Abstract
Mood disorders are a spectrum of neuropsychiatric disorders characterized by changes in the emotional state. In particular, major depressive disorder is expected to have a worldwide prevalence of 20% in 2020, representing a huge socio‐economic burden. Currently used antidepressant drugs have poor efficacy with only 30% of the patients in remission after the first line of treatment. Importantly, mood disorder patients present uncoupling of circadian rhythms. In this regard, melatonin (5‐methoxy‐N‐acetyltryptamine), an indolamine synthesized by the pineal gland during the night, contributes to synchronization of body rhythms with the environmental light/dark cycle. In this review, we describe evidence supporting antidepressant‐like actions of melatonin related to the circadian modulation of neuroplastic changes in the hippocampus. We also present evidence for the role of melatonin receptors and their signalling pathways underlying modulatory effects in neuroplasticity. Finally, we briefly discuss the detrimental consequences of circadian disruption on neuroplasticity and mood disorders, due to the modern human lifestyle. Together, data suggest that melatonin's stimulation of neurogenesis and neuronal differentiation is beneficial to patients with mood disorders.
Linked Articles
This article is part of a themed section on Recent Developments in Research of Melatonin and its Potential Therapeutic Applications. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.16/issuetoc
Abbreviations
- BDNF
brain‐derived neurotrophic factor
- CA
cornus ammonis
- CaM
calmodulin
- CMS
chronic mild stress
- FST
forced swimming test
- LAN
light at night
- LL
constant light
- MDD
major depression disorder
- SCN
suprachiasmatic nucleus
- TST
tail suspension test
- ZT
Zeitgeber Time
Introduction
According to the latest version of the Diagnostic and Statistical Manual of Mental Disorders from the American Psychiatric Association (DSM‐5, 2013), mood disorders are defined as a spectrum of neuropsychiatric disorders characterized by disturbances in the emotional state such as prolonged and persistent periods of extreme sadness or extreme happiness. They comprise bipolar disorder, anxiety disorder, seasonal affective disorder, as well as minor, mild and major depression disorder (MDD). Depression is characterized by the loss of interest and pleasure (anhedonia), the lack of energy (anergia), loss of appetite and alterations in sleep patterns that decrease the patients' occupational and social functioning. The aetiology of MDD is multifactorial with genetic, environmental and neuroendocrine factors, all participating in its development.
One important feature of mood disorders is the uncoupling of circadian rhythms. Patients with seasonal affective disorder, MDD and bipolar disorder show a misalignment in the sleep/wake cycle. Also, core body temperature, appetite and hormonal release cycles are altered, including advance of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224 secretion onset in some patients and blunted rhythms of clock genes expression in the brain (Beck‐Friis et al., 1985; Souêtre et al., 1989; Landgraf et al., 2014). Importantly, both low melatonin doses and bright light exposure entrain the sleep–wake cycle and increase the amplitude of the melatonin secretion peak at night (Emens and Burgess, 2015). Many different strategies aimed at counteracting circadian misalignment have been beneficial for the clinical management of mood disorders.
In mammals, the circadian system regulates the timing of physiological and behavioural processes. The hypothalamic biological clock, the suprachiasmatic nucleus (SCN), is considered the master pacemaker. The SCN is synchronized by environmental cues, such as the light/dark cycle, and regulates the circadian rhythm of melatonin synthesis by the pineal gland. Pineal melatonin synthesized during the dark phase is released into the CSF of the third ventricle from which it diffuses to different regions of the brain to signal the environmental photoperiodic changes. Cellular circadian oscillators that control physiological functions might in turn be regulated by melatonin (see Hardeland, 2013).
Melatonin modulates circadian rhythms through signalling pathways coupled to the melatonin receptors, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=287 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=288 (Hardeland, 2013; Liu et al., 2017). Activation of the MT2 receptor phase advances the circadian rhythm of neuronal firing in SCN slices (Hunt et al., 2001), although a role for the MT1 receptor cannot be excluded (Liu et al., 1997). The MT1 receptor appears to be involved in melatonin‐mediated phase advances of circadian rhythms in the mouse in vivo (Dubocovich et al., 2005). Both the MT1 and MT2 receptors are involved in mediating anhedonic‐ and anxiety‐like behaviours (Liu et al., 2017). Recently, melatonin was reported to increase the expression of the clock genes Per1 and Per2, which play a critical role in circadian clock resetting (Kandalepas et al., 2016).
In MDD patients, plasma levels of melatonin are decreased. This is related in part to a persistent brain inflammatory response mediated by the infiltration of immune cells through the blood brain barrier and increased levels of soluble pro‐inflammatory proteins (Anderson, 2017). Decreased levels of melatonin can also be related to a gene variant that codifies for N‐acetylserotonin O‐methyltransferase, a key enzyme in the melatonin biosynthesis that has low mRNA expression and low activity in bipolar disorder (Etain et al., 2011). In addition, patients with either MDD or bipolar disorder may have lower responses to melatonin due to a single nucleotide polymorphism located near the MTNR1A gene for the MT1 receptor (Demirkan et al., 2016).
Another important feature in patients with MDD is a decrease in cognitive functions associated with a reduction in hippocampal volume (Drevets et al., 2008). Hippocampal atrophy implies an overall impairment of neuroplastic processes, which in preclinical studies can be modulated by melatonin. Additionally, in MDD, little attention has been paid to alterations in neuroplasticity due to photoperiod changes, which are environmental cues that entrain circadian rhythms. Therefore, this review focuses on evidence supporting the antidepressant effects of melatonin in relation to the light/dark cycle, and its modulatory role in hippocampal neuroplasticity.
The neurotrophic and glutamatergic hypotheses in the aetiology of major depression: importance for neuroplasticity
In 2000, the neurotrophic hypothesis of MDD was put forward, in place of the monoaminergic hypothesis and highlighting the importance of hippocampal neuroplasticity for the treatment of this disorder. This hypothesis postulates that hippocampal neurogenesis is impaired in depression due to decreased amounts of neurotrophins such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4872 (BDNF), causing a decrease in neuronal survival and alterations in the neuronal connections. In support of this hypothesis, a reduction in the hippocampal volume has been observed in patients with MDD together with decreased levels of BDNF. Also, chronic administration of antidepressants increases BDNF expression in the prefrontal cortex and hippocampus. Importantly, the selective http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=928 inhibitors (SSRIs) besides their antidepressant effects, also stimulate neuroplasticity (Castren and Rantamaki, 2010).
Recent evidence showed that the glutamatergic system is also involved in the aetiology of MDD disease and that http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 antagonists used as antidepressants enhance neurogenesis in the prefrontal cortex and the hippocampus in rodents (Hillhouse and Porter, 2015). High levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1369 both in plasma and in CNS structures have been described in MDD patients (Hashimoto, 2009). High concentrations of glutamate in the CNS induce receptor hyperactivation, particularly of the ionotropic NMDA receptors which increase intracellular Ca2+, leading to neuronal excitotoxicity and cellular death. Also, glutamate inhibits BDNF signalling and, as a consequence, neurogenesis. Melatonin exerts neuroprotective actions by counteracting the NMDA‐mediated excitotoxic effects of glutamate, including the impaired BDNF signalling and cell death (Bavithra et al., 2015). Exogenous melatonin can differentially modulate glutamate release in CNS structures in rodents (Evely et al., 2016), suggesting it might balance the disruption in the glutamatergic system in patients with mood disorders. Together, these data suggest that melatonin may increase neuroplasticity in the CNS acting on the glutamatergic system.
Antidepressant‐like actions of melatonin in animal preclinical studies and clinical studies in humans
Behavioural tests in animals have been a useful tool to study antidepressant effects of drugs and their biochemical mechanisms. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=198, an agonist at MT1 and MT2 receptors, was the first melatonin receptor ligand showing antidepressant‐like activity in animal drug screening tests (Overstreet et al., 1998). Soon it was discovered that its mechanism of antidepressant action also involved blockade of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=8 receptors, with efficacy as a modulator of the monoaminergic system including enhancement of dopaminergic neurotransmission (Chenu et al., 2013). In fact, the discovery that agomelatine had antidepressant effects in humans prompted preclinical research to validate the melatonin antidepressant effects.
The antidepressant‐like effects of melatonin were demonstrated with the forced swimming test (FST), which is considered the gold standard of the antidepressant drug screening tests. Also, the tail suspension test (TST) and the chronic mild stress (CMS) test have provided evidence for the antidepressant‐like actions of melatonin. Both FST and TST are learned helplessness paradigms where animals are exposed to unpredictable and uncontrollable stress, subsequently developing coping deficits for aversive but escapable situations. In male Swiss mice maintained under a 12:12 h light/dark cycle, the immobility time in the TST was reduced by the acute administration of melatonin (Mantovani et al., 2003). This effect appears to be mediated through an interaction with NMDA receptors and the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=721‐http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509 pathway. In contrast, acute administration of melatonin (2.5–10 mg·kg−1) failed to induce an antidepressant‐like effect in mice subjected to the FST for the first time. However, daily administration of melatonin prior to the swimming test significantly reduced the immobility time in the FST (Raghavendra et al., 2000). On the other hand, CMS paradigms are based on the fact that chronic stress contributes to trigger depression in humans in a similar way that exposure to unpredictable stressors elicits altered behaviours in rodents that could be counteracted with antidepressant drugs. Moreover, these paradigms induce high basal http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2869 levels, which are reversed by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=357 and melatonin (Detanico et al., 2009).
The antidepressant actions of melatonin have been related to the activation of melatonin receptors. The administration of the non‐selective and competitive MT1/MT2 receptor antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1363 to wild‐type mice reversed melatonin's antidepressant‐like activity in the FST (Micale et al., 2006). In contrast, mice lacking MT1 receptors display increased behavioural despair in the FST when compared with wild‐type mice (Weil et al., 2006). Also, several studies suggest a hypofunction of the GABAergic system in depression and therefore administration of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1067 agonists should produce antidepressant effects. In this regard, melatonin has been shown to increase the number of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72 receptors in rat brain suggesting this up‐regulation may contribute to its antidepressant‐like action.
In summary, there is sound evidence for the anti‐stress and antidepressant‐like actions of melatonin, suggesting its interaction with several neurotransmitter systems, including the GABAergic, serotoninergic (Micale et al., 2006), glutamatergic and nitrergic systems (Mantovani et al., 2003), as well as the modulation of the hypothalamic–pituitary–adrenal axis (Detanico et al., 2009).
Current clinical studies do not allow clear conclusions regarding melatonin effects as an antidepressant in humans. Most research has used melatonin in humans to alleviate sleep disorders associated with depressed mood (Serfaty et al., 2010). However, patients participating in these clinical trials involve diverse psychiatric diagnoses making comparisons and evaluation of melatonin effectiveness as antidepressant in humans difficult (De Crescenzo et al., 2017). A few clinical studies using better controlled variables and a larger number of patients support the use of melatonin to alleviate the symptoms associated with mood disorders (Beck‐Friis et al., 1985; Garzón et al., 2009; Madsen et al., 2017). However, carefully controlled and double‐blind studies are needed to fully understand the effectiveness of melatonin in mood disorders either alone or as co‐adjuvant to improve mood.
The hippocampus: a neuroplastic brain structure as a target for melatonin antidepressant‐like actions
The human limbic system appears to be the main brain area affected in mood disorders (Figure 1). Brain imaging studies show structural abnormalities including decreased volumetric size in the prefrontal cortex and the hippocampus in these patients. The hippocampus is the major site of action for typical and atypical antidepressants and the structure where cognitive functions such as memory and learning are integrated (Drevets et al., 2008). At the cellular level, structural hippocampal alterations are associated with the loss of subicular dendrites and their arborizations, and diminished neurogenesis in the dentate gyrus. At the behavioural level, the alterations in the hippocampal formation are associated with decreased adaptive responses manifested as a poor social integration and deficits in cognition and memory (Treadway et al., 2015).
Figure 1.
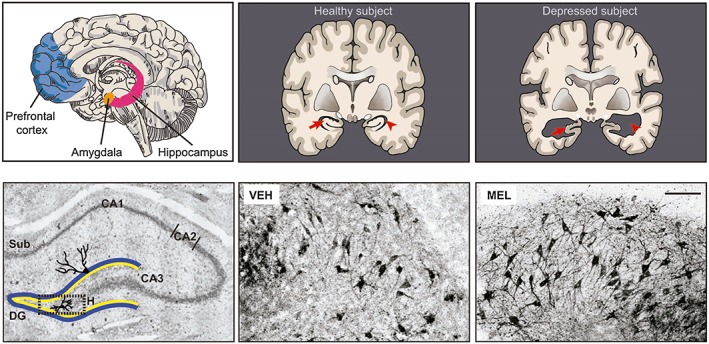
Potential targets for melatonin antidepressant actions. The prefrontal cortex, the amygdala and the hippocampus are altered in mood disorders. In rodents, these brain structures are targets for melatonin actions. In upper panel, schemes of the brain of depressive subjects show that the volume of the hippocampus (red arrows) is decreased and the lateral ventricles (red arrowheads) are enlarged in comparison with healthy subjects. Left lower panel shows the hilar zone of the rat hippocampus (H, dotted square), and the trisynaptic circuitry formed by the subiculum (Sub), the dentate gyrus (DG), and the CA1, CA2 and CA3 regions (Nissl stain). Amplifications of the hilar zone of hippocampal organotypic slices incubated for 6 h with the vehicle (VEH) or 10−7 M melatonin (MEL) are shown in the lower middle and right panels. Dendrites were immunodetected with a MAP2 antibody followed by a secondary antibody coupled to biotin‐avidin‐peroxidase. The anti‐MAP2 antibody stains the soma and the dendrites. Also, melatonin prevents apoptosis (manuscript in preparation). Therefore, hippocampal slices incubated with melatonin showed an increased number of immunopositive cells. Scale bar: 100 μm (Domínguez‐Alonso et al., 2012).
Preclinical evidence has shown that the hippocampus is a main target for melatonin actions (Figure 1), protecting this structure from damage caused by oxidative stress or ischaemia. Also, it promotes dendrite ramifications in cortical pyramidal neurons in rats exposed to toluene (Pascual and Bustamante, 2010) and prevents the loss of neurons in the cornus ammonis (CA)1 and CA3 hippocampal regions in pinealectomized rats (De Butte and Pappas, 2007).
Neuroplasticity is a complex process by which the neurons form intricate networks. It includes the formation of new neurons (neurogenesis), their differentiation (axogenesis and dendritogenesis) and the establishment of proper connectivity into neural circuits (synaptogenesis) allowing the survival and the good adaptation of the organism to the environment. In rodents, melatonin stimulates all stages of neuroplasticity. In particular, the indolamine stimulates neurogenesis in the dentate gyrus in pinealectomized rats (Rennie et al., 2009) and increases neuronal survival in C57BL/6 mice (Ramirez‐Rodriguez et al., 2009). Also, wheel running activity in combination with melatonin treatment stimulates neurogenesis in adult C3H/Hen mice (Liu et al., 2013). Additionally, exogenous melatonin administration may elicit neurogenesis in sleep‐deprived mice.
Melatonin is also known to stimulate dendritogenesis and synaptogenesis in the hippocampus. These processes are disrupted by chronic psychosocial stress as well as in depression (Kendler et al., 1999) and can be reversed by antidepressants. In this regard, in newly formed neurons in the dentate gyrus and in differentiated hilar neurons in cultured hippocampal organotypic slices, melatonin enhanced dendrite formation and the number of synapses after 6 h of incubation (Domínguez‐Alonso et al., 2012) (Figure 1). Moreover, melatonin increased axonal formation in cultured rat hippocampal neurons (Liu et al., 2015), and recently, we reported that melatonin enhances both axonal and synapse formation as well as extrasynaptic secretion in human olfactory neuronal precursors. Together, this evidence indicates that melatonin induces neuroplastic changes in the hippocampus.
The mechanism(s) by which melatonin modulates neuroplastic responses in the hippocampus involves activation of melatonin receptors that have been localized in the dentate gyrus, CA3 and CA1 regions as well as in the subiculum. Activation of MT1 and MT2 receptors modulates cell proliferation in the subgranular zone of the dentate gyrus (Fredrich et al., 2017). The involvement of MT1 and MT2 receptors in axonal formation in human olfactory neuronal precursors and in dendrite formation in organotypic hippocampal slices was recently reported by our group. In addition, melatonin induced dendrite formation by increasing http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2351 (CaM) availability and through the activation of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1554, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=286 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=5141/2 (Domínguez‐Alonso et al., 2015). Relevant for neuroplasticity is the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2030/CRMP‐2 cascade that is involved in axogenesis and can be triggered by melatonin (Liu et al., 2015). Importantly, melatonin has neuroprotective effects mediated by its antioxidant action as a free radical scavenger. In this regard, many in vivo and in vitro bioassays have been used to increase the oxidative status of brain tissues and to test the antioxidant protective effects of melatonin.
It is important to mention that, in addition to improving neuroplasticity in the hippocampus, melatonin attenuates the cognitive and memory deficiencies caused by sleep deprivation and ischaemia reperfusion (Zhang et al., 2013). Also, melatonin receptors are involved in modulating mood behaviour. Thus, genetic deletion of the MT2 receptor but not the MT1 receptor in C3H/HeN mice induced anhedonic behaviour and decreased social interaction while increasing anxiety‐like behaviours (Liu et al., 2017). Conversely, this genetic deletion blocked the antidepressant effects induced by the competitive MT1/MT2 receptor antagonist luzindole (Sumaya et al., 2005). Further research is needed to fully understand the underlying mechanisms by which melatonin modulates behaviour.
Neuroplasticity shows a circadian rhythm: modulatory role of melatonin
The antidepressant effect of bright light in patients with seasonal affective disorder supports the importance of circadian rhythm entrainment in the treatment of MDD (Golden et al., 2005). Preclinical studies have shown that neuroplastic changes in the adult brain follow a circadian rhythm and are also influenced by the light/dark cycle. In most cases, the maximum amplitude of neuroplastic rhythms is observed during the dark phase of the light/dark cycle, regardless of the activity pattern of the species under study. In the subgranular zone of the murine adult hippocampus, proliferation of immature neurons is increased at the middle of the dark phase (Zeitgeber Time 18: ZT18) coincident with the maximum amplitude of melatonin secretion. Neuronal apoptosis has an anti‐phase relationship with proliferation, having a peak at the middle of the light phase (ZT6) when melatonin levels are low. These rhythmic patterns were present only in mice with functional melatonin MT1 and MT2 receptors (Fredrich et al., 2017). Interestingly, in zebrafish, which is a diurnal‐active vertebrate, and in mice who are nocturnal‐active, the different stages of the cell cycle in neurogenic niches display a circadian pattern that reaches its maximum levels during the dark phase (Bouchard‐Cannon et al., 2013; Akle et al., 2017). These patterns correlate with the expression of clock genes Clock1 and Per1 in zebrafish (Akle et al., 2017), and with Per2 and Bmal1 in mice (Bouchard‐Cannon et al., 2013). It is well known that cell proliferation is under the control of clock genes in most mammalian cells, and melatonin can modulate clock proteins at the level of gene expression (Imbesi et al., 2009). Moreover, melatonin might regulate the half‐life of clock proteins by timing their degradation in the proteasome.
Neuroplastic changes in dendrites also display a circadian rhythm. A temporal analysis of hippocampal dendritic structure in Siberian hamsters revealed photoperiod length‐dependent changes that occur during the dark phase of the cycle and were more significant in basilar dendrites of CA1 pyramidal neurons. During short days (8 h L : 16 h D), the number of branch points significantly increases, indicating a more complex dendritic tree, while during long days (16 h L : 8 h D), there is an increase in dendrite length. Exogenous melatonin administration in the late hours of the light phase induces the nocturnal dendritic morphology in CA1 neurons within 4 h. These changes occur together with an increase in the expression of clock genes Per1 and Bmal1 in the hippocampus (Ikeno and Nelson, 2015). Either in short or long photoperiod schemes, the duration of endogenous melatonin secretion peak would be proportional to the length of the dark phase, providing seasonal information to the organism. Whether the differential effects observed in dendrite morphology are modulated by a shorter or longer exposure to endogenous melatonin would be very interesting to find out.
Circadian rhythms in synaptic plasticity have been studied by both structural and electrophysiological approaches. The former showed a circadian pattern reaching a maximum number or size of hippocampal dendritic spines during the dark phase in mice (Ikeda et al., 2015). However, studies in Siberian hamsters indicate a decrease in dendritic spine density during the dark phase in the dentate gyrus (Ikeno and Nelson, 2015). Electrophysiological studies have shown that hippocampal LTP, which is indicative of synaptic strength, was greater in magnitude and had an increased stability in slices obtained from mice killed during the dark phase (Chaudhury et al., 2005). This pattern persisted even in constant darkness conditions or in slices obtained at daytime and LTP‐induction assessed at night‐time, suggesting an endogenous rhythm in synaptic plasticity that could be maintained ex vivo in a brain slice. Melatonin exerts an inhibitory effect on hippocampal induced‐LTP, over a wide range of concentrations (1 nM to 100 μM) (Wang et al., 2005). It is important to mention that these experiments (i.e. hippocampal slices preparation and electrophysiological recordings) were completed during the light phase of the cycle. Thus, interpretation of results is difficult in the context of the possible role of endogenous melatonin in modulating the circadian synaptic plasticity. In summary, the data suggest that hippocampal neuroplasticity is under the influence of the light/dark cycle and melatonin modulation.
Chronodisruption
The light/dark cycle is the most predictable of the environmental cycles and constitutes the strongest Zeitgeber indicating external time for synchronization of circadian rhythms. The lifestyle of modern humans demands that activity is no longer restricted to the natural cycle of illumination. Instead, most of the world's populations nowadays are exposed to non‐natural conditions of light at night (LAN), disrupting circadian rhythms including the nocturnal synthesis and secretion of melatonin (Navara and Nelson, 2007). Other important Zeitgeber systems, such as food intake and social activity, are also affected in our 24/7 society. Detrimental consequences for human health associated with misalignment or disruption between external time cues and endogenous rhythms have become a matter of scientific concern in recent years. In this sense, the term chronodisruption was coined to define ‘a relevant disturbance of the circadian organization of physiology, endocrinology, metabolism and behavior’ (Erren and Reiter, 2013).
Chronodisruption has been associated with an increased number of pathologies including mood disorders (Bedrosian and Nelson, 2017). In animal paradigms of chronodisruption, that is, exposure to constant light (LL) or dim‐LAN, both behaviour and hippocampal neuroplasticity are altered. In mice, LL exposure leads to impaired hippocampal neurogenesis and defective cognitive performance (Fujioka et al., 2011), as well as an increase in behavioural despair in the FST (Fonken et al., 2009). Rats subjected to chronic LL‐exposure displayed both anhedonic‐ and anxiety‐like behaviours and altered patterns of cellular activation in the SCN (Tapia‐Osorio et al., 2013). A similar effect was found in dim‐LAN‐exposed mice (Fonken and Nelson, 2013) and hamsters that presented increased immobility time in the FST and diminished preference to sucrose solution. These altered behaviours correlated with reduced dendritic spine density in hippocampal CA1 neurons and with decreased BDNF gene expression in the hippocampus, relative to animals exposed to a standard light/dark cycle. Interestingly, the biochemical, morphological and behavioural alterations caused by a chronic 4‐week dim‐LAN exposure were reversible after a 2‐week exposure to the regular light/dark cycle (Bedrosian et al., 2013). Similar alterations induced by dim‐LAN exposure have been observed in diurnal‐active rodent species.
Increased helplessness, behavioural despair and anxiety‐like behaviour were observed after knocking down the expression of clock genes at the SCN in mice (Landgraf et al., 2016). Also, it has been recognized that when the SCN is affected, the rhythm of melatonin release is perturbed (Bonmati‐Carrion et al., 2014), which might imply impairment of the oscillatory functions modulated by them. In this regard, dysfunction in hippocampal glutamate signalling and in brain plasticity mechanisms might be produced in the offspring as a consequence of gestational disruption of circadian rhythms by constant illumination.
Together, the evidence suggests that the light/dark cycle and melatonin are two important factors that influence neuroplastic changes in the hippocampus and mood (Figure 2). The increased number of nocturnal workers and the use of electronic devices lead to inappropriate exposure to light during the night with detrimental effects in health. Therefore, it is important to generate public health strategies for light hygiene, that is, a ‘circadian healthy light exposure’ (Bonmati‐Carrion et al., 2014). From our point of view, a thorough re‐evaluation of the potential of melatonin chronotherapy as an alternative for depressive patients, given alone or in combination with current antidepressant treatments, is worthwhile and needs to be undertaken.
Figure 2.
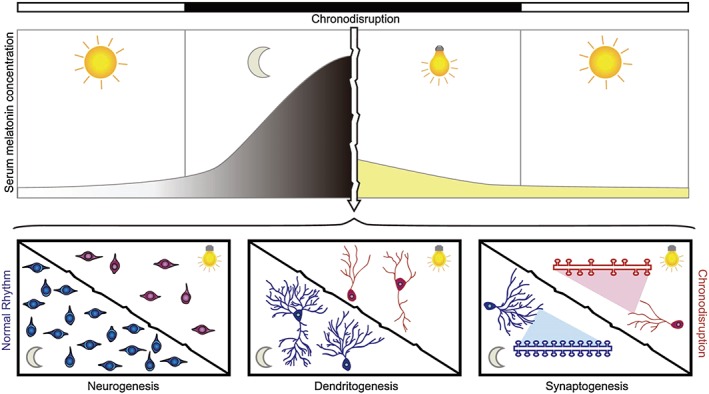
Chronodisruption in neuroplasticity processes related to mood disorders. Our modern society is subjected to artificial light at night (LAN) among other conditions that cause an overall disruption of circadian rhythms. The horizontal bars indicate the phases of the environmental light/dark cycle (light: white bar; dark: black bar). The upper panel represents the serum melatonin concentration, which would reach its higher amplitude during the dark phase. The white arrow indicates chronodisruption by exposure to LAN that inhibits melatonin synthesis and secretion and thus the rhythm of its circulating levels is blunted. In preclinical studies, this condition causes detrimental effects in neuroplasticity processes such as neurogenesis, dendritogenesis and synaptogenesis (represented in lower panels), which have been related to the aetiology of mood disorders and their increasing prevalence in the last decades.
In summary, neuroplasticity is an important process that follows a circadian rhythm for maintaining appropriate mental health. The hippocampus is one of the most affected structures in mood disorders and an important target of the modulatory effects of melatonin. Administration of exogenous melatonin prevents neuronal damage and may stabilize circadian rhythms of neuroplasticity. The preclinical evidence indicates that melatonin augmented the neuroplastic changes in the hippocampus. However, it is necessary to demonstrate its effectiveness in the treatment of depressive patients to obtain translational evidence. Therefore, MRI studies in healthy subjects and in patients with mood disorders are necessary to know if reduced densities and sizes of neurons and reduced hippocampal volumes in depressive patients could be reversed by melatonin administration. Also, it is necessary to know by clinical studies if melatonin co‐administered with conventional antidepressant treatments causes the reversal of symptoms and of the abnormalities in the hippocampal volume.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by Consejo Nacional de Ciencia y Tecnología grant 178075 to G.B.‐K. and grant 252935 to C.T.
Valdés‐Tovar, M. , Estrada‐Reyes, R. , Solís‐Chagoyán, H. , Argueta, J. , Dorantes‐Barrón, A. M. , Quero‐Chávez, D. , Cruz‐Garduño, R. , Cercós, M. G. , Trueta, C. , Oikawa‐Sala, J. , Dubocovich, M. L. , and Benítez‐King, G. (2018) Circadian modulation of neuroplasticity by melatonin: a target in the treatment of depression. British Journal of Pharmacology, 175: 3200–3208. 10.1111/bph.14197.
References
- Akle V, Stankiewicz AJ, Kharchenko V, Yu L, Kharchenko PV, Zhdanova IV (2017). Circadian kinetics of cell cycle progression in adult neurogenic niches of a diurnal vertebrate. J Neurosci 37: 1900–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G (2017). Linking the biological underpinnings of depression: role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog Neuropsychopharmacol Biol Psychiatry 80: 255–266. [DOI] [PubMed] [Google Scholar]
- Bavithra S, Sugantha Priya E, Selvakumar K, Krishnamoorthy G, Arunakaran J (2015). Effect of melatonin on glutamate: BDNF signaling in the cerebral cortex of polychlorinated biphenyls (PCBs)‐exposed adult male rats. Neurochem Res 40: 1858–1869. [DOI] [PubMed] [Google Scholar]
- Beck‐Friis J, Kjellman BF, Aperia B, Undén F, von Rosen D, Liunggren JG et al (1985). Serum melatonin in relation to clinical variables in patients with major depressive disorder and a hypothesis of a low melatonin syndrome. Acta Psychiatr Scand 71: 319–330. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ (2017). Timing of light exposure affects mood and brain circuits. Transl Psychiatry 7: e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Weil ZM, Nelson RJ (2013). Chronic dim light at night provokes reversible depression‐like phenotype: possible role for TNF. Mol Psychiatry 18: 930–936. [DOI] [PubMed] [Google Scholar]
- Bonmati‐Carrion MA, Arguelles‐Prieto R, Martinez‐Madrid MJ, Reiter R, Hardeland R, Rol MA et al (2014). Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci 15: 23448–23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard‐Cannon P, Mendoza‐Viveros L, Yuen A, Kaern M, Cheng HY (2013). The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell‐cycle entry and exit. Cell Rep 5: 961–973. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T (2010). The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70: 289–297. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS (2005). Circadian regulation of hippocampal long‐term potentiation. J Biol Rhythms 20: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu F, El Mansari M, Blier P (2013). Electrophysiological effects of repeated administration of agomelatine on the dopamine, norepinephrine, and serotonin systems in the rat brain. Neuropsychopharmacology 38: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Butte M, Pappas BA (2007). Pinealectomy causes hippocampal CA1 and CA3 cell loss: reversal by melatonin supplementation. Neurobiol Aging 28: 306–313. [DOI] [PubMed] [Google Scholar]
- De Crescenzo F, Lennox A, Gibson JC, Cordey JH, Stockton S, Cowen PJ et al (2017). Melatonin as a treatment for mood disorders: a systematic review. Acta Psychiatr Scand 136: 549–558. [DOI] [PubMed] [Google Scholar]
- Demirkan A, Lahti J, Direk N, Viktorin A, Lunetta KL, Terracciano A et al (2016). Somatic, positive and negative domains of the Center for Epidemiological Studies Depression (CES‐D) scale: a meta‐analysis of genome‐wide association studies. Psychol Med 46: 1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detanico BC, Piato AL, Freitas JJ, Lhullier FL, Hidalgo MP, Caumo W et al (2009). Antidepressant‐like effects of melatonin in the mouse chronic mild stress model. Eur J Pharmacol 607: 121–125. [DOI] [PubMed] [Google Scholar]
- Domínguez‐Alonso A, Ramírez‐Rodriguez G, Benítez‐King G (2012). Melatonin increases dendritogenesis in the hilus of hippocampal organotypic cultures. J Pineal Res 52: 427–436. [DOI] [PubMed] [Google Scholar]
- Domínguez‐Alonso A, Valdés‐Tovar M, Solís‐Chagoyán H, Benítez‐King G (2015). Melatonin stimulates dendrite formation and complexity in the hilar zone of the rat hippocampus: participation of the Ca++/Calmodulin complex. Int J Mol Sci 16: 1907–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213: 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E (2005). Effect of MT1 melatonin receptor deletion on melatonin‐mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res 39: 113–120. [DOI] [PubMed] [Google Scholar]
- Emens JS, Burgess HJ (2015). Effect of light and melatonin and other melatonin receptor agonists on human circadian physiology. Sleep Med Clin 10: 435–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erren TC, Reiter RJ (2013). Revisiting chronodisruption: when the physiological nexus between internal and external times splits in humans. Naturwissenschaften 100: 291–298. [DOI] [PubMed] [Google Scholar]
- Etain B, Milhiet V, Belliver F, Leboyer M (2011). Genetics of circadian rhythms and mood spectrum disorders. Eur Neuropsychopharmacol 21 (Suppl 4): S676–S682. [DOI] [PubMed] [Google Scholar]
- Evely KM, Hudson RL, Dubocovich ML, Haj‐Dahmane S (2016). Melatonin receptor activation increases glutamatergic synaptic transmission in the rat medial lateral habenula. Synapse 70: 181–186. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J et al (2009). Influence of light at night on murine anxiety‐ and depressive‐like responses. Behav Brain Res 205: 349–354. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ (2013). Dim light at night increases depressive‐like responses in male C3H/HeNHsd mice. Behav Brain Res 243: 74–78. [DOI] [PubMed] [Google Scholar]
- Fredrich M, Hampel M, Seidel K, Christ E, Korf HW (2017). Impact of melatonin receptor‐signaling on Zeitgeber time‐dependent changes in cell proliferation and apoptosis in the adult murine hippocampus. Hippocampus 27: 495–506. [DOI] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Tsuruta R, Izumi T, Kasaoka S, Maekawa T (2011). Effects of a constant light environment on hipocampal neurogenesis and memory in mice. Neurosci Lett 488: 41–44. [DOI] [PubMed] [Google Scholar]
- Garzón C, Guerrero JM, Aramburu O, Guzmán T (2009). Effect of melatonin administration on sleep, behavioral disorders and hypnotic drug discontinuation in the elderly: a randomized, double‐blind, placebo‐controlled study. Aging Clin Exp Res 21: 38–42. [DOI] [PubMed] [Google Scholar]
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T et al (2005). The efficacy of light therapy in the treatment of mood disorders: a review and meta‐analysis of the evidence. Am J Psychiatry 162: 656–662. [DOI] [PubMed] [Google Scholar]
- Hardeland R (2013). Chronobiology of melatonin beyond the feedback to the suprachiasmatic nucleus‐consequences to melatonin dysfunction. Int J Mol Sci 14: 5817–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K (2009). Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 61: 105–123. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH (2015). A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol 23: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt AE, Al‐Ghoul WM, Gillette MU, Dubocovich ML (2001). Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol 280: C110–C118. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Hojo Y, Komatsuzaki Y, Okamoto M, Kato A, Takeda T et al (2015). Hippocampal spine changes across the sleep‐wake cycle: corticosterone and kinases. J Endocrinol 226: M13–M27. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Nelson RJ (2015). Acute melatonin treatment alters dendritic morphology and circadian clock gene expression in the hippocampus of Siberian hamsters. Hippocampus 25: 142–148. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Arslan AD, Yildiz S, Sharma R, Gavin D, Tun N et al (2009). The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal 'clock' gene expression in striatal neurons in vitro. J Pineal Res 46: 87–94. [DOI] [PubMed] [Google Scholar]
- Kandalepas PC, Mitchell JW, Gillette MU (2016). Melatonin signal transduction pathways require E‐box‐mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLos One 11: e0157824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA (1999). Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156: 837–841. [DOI] [PubMed] [Google Scholar]
- Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, Welsh DK (2016). Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety‐like behavior in mice. Biol Psychiatry 80: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, McCarthy MJ, Welsh DK (2014). Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr Psychiatry Rep 16: 483. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK et al (1997). Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19: 91–102. [DOI] [PubMed] [Google Scholar]
- Liu D, Wei N, Man HY, Lu Y, Zhu LQ, Wang JZ (2015). The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ 22: 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Clough SJ, Dubocovich ML (2017). Role of the MT1 and MT2 melatonin receptors in mediating depressive‐ and anxiety‐like behaviors in C3H/HeN mice. Genes Brain Behav 16: 546–553. [DOI] [PubMed] [Google Scholar]
- Liu J, Somera‐Molina KC, Hudson RL, Dubocovich ML (2013). Melatonin potentiates running wheel‐induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J Pineal Res 54: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MT, Isbrand A, Andersen UO, Andersen LJ, Taskiran M, Simonsen E et al (2017). The effect of MElatonin on Depressive symptoms, Anxiety, CIrcadian and Sleep disturbances in patients after acute coronary syndrome (MEDACIS): study protocol for a randomized controlled trial. Trials 18: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani M, Pertile R, Calixto JB, Santos AR, Rodrigues AL (2003). Melatonin exerts an antidepressant‐like effect in the tail suspension test in mice: evidence for involvement of N‐methyl‐D‐aspartate receptors and the L‐arginine‐nitric oxide pathway. Neurosci Lett 343: 1–4. [DOI] [PubMed] [Google Scholar]
- Micale V, Arezzi A, Rampello L, Drago F (2006). Melatonin affects the immobility time of rats in the forced swim test: the role of serotonin neurotransmission. Eur Neuropsychopharmacol 16: 538–545. [DOI] [PubMed] [Google Scholar]
- Navara KJ, Nelson RJ (2007). The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res 43: 215–224. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Pucilowski O, Retton MC, Delagrange P, Guardiola‐Lemaitre B (1998). Effects of melatonin receptor ligands on swim test immobility. Neuroreport 9: 249–253. [DOI] [PubMed] [Google Scholar]
- Pascual R, Bustamante C (2010). Melatonin promotes distal dendritic ramifications in layer II/III cortical pyramidal cells of rats exposed to toluene vapors. Brain Res 1355: 214–220. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Kaur G, Kulkarni SK (2000). Anti‐depressant action of melatonin in chronic forced swimming‐induced behavioral despair in mice, role of peripheral benzodiazepine receptor modulation. Eur Neuropsychopharmacol 10: 473–481. [DOI] [PubMed] [Google Scholar]
- Ramirez‐Rodriguez G, Klempin F, Babu H, Benítez‐King G, Kempermann G (2009). Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 34: 2180–2191. [DOI] [PubMed] [Google Scholar]
- Rennie K, De Butte M, Pappas BA (2009). Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J Pineal Res 47: 313–317. [DOI] [PubMed] [Google Scholar]
- Serfaty MA, Osborne D, Buszewicz MJ, Blizard R, Raven PW (2010). A randomized double‐blind placebo‐controlled trial of treatment as usual plus exogenous slow‐release melatonin (6 mg) or placebo for sleep disturbance and depressed mood. Int Clin Psychopharmacol 25: 132–142. [DOI] [PubMed] [Google Scholar]
- Souêtre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B et al (1989). Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res 28: 263–278. [DOI] [PubMed] [Google Scholar]
- Sumaya IC, Masana MI, Dubocovich ML (2005). The antidepressant‐like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J Pineal Res 39: 170–177. [DOI] [PubMed] [Google Scholar]
- Tapia‐Osorio A, Salgado‐Delgado R, Angeles‐Castellanos M, Escobar C (2013). Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety‐like behaviors in the rat. Behav Brain Res 252: 1–9. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MT, Chakravarty MM et al (2015). Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 77: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS (2005). Melatonin inhibits hippocampal long‐term potentiation. Eur J Neurosci 22: 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Hotchkiss AK, Gatien ML, Pieke‐Dahl S, Nelson RJ (2006). Melatonin receptor (MT1) knockout mice display depression‐like behaviors and deficits in sensorimotor gating. Brain Res Bull 68: 425–429. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang HQ, Liang XY, Zhang HF, Zhang T, Liu FE (2013). Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress, BDNF and CaMKII. Behav Brain Res 256: 72–81. [DOI] [PubMed] [Google Scholar]


