Based on our findings it appears that MSKUS cannot reliably detect hemosiderin, and if clinically relevant, requires MRI to make the distinction.
With respect to use of POC MSKUS for the evaluation of hemophilic arthropathy in everyday clinical practice, our findings support MSKUS as a highly sensitive modality to detect the presence of soft tissue alterations, albeit without discrimination between synovial, fatty, and blood origins. Recognizing this limitation appears valuable and relevant when comparing findings to baseline examinations in clinical follow-up over time, as well as for the development of ultrasound scales to quantify and describe the progression of arthropathic changes. Based on our findings we suggest that soft tissue proliferation is best reported nonspecifically as “soft tissue expansion” rather than “synovial proliferation”, and that soft tissue alterations are described applying usual ultrasound nomenclature, but without assignment to specific structures such as synovium. The discrete tissue delineation using ultrasound and MRI, employing conventional and UTE MR sequences, revealed a key finding – namely, that expanding tissues in hemophilic joints are not just synovial, as widely assumed. However, the clinical and pathobiological meaning of this finding is unknown and requires further study.
This study reinforces the importance of following OMERACT guidelines when developing new imaging modalities for arthritic conditions, and highlights the importance of providing objective evidence regarding advantages and limitations of MSKUS. The integration of POC MSKUS to afford insightful, timely, convenient and targeted management of hemophilic arthropathy remains a major advancement for hemophilia care, but requires ongoing validation and standardization. It is imperative that providers recognize the advantages and limitations of MSKUS in order to decide which imaging modality may be most appropriate based on current knowledge to answer a specific question.
Acknowledgments
The authors acknowledge Christine B. Chung, M.D. and Sheronda Statum, M.S., for their assistance with acquisition of the magnetic resonance imaging examinations, and Thomas J. Cramer for logistic study support. This study is supported by Bioverativ (A.v.D., E.C.), and by a career development award from the National Hemophilia Foundation/Novo Nordisk (A.v.D.), and by funding from the Health Resource and Service Agency (HRSA) H30MC24045. E.C. acknowledges support from the Veterans Affairs Clinical Science Research and Development Service (I01CX001388).
References
- 1.Luck JV, Jr, Silva M, Rodriguez-Merchan EC, et al. Hemophilic arthropathy. J Am Acad Orthop Surg. 2004;12(4):234–45. doi: 10.5435/00124635-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Muca-Perja M, Riva S, Grochowska B, et al. Ultrasonography of haemophilic arthropathy. Haemophilia. 2012;18(3):364–8. doi: 10.1111/j.1365-2516.2011.02672.x. [DOI] [PubMed] [Google Scholar]
- 3.Ceponis A, Wong-Sefidan I, Glass CS, et al. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19(5):790–8. doi: 10.1111/hae.12175. [DOI] [PubMed] [Google Scholar]
- 4.Acharya SS, Schloss R, Dyke JP, et al. Power Doppler sonography in the diagnosis of hemophilic synovitis–a promising tool. J Thromb Haemost. 2008;6(12):2055–61. doi: 10.1111/j.1538-7836.2008.03160.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhat V, Olmer M, Joshi S, et al. Vascular remodeling underlies rebleeding in hemophilic arthropathy. Am J Hematol. 2015;90(11):1027–35. doi: 10.1002/ajh.24133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyseure T, Mosnier LO, von Drygalski A. Advances and challenges in hemophilic arthropathy. Semin Hematol. 2016;53(1):10–9. doi: 10.1053/j.seminhematol.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Hua B, Livingston EW, et al. Abnormal joint and bone wound healing in hemophilia mice is improved by extending factor IX activity after hemarthrosis. Blood. 2017;129(15):2161–71. doi: 10.1182/blood-2016-08-734053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung CB. Miscellaneous Disorders of the Elbow. In: Chung CB, Steinbach LS, editors. MRI of the upper extremity : shoulder, elbow, wrist and hand. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010. p. 501. [Google Scholar]
- 9.Loeuille D, Rat AC, Goebel JC, et al. Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthr Cartilage. 2009;17(9):1186–92. doi: 10.1016/j.joca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Roemer FW, Kassim Javaid M, Guermazi A, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18(10):1269–74. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Ohrndorf S, Backhaus M. Advances in sonographic scoring of rheumatoid arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii69–75. doi: 10.1136/annrheumdis-2012-202197. [DOI] [PubMed] [Google Scholar]
- 12.Kidder W, Nguyen S, Larios J, et al. Point-of-care musculoskeletal ultrasound is critical for the diagnosis of hemarthroses, inflammation and soft tissue abnormalities in adult patients with painful haemophilic arthropathy. Haemophilia. 2015;21(4):530–7. doi: 10.1111/hae.12637. [DOI] [PubMed] [Google Scholar]
- 13.Melchiorre D, Linari S, Innocenti M, et al. Ultrasound detects joint damage and bleeding in haemophilic arthropathy: a proposal of a score. Haemophilia. 2011;17(1):112–7. doi: 10.1111/j.1365-2516.2010.02380.x. [DOI] [PubMed] [Google Scholar]
- 14.Coris EE, Pescasio M, Zwygart K, et al. Office-based ultrasound in sports medicine practice. Clin J Sport Med. 2011;21(1):57–61. doi: 10.1097/JSM.0b013e31820758aa. [DOI] [PubMed] [Google Scholar]
- 15.Hall M, Doherty S, Courtney P, et al. Ultrasound detected synovial change and pain response following intra-articular injection of corticosteroid and a placebo in symptomatic osteoarthritic knees: a pilot study. Ann Rheum Dis. 2014;73(8):1590–1. doi: 10.1136/annrheumdis-2014-205206. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol. 2009;193(3):619–27. doi: 10.2214/AJR.09.2841. [DOI] [PubMed] [Google Scholar]
- 17.Aznar JA, Abad-Franch L, Perez-Alenda S, et al. Ultrasonography in the monitoring of management of haemarthrosis. Haemophilia. 2011;17(5):826–8. doi: 10.1111/j.1365-2516.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 18.Aznar JA, Perez-Alenda S, Jaca M, et al. Home-delivered ultrasound monitoring for home treatment of haemarthrosis in haemophilia A. Haemophilia. 2015;21(2):e147–50. doi: 10.1111/hae.12622. [DOI] [PubMed] [Google Scholar]
- 19.Martinoli C, Della Casa Alberighi O, Di Minno G, et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) Thromb Haemost. 2013;109(6):1170–9. doi: 10.1160/TH12-11-0874. [DOI] [PubMed] [Google Scholar]
- 20.Doria AS, Keshava SN, Mohanta A, et al. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. AJR Am J Roentgenol. 2015;204(3):W336–47. doi: 10.2214/AJR.14.12501. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson JA. Musculoskeletal ultrasound update. Semin Musculoskelet Radiol. 2013;17(1):1–2. doi: 10.1055/s-0033-1333907. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485–7. [PubMed] [Google Scholar]
- 23.Martinoli C, Di Minno MN, Pasta G, et al. Hemosiderin Detection With Ultrasound: Reality or Myth? AJR Am J Roentgenol. 2016;206(1):W30. doi: 10.2214/AJR.15.14981. [DOI] [PubMed] [Google Scholar]
- 24.Doria AS, Keshava SN, Gibikote S. Reply to “Hemosiderin Detection With Ultrasound: Reality or Myth?”. AJR Am J Roentgenol. 2016;206(1):W31–5. doi: 10.2214/AJR.15.15535. [DOI] [PubMed] [Google Scholar]
- 25.Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12(5):518–25. doi: 10.1111/j.1365-2516.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 26.Pettersson H, Ahlberg A, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res. 1980;149:153–9. [PubMed] [Google Scholar]
- 27.Lundin B, Manco-Johnson ML, Ignas DM, et al. An MRI scale for assessment of haemophilic arthropathy from the International Prophylaxis Study Group. Haemophilia. 2012;18(6):962–70. doi: 10.1111/j.1365-2516.2012.02883.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang EY, Du J, Bae WC, et al. Qualitative and Quantitative Ultrashort Echo Time Imaging of Musculoskeletal Tissues. Semin Musculoskelet Radiol. 2015;19(4):375–86. doi: 10.1055/s-0035-1563733. [DOI] [PubMed] [Google Scholar]
- 29.Stadler A, Schima W, Ba-Ssalamah A, et al. Artifacts in body MR imaging: their appearance and how to eliminate them. Eur Radiol. 2007;17(5):1242–55. doi: 10.1007/s00330-006-0470-4. [DOI] [PubMed] [Google Scholar]
- 30.Beltran J, Noto AM, Herman LJ, et al. Joint effusions: MR imaging. Radiology. 1986;158(1):133–7. doi: 10.1148/radiology.158.1.3940370. [DOI] [PubMed] [Google Scholar]
- 31.Blackmore CC, Francis CW, Bryant RG, et al. Magnetic resonance imaging of blood and clots in vitro. Invest Radiol. 1990;25(12):1316–24. doi: 10.1097/00004424-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Jeong J, Park S, Jeong E, et al. Time-dependent low-field MRI characteristics of canine blood: an in vitro study. J Vet Sci. 2016;17(1):103–9. doi: 10.4142/jvs.2016.17.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMurdo SK, Jr, Brant-Zawadzki M, Bradley WG, Jr, et al. Dural sinus thrombosis: study using intermediate field strength MR imaging. Radiology. 1986;161(1):83–6. doi: 10.1148/radiology.161.1.3763888. [DOI] [PubMed] [Google Scholar]
- 34.Clark RA, Watanabe AT, Bradley WG, Jr, et al. Acute hematomas: effects of deoxygenation, hematocrit, and fibrin-clot formation and retraction on T2 shortening. Radiology. 1990;175(1):201–6. doi: 10.1148/radiology.175.1.2315481. [DOI] [PubMed] [Google Scholar]
- 35.Kursunoglu-Brahme S, Riccio T, Weisman MH, et al. Rheumatoid knee: role of gadopentetate-enhanced MR imaging. Radiology. 1990;176(3):831–5. doi: 10.1148/radiology.176.3.2389044. [DOI] [PubMed] [Google Scholar]
- 36.Adam G, Dammer M, Bohndorf K, et al. Rheumatoid-Arthritis of the Knee - Value of Gadopentetate Dimeglumine Enhanced Mr Imaging. Am J Roentgenol. 1991;156(1):125–9. doi: 10.2214/ajr.156.1.1898545. [DOI] [PubMed] [Google Scholar]
- 37.Eshed I, Krabbe S, Ostergaard M, et al. Influence of field strength, coil type and image resolution on assessment of synovitis by unenhanced MRI - a comparison with contrast-enhanced MRI. Eur Radiol. 2015;25(4):1059–67. doi: 10.1007/s00330-014-3470-9. [DOI] [PubMed] [Google Scholar]
- 38.Mainardi CL, Levine PH, Werb Z, et al. Proliferative synovitis in hemophilia: biochemical and morphologic observations. Arthritis Rheum. 1978;21(1):137–44. doi: 10.1002/art.1780210122. [DOI] [PubMed] [Google Scholar]
- 39.Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis. 2010;2(6):349–59. doi: 10.1177/1759720X10378373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doria AS, Lundin B, Kilcoyne RF, et al. Reliability of progressive and additive MRI scoring systems for evaluation of haemophilic arthropathy in children: expert MRI Working Group of the International Prophylaxis Study Group. Haemophilia. 2005;11(3):245–53. doi: 10.1111/j.1365-2516.2005.01097.x. [DOI] [PubMed] [Google Scholar]
- 41.Lundin B, Pettersson H, Ljung R. A new magnetic resonance imaging scoring method for assessment of haemophilic arthropathy. Haemophilia. 2004;10(4):383–9. doi: 10.1111/j.1365-2516.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 42.Kidder W, Chang EY, C MM, et al. Persistent Vascular Remodeling and Leakiness are Important Components of the Pathobiology of Re-bleeding in Hemophilic Joints: Two Informative Cases. Microcirculation. 2016;23(5):373–8. doi: 10.1111/micc.12273. [DOI] [PubMed] [Google Scholar]
- 43.Bruyn GA, Naredo E, Iagnocco A, et al. The OMERACT Ultrasound Working Group 10 Years On: Update at OMERACT 12. Journal of Rheumatology. 2015;42(11):2172–6. doi: 10.3899/jrheum.141462. [DOI] [PubMed] [Google Scholar]
- 44.Filippucci E, Di Geso L, Grassi W. TIMELINE Progress in imaging in rheumatology. Nat Rev Rheumatol. 2014;10(10):628–34. doi: 10.1038/nrrheum.2014.145. [DOI] [PubMed] [Google Scholar]
- 45.Keshava SN, Gibikote SV, Mohanta A, et al. Ultrasound and magnetic resonance imaging of healthy paediatric ankles and knees: a baseline for comparison with haemophilic joints. Haemophilia. 2015;21(3):e210–22. doi: 10.1111/hae.12614. [DOI] [PubMed] [Google Scholar]
- 46.Learch TJ, Braaton M. Lipoma arborescens: high-resolution ultrasonographic findings. J Ultrasound Med. 2000;19(6):385–9. doi: 10.7863/jum.2000.19.6.385. [DOI] [PubMed] [Google Scholar]
- 47.Sarawagi R, Vijay S, Kumar Reddy A, et al. Lipoma arborescens: an unusual case of knee swelling. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-203686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vilanova JC, Barcelo J, Villalon M, et al. MR imaging of lipoma arborescens and the associated lesions. Skeletal Radiol. 2003;32(9):504–9. doi: 10.1007/s00256-003-0654-9. [DOI] [PubMed] [Google Scholar]
- 49.Roemer FW, Jarraya M, Felson DT, et al. Magnetic resonance imaging of Hoffa’s fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthritis Cartilage. 2016;24(3):383–97. doi: 10.1016/j.joca.2015.09.018. [DOI] [PubMed] [Google Scholar]


