Abstract
Background and Purpose
The aim of this study was to explore if the administration of naltrexone together with cannabidiol (CBD) may improve the efficacy in reducing alcohol consumption and motivation rather than any of the drugs given separately.
Experimental Approach
The effects of low doses of naltrexone (0.7 mg·kg−1, p.o.) and/or CBD (20 mg·kg−1·day−1, s.c.) on ethanol consumption and motivation to drink were evaluated in the oral‐ethanol self‐administration paradigm in C57BL/6 mice. Gene expression analyses of the opioid μ receptor (Oprm1) in the nucleus accumbens (NAc), tyrosine hydroxylase (TH) in the ventral tegmental area (VTA) and the 5‐HT1A receptor in the dorsal raphe nucleus (DR) were carried out by real‐time PCR. The role of 5‐HT1A receptors in the ethanol reduction induced by the administration of CBD + naltrexone was analysed by using the 5‐HT1A receptor antagonist WAY100635 (0.3 mg·kg−1, i.p.).
Key Results
The administration of CBD + naltrexone significantly reduced motivation and ethanol intake in the oral self‐administration procedure in a greater proportion than the drugs given alone. Only the combination of both drugs significantly reduced Oprm1, TH and 5‐HT1A gene expressions in the NAc, VTA and DR respectively. Interestingly, the administration of WAY100635 significantly blocked the actions of CBD + naltrexone but had no effects by itself.
Conclusion and Implications
The combination of low doses of CBD plus naltrexone were more effective than either CBD or naltrexone alone at reducing ethanol consumption and the motivation to drink. These effects appear to be mediated, at least in part, by 5‐HT1A receptors.
Abbreviations
- AUD
alcohol use disorders
- CB receptor
cannabinoid receptor
- CBD
cannabidiol
- DR
dorsal raphe nucleus
- NAc
nucleus accumbens
- OEA
oral ethanol self‐administration
- Oprm1
opioid μ receptor gene
- VTA
ventral tegmental area
Introduction
The limited efficacy of the current pharmacological treatments for alcohol use disorders (AUD) justifies the development of alternative drugs. In this respect, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150 (CBD), one of the main compounds present in the plant Cannabis sativa, which lacks activity as a drug of abuse (Fusar‐Poli et al., 2009; Manzanares et al., 2016; Martin‐Santos et al., 2012; Mechoulam et al., 2002; Manzanares and Garcia‐Gutierrez, 2017; Winton‐Brown et al., 2011; Zlebnik and Cheer, 2016), has been pointed out as a new potential therapeutic drug for the treatment of drug use disorders due to its anxiolytic (Guimaraes et al., 1990; Moreira et al., 2006; Resstel et al., 2006; Lemos et al., 2010; de Mello Schier et al., 2014; Blessing et al., 2015), antidepressant (El‐Alfy et al., 2010; Zanelati et al., 2010), antipsychotic (Zuardi et al., 1991; Moreira and Guimaraes, 2005; Long et al., 2006; Leweke et al., 2012; Levin et al., 2014; Peres et al., 2016) and neuroprotective properties (Hamelink et al., 2005; Campos et al., 2016).
Interestingly, recent evidence revealed that CBD reduces http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9082 craving and relapse (Ren et al., 2009) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2286 intake (Weiss et al., 2016). Furthermore, our group has demonstrated that CBD also decreases http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2299 intake and ethanol preference in the two‐bottle choice paradigm in mice. In addition, a single administration of a controlled release formulation of CBD (30 mg·kg−1·day−1, s.c.) that lasted for up to 2 weeks significantly decreased motivation to drink and ethanol consumption in the oral ethanol self‐administration (OEA) paradigm. CBD also reduced ethanol‐induced relapse but had no effect over non‐reinforcing substances, such as water. These behavioural effects were associated with alterations in key targets and brain regions closely related with alcohol consumption, such as http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1243 in the ventral tegmental area (VTA), the opioid http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319 (Oprm1) and cannabinoid receptors (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=109) in the nucleus accumbens (NAc), both critical regions for reward, goal‐directed behaviour and habit formation (Viudez‐Martinez et al., 2018). Altogether, these results supported the potential therapeutic use of CBD in the treatment of AUD.
Despite the devastating impact of AUD on society, current options for treatment are scarce and have limited efficacy (Lee and Leggio, 2014). To date, there are just three drug‐based treatments approved for AUD by the Food and Drug Administration and the European Medicines Agency: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1639, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7168 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7106 (Rosner et al., 2010; Jarosz et al., 2013; Skinner et al., 2014). Other drug‐based therapies are usually employed off‐label, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6849, an anticonvulsant drug with a broad‐spectrum activity that seems effective in treating alcohol dependence (Johnson et al., 2003). Nevertheless, naltrexone is still the most effective drug available for the treatment of AUD since it reduces heavy drinking and ethanol craving by antagonizing the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3723‐stimulated http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940 release in the NAc induced by alcohol (Nicholson et al., 2018).
The combination of different drugs is also a commonly used procedure for the treatment of AUD to achieve a greater effect than individual drug therapies by using lower doses of each drug than the ones employed in monotherapy. This strategy also prevents certain dose‐related side effects. In this respect, the combination of naltrexone with other drugs, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5483 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5 reuptake inhibitors, showed a greater clinical outcome in several clinical trials (Anton et al., 2011; Froehlich et al., 2013) and animal studies (Froehlich et al., 2013). However, no combination has been proposed to be superior due to the variability among studies (Lee and Leggio, 2014).
In the present study, we further explored whether CBD improves the efficacy of naltrexone to reduce alcohol consumption and motivation to drink in mice. With this aim, the effects of a sub‐effective dose of naltrexone (0.7 mg·kg−1, p.o.), CBD (20 mg·kg−1·day−1, s.c., poly‐ε‐caprolactone spherical microparticles with small pores providing a continuous controlled release during 3 weeks) or their combination were employed. Dose selection was made according to published evidence showing that naltrexone (0.7 mg·kg−1, p.o.), a lower dose than the one commonly used in most studies, is able to reduce ethanol intake in mice (Navarrete et al., 2014), although it is not always effective (Oliva and Manzanares, 2007). For CBD, a lower dose than the one our group previously reported as effective (Viudez‐Martinez et al., 2018) was evaluated in the OEA paradigm in C57BL/6J male mice. Subsequent real‐time PCR experiments were performed to analyse gene expression changes in Oprm1 in the NAc, TH in the VTA and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1 in the dorsal raphe nucleus (DR) respectively.
Furthermore, to explore the role of 5‐HT1A receptors, one of the main targets for CBD (Blessing et al., 2015; Ibeas Bih et al., 2015), in the effects of CBD plus naltrexone, the 5‐HT1A antagonist, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=80 (0.3 mg·kg−1, i.p.), was administered previously to CBD and naltrexone in the OEA paradigm. To this purpose, the dose of WAY100635 was chosen according to published studies showing that administration of this compound (0.5 mg·kg−1, i.p.) seems to prevent the reduction of ethanol intake produced by 5‐HT1A receptor agonists, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7, in male C57BL/6J mice (Kelai et al., 2006), but did not present effects on its own. Additionally, another group reported that the antipanic‐like effects of CBD were blocked when WAY100635 (0.3 mg·kg−1, i.p.) was given to male Swiss mice (Twardowschy et al., 2013) and was without effect when given alone.
Methods
Mice
One hundred and forty C57BL/6J male mice from Charles River (Lille, France), 70 for each experiment, weighing 20–25 g, were housed in groups of six per cage (40 × 25 × 22 cm) under controlled conditions (temperature, 23 ± 2°C; relative humidity, 60 ± 10%; 12 h light/dark cycle, lights on from 8:00 to 20:00 h). The strain and gender of mice were selected based on the results previously reported by our group (Viudez‐Martinez et al., 2018). Behavioural analyses were initiated 1 week after acclimatization to the animal room and were performed by placing the home cage in the operant‐task room during the development of conditioning experiments. All the studies were conducted in compliance with the Spanish Royal Decree 1201/2005, the Spanish Law 32/2007 and the European Union Directive of the 22nd of September 2010 (2010/63/UE) regulating the care of experimental animals, and the University Miguel Hernández Research Ethics Committee approved the experiments. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Behavioural analyses
All the experiments were analysed by observers blind to treatment.
Experiment 1: to evaluate the effects of the combination of CBD plus naltrexone on ethanol consumption and motivation to drink
Oral ethanol self‐administration paradigm
Our group performed the OEA following a published protocol (Navarrete et al., 2014). The OEA was carried out in 18 modular operant chambers (Panlab) placed inside 18 noise isolation boxes equipped with a chamber light, two levers, one receptacle to drop liquid solution, one syringe pump, one stimulus light and one buzzer. Packwin software (Panlab) controlled the stimulus and fluid delivery and recorded operant responses.
Pressing on one of the levers did not have any consequences (inactive lever), whereas pressing the other lever (active lever) delivered 36 μL fluid combined with a 0.5 s bright stimulus and a 0.5 s, 2850 Hz, 85 dB buzzer beep, followed by a time‐out period of 6 s, in which no fluid was delivered. After the 6 s time out, an intertrial interval started, the duration of which depended on each subject's spontaneous waiting time before an active lever press. The experiment was divided into three phases: training, saccharin substitution and ethanol 8% (v.v‐1) consumption (see Figure 1).
Training phase (9 days): Two days before beginning the experiment, standard chow was restricted to only 1 h access a day. Before the first training session, water access was restricted for 24 h to increase the motivation for lever pressing during the first training session according to protocols previously described (Cohen et al., 1999; Middaugh and Kelley, 1999; Orru et al., 2012; Navarrete et al., 2014; Garcia‐Gutierrez et al., 2016; Viudez‐Martinez et al., 2018). During this deprivation period, no behavioural or physical decline was observed. The body weight fluctuation was never higher than 10% (see Supporting Information Data S1). Food allotment was provided 1 h prior to the 60 min session to also increase the motivation for lever pressing. During the subsequent 4 days, water was provided ad libitum except during food access for 1 h before beginning each session, in which the water bottle was removed from the cages (postprandial). The following 5 days and during the rest of the experiment, food access was provided for 1 h after the end of each daily session, and water was available ad libitum to avoid ethanol consumption due to thirst (preprandial). C57BL/6J mice were trained to press on the active lever to receive 36 μL of 0.2% (w.v‐1) saccharin reinforcement.
Saccharin substitution (9 days): The saccharin (Sac) concentration was gradually faded out as the ethanol concentration was gradually increased (Grant and Samson, 1986). Each solution combination was fixed to three consecutive sessions per combination (0.15% Sac–2.5% EtOH, 0.10% Sac–5% EtOH, 0.05% Sac–8% EtOH).
Basal 8% (v.v‐1) ethanol consumption (11 days): The number of responses on the active lever, the 8% ethanol (v.v‐1) consumption and the motivation to drink in C57BL/6 mice without pharmacological treatment were measured. There were three phases: (i) Fixed ratio 1 (FR1), mice responding on the active lever to obtain 8% ethanol and no saccharin were evaluated using an FR1 reinforcement schedule during five daily consecutive 1 h sessions; (ii) FR3, after FR1 mice underwent five daily 1 h sessions using an FR3 reinforcement schedule (mice have to respond three times on the active lever to achieve one reinforcement); and (iii) progressive ratio (PR), on the day subsequent to FR3, a PR session was carried out. In this session, the response requirement to earn reinforcements escalated according to the following series: 1‐2‐3‐5‐12‐18‐27‐40‐60‐90‐135‐200‐300‐450‐675‐1000. The PR session lasted for 2 h and the ‘breaking point’ (the maximum number of lever presses each animal was able to perform to achieve one reinforcement) was determined in each animal.
Effects of pharmacological treatment on 8% ethanol consumption (9 days): Once the animals underwent the FR1, FR3 and PR phases, they were selected according to the following learning task criteria: (i) reaching ≥70% of preference for the active lever; (ii) ≥10 reinforced trials by session in FR1 and FR3, and ≥5 reinforced trials in PR; (iii) ≤30% deviation in the number of reinforced trials, during the last three consecutive days (FR1 and FR3); (iv) mean 8% ethanol consumption ≥500 μL (1.5 g·kg−1) in FR1, ≥300 μL (0.9 g·kg−1) in FR3 and ≥117.5 μL (0.35 g·kg−1) in PR; and (v) a breaking point ≥12 in PR. Mice reaching these criteria were randomly distributed into the following groups: vehicle + vehicle (VEH + VEH) (n = 11), vehicle + naltrexone (0.7 mg·kg−1, p.o) (VEH + NTX) (n = 11), CBD (20 mg·kg−1, s.c.) + vehicle (CBD + VEH) (n = 11) and CBD (20 mg·kg−1, s.c.) + naltrexone (0.7 mg·kg−1, p.o) (CBD + NTX) (n = 11). Selected mice underwent an FR1 stabilization phase (5 days), in order to stabilize the ethanol intake after the PR stage. Then they were exposed again to FR1 (4 days), FR3 (4 days) and PR (1 day) stages receiving the corresponding treatment as explained in Figure 1. For all the different stages, the ethanol left in the receptacle was detracted from the total amount of ethanol delivered, getting the real amount of ethanol consumed [Ethanol solution intake = 37 μL volume delivered – volume left on the receptacle].
Figure 1.
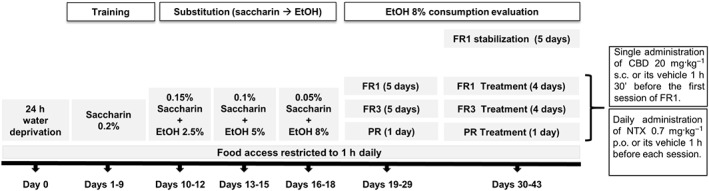
Schematic diagram of the ethanol oral self‐administration schedule including the different experimental phases. FR1; FR3; PR.
Gene expression studies by real‐time PCR
Mice were killed by cervical dislocation 2 h and 30 min after the vehicle or corresponding drug administration of the last OEA session. Brains were removed from the skull and frozen at −80°C. Briefly, brain sections were cut (500 μm) in a cryostat (−10°C) containing the regions of interest (NAc, VTA and DR) according to Paxinos and Franklin (2001), mounted onto slides and stored at −80°C. Sections were microdissected following the method described by Palkovits and previously performed by our group (Palkovits, 1983; Garcia‐Gutierrez et al., 2010). Total RNA was obtained from brain micropunches with TRI Reagent extraction reagent (Applied Biosystems, Madrid, Spain). Reverse transcription was carried out following the instructions of the manufacturer (Applied Biosystems, Madrid, Spain). Quantitative analysis of the relative abundance of TH (Mm00447546_m1), Oprm1 (Mm01188089_m1) and 5‐HT1A receptor (Mm00434106_s1) gene expressions was performed on the StepOne Sequence Detector System (Applied Biosystems, Madrid, Spain). All of the reagents were obtained from Life Technologies, and the manufacturer's protocols were followed. The reference gene used was 18S rRNA (Mm03928990_g1). Data for each target gene were normalized to the endogenous reference gene, and the fold change in target gene expression was determined using the 2‐ΔΔCt method (Livak and Schmittgen, 2001).
Experiment 2: to evaluate the role of 5‐HT1A receptors in the effects exerted by the combination of CBD plus naltrexone on ethanol consumption and motivation to drink
A further OEA experiment was carried out following the protocol described above. For this experiment, once the animals underwent the FR1, FR3 and PR phases, they were selected according to the learning criteria previously specified and randomly distributed into the following groups: (i) vehicle + vehicle (VEH + VEH) (n = 10); (ii) WAY 100635 (0.3 mg·kg−1, i.p.) + vehicle (WAY + VEH) (n = 10); (iii) WAY 100635 (0.3 mg·kg−1, i.p.) + CBD (20 mg·kg−1, s.c.) + naltrexone (0.7 mg·kg−1, p.o.) (WAY + CBD + NTX) (n = 10); and (iv) vehicle + CBD (20 mg·kg−1, s.c.) + naltrexone (0.7 mg·kg−1, p.o) (VEH + CBD + NTX) (n = 10). The selected mice underwent the FR1 (4 days), FR3 (4 days) and PR (1 day) stages receiving the corresponding treatment as explained in the Materials section.
Group sizes
Group sizes were determined after performing different power calculations. The results of these tests showed that, in order to obtain a power >0.8, between 8 and 10 subjects were needed for each group. Taking into account that only around a 60% of mice that underwent the OEA would match the learning and consumption criteria needed to undergo the treatment evaluation phase, we employed 70 mice for each experiment. After undergoing the training, substitution, FR1, FR3 and PR phases, 44 mice matched the selection criteria (n = 11 per group) for receiving treatment in experiment 1 and 40 subjects (n = 10 per group) in experiment 2.
Statistical analyses
Statistical analyses were performed using two‐way ANOVA with repeated measures followed by the Student–Newman–Keuls test to compare the treatment and control groups at different time points on the OEA paradigms; post hoc tests were only applied when ANOVA (F value) indicated significance. The data obtained from the gene expression studies and PR phase in OEA were statistically analysed using the two‐way ANOVA test. Statistical analyses were performed with SigmaPlot v11.0 (Systat Software Inc., Chicago, IL, USA) software. Differences were considered significant if the probability of error was less than 5%. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018).
Materials
Poly‐ε‐caprolactone spherical microparticles with small pores providing a CBD continuous controlled release (20 mg·kg−1·day−1, s.c.) that lasts for more than 2 weeks and its respective vehicle (empty controlled release microparticles) were obtained from the Pharmaceutical Technology Department (Complutense University of Madrid, Madrid, Spain) (Viudez‐Martinez et al., 2018), suspended in PBS 1X (pH 7.4) + 1% Pluronic F‐68 (w.v‐1) and then administered (0.4 mL). After the fixed ratio 1 (FR1) stabilization phase, CBD continuous controlled release or its vehicle were administered only once on the first day after the stabilization phase, half an hour after administering WAY 100635 or VEH and half an hour before administering naltrexone or VEH (1 h and a half before starting the experimental session).
Naltrexone (naltrexone hydrochloride, Accord Healthcare S.L.U., Barcelona, Spain) was daily dissolved in tap water to obtain the desired concentration (0.7 mg·kg−1, p.o., 0.3 mL). The naltrexone solution or its vehicle was administered once daily after the FR1 stabilization phase, 1 h before the beginning of each session.
WAY 100635 (WAY 100635 maleate; TOCRIS, Madrid, Spain) was daily dissolved in saline 0.9% w.v‐1 to obtain the required concentration (0.3 mg·kg−1, i.p., 0.3 mL). The WAY 100635 solution or its vehicle was administered once daily after the FR1 stabilization phase, 2 h before the beginning of each session and 1 h prior the administration of naltrexone or VEH.
For the oral self‐administration procedure, absolute ethanol (Merck, Spain) and saccharin sodium salt were dissolved in tap water [8% (v.v‐1) ethanol solution (EtOH)].
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Results
Experiment 1: to evaluate the effects of the combination of CBD plus naltrexone on ethanol consumption and motivation to drink
Oral ethanol self‐administration
During the stabilization phase, no significant differences were observed in the number of active lever presses (Figure 2A) (P > 0.05, two‐way ANOVA repeated measures) nor in the ethanol intake (Figure 2B) (P > 0.05, two‐way ANOVA repeated measures) between the different groups before being treated.
Figure 2.
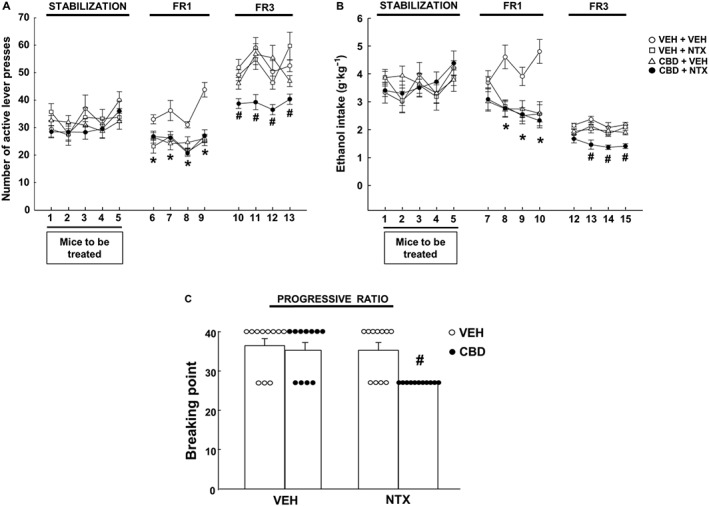
Evaluation of the effects of the combination of CBD plus naltrexone (NTX) on ethanol oral self‐administration in C57BL/6J mice. (A) Number of active responses (active levers presses that release ethanol solution) during the FR1 stabilization, FR1 + treatment and FR3 + treatment phases; (B) ethanol intake expressed as g·kg−1 of all groups during the FR1 stabilization, FR1 + treatment and FR3 + treatment phases; (C) breaking point achieved during PR. The dots and the columns represent the means and vertical lines ± the SEM. * Represents the values from VEH + NTX, CBD + VEH and CBD + NTX mice that are significantly different (P < 0.05) from VEH + VEH group. # Represents the values from CBD + NTX‐treated group that are significantly different (P < 0.05) from VEH + VEH, NTX + VEH and CBD + VEH groups (n = 11 for each group).
The administration of CBD controlled release microparticle s.c. formulation (20 mg·kg−1, s.c.), naltrexone (0.7 mg·kg−1, p.o.) or their combination significantly reduced the number of active lever presses during FR1 (Figure 2A) (P < 0.05, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test). Both treatments and their combination successfully reduced ethanol intake on days 8, 9 and 10 during FR1 phase when compared with the control group VEH + VEH (Figure 2B) (P < 0.001, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test).
The combination of CBD plus naltrexone was the only treatment successful in reducing the number of active lever presses during FR3 (Figure 2A) (P < 0.05, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test). Furthermore, only the combination CBD + naltrexone was able to reduce ethanol intake on days 13, 14 and 15 during FR3 stage (Figure 2B) (P < 0.05, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test).
Interestingly, during the PR phase, only the CBD + NTX group presented a lower breaking point compared to the VEH + VEH, VEH + NTX and CBD + VEH groups (Figure 2C) (P < 0.05, two‐way ANOVA followed by Student–Newman–Keuls test).
Gene expression analyses
The administration of CBD (20 mg·kg−1, s.c.) reduced the relative gene expression of TH (−35%) in the VTA when compared to VEH + VEH. Interestingly, the combination of CBD + NTX reduced the gene expression of TH in a 50% when compared to VEH + VEH groups (Figure 3A) (P < 0.05, two‐way ANOVA followed by Student–Newman–Keuls test). However, naltrexone failed to modify relative gene expression of TH.
Figure 3.
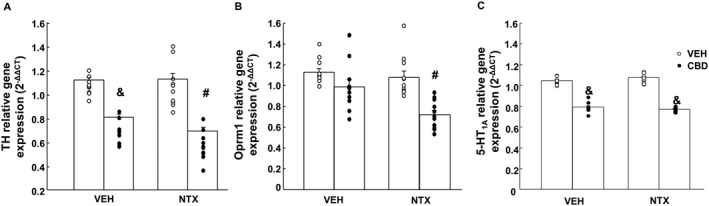
Gene expression studies of (A) TH in the VTA, (B) μ receptor (Oprm1) in the NAc and (C) 5‐HT1A receptor in the DR of C57BL/6J mice treated with naltrexone (NTX; 0.7 mg·kg−1, p.o.), CBD [a single administration of a microparticle formulation providing CBD continuous controlled release (20 mg·kg−1·day−1, s.c.)] or their combination. The columns represent the means and vertical lines ± the SEM. & Represents the values from the groups that are significantly different (P < 0.05) from VEH + VEH and VEH + NTX groups. # Represents the values from the groups that are significantly different (P < 0.005) from CBD + VEH, VEH + NTX and VEH + VEH groups (n = 11 for each group).
In addition, only the combination of CBD + NTX significantly reduced Oprm1 expression (−37%) in the NAc compared with its corresponding control group VEH + VEH (P < 0.05, two‐way ANOVA followed by Student–Newman–Keuls test). Nevertheless, the administration of CBD or naltrexone did not induce any alteration (Figure 3B).
5‐HT1A receptor gene expression was reduced in the DR of CBD + VEH (−22%) and CBD + NTX (−27%) groups compared with their corresponding control group VEH + VEH (P < 0.05, two‐way ANOVA followed by Student–Newman–Keuls test). The administration of naltrexone failed to induce any modification (Figure 3C).
Experiment 2: to evaluate the role of 5‐HT1A on the effects produced by the combination of CBD plus naltrexone on ethanol consumption and motivation to drink
During the stabilization phase, no significant differences were observed in the number of active lever presses (Figure 4A) (P > 0.05, two‐way ANOVA repeated measures) or in the ethanol intake (P > 0.05, two‐way ANOVA repeated measures) (Figure 4B) between groups before being treated.
Figure 4.
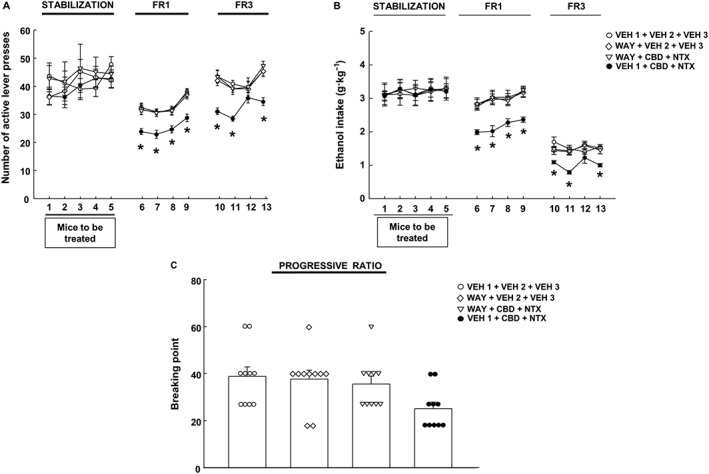
Evaluation of the effects of the 5‐HT1A antagonist WAY 100635 on the actions exerted by the combination of CBD plus naltrexone (NTX) on ethanol self‐administration in C57BL/6J mice. (A) Number of active responses during the FR1 stabilization, FR1 + treatment and FR3 + treatment phases; (B) ethanol intake expressed as g·kg−1 of all groups during the FR1 stabilization, FR1 + treatment and FR3 + treatment; (C) breaking point achieved during PR. The dots and the columns represent the means and vertical lines ± the SEM. * Represents the values from CBD + NTX that are significantly different (P < 0.05) from VEH + VEH, WAY + VEH and CBD + NTX + WAY groups (n = 10 for each group).
Again, the association of CBD plus naltrexone diminished the number of active lever presses during FR1 (P < 0.05, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test) (Figure 4A) and the ethanol intake throughout this phase (P < 0.05, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test) (Figure 4B).
In the same direction, the combination of CBD plus naltrexone was successful in reducing the number of active lever presses (P < 0.05, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test) (Figure 4A) and the ethanol intake on days 10, 11 and 13 during FR3 (P < 0.05, two‐way ANOVA repeated measures followed by Student–Newman–Keuls test) (Figure 4B). Interestingly, the administration of WAY 100635 significantly blocked the effects induced by the combination of CBD + NTX on the number of active lever presses.
In agreement with the results obtained from the FR1 and FR3 phases, during the PR phase, WAY 100635 blocked the reduction in the breaking point induced by CBD + NTX (Figure 4C) (P < 0.05, two‐way ANOVA). No effects were observed with the administration of WAY 100635 when it is given alone in any of the phases evaluated (FR1, FR3 and PR).
Discussion
The results of the present study reveal the efficacy of combining low doses of CBD and naltrexone in regulating the reinforcing actions of ethanol consumption. This statement is supported by the following observations: (i) the administration of CBD (20 mg·kg−1, s.c.) plus naltrexone (0.7 mg·kg−1, p.o.) was the only treatment able to reduce motivation and ethanol intake in all the phases of OEA evaluated (FR1, FR3 and PR); (ii) the administration of CBD plus naltrexone produced a greater reduction of the TH gene expression (50%) and was the only treatment that reduced the expression of Oprm1 (30%); (iii) the administration of CBD alone or in combination with naltrexone reduced the 5‐HT1A receptor gene expression (22 and 27% respectively); and (iv) the administration of the 5‐HT1A receptor antagonist, WAY 100635, blocked the reduction of motivation and ethanol intake induced by the combination of CBD plus naltrexone in the OEA paradigm.
Combination of drugs is a common clinical approach used to obtain a dual benefit: improve the clinical response to the pharmacological treatment and reduce the associated side effects. Regarding AUD, during the past few years, different studies have focused on the efficacy evaluation of the association of different drugs with naltrexone, the most effective current drug used for the treatment of alcohol dependence (Kim et al., 2004; Lee and Leggio, 2014; Navarrete et al., 2014; Nicholson et al., 2018). Despite the efforts made, no association has been proposed as superior due to the variability of results among studies.
Given the efficacy of CBD to reduce the reinforcing effects of ethanol and its neurodegenerative effects when administered at high doses in mice, in this study, we further explored if the combination of CBD plus naltrexone may present greater potential benefits for the treatment of AUD. For this purpose, a low dose of naltrexone, not always successful in reducing ethanol intake (Oliva and Manzanares, 2007), and a lower dose of CBD than the one we previously reported as effective for AUD (Viudez‐Martinez et al., 2018) were selected to evaluate potential either additive or synergistic effects achieved by combining both drugs.
The results of the present study revealed that the combination of lower doses of CBD plus naltrexone presented greater efficacy to reduce the reinforcing properties of ethanol in OEA compared with either of them when given alone. Despite CBD and naltrexone reducing ethanol consumption and motivation to drink (measured as active lever presses) in FR1 when given alone, only their combination reduced ethanol consumption and motivation to drink as the effort to get the reward increased (FR3 and PR). These results revealed that, as the effort required to obtain the drug increased, only the combination of both drugs was effective. The lack of effect of naltrexone at the dose selected (0.7 mg·kg−1) to reduce ethanol consumption in the FR3 and PR phases differs from what was previously demonstrated by our group (Navarrete et al., 2014). These discrepancies regarding the efficacy of naltrexone may be due, at least in part, to the strain of mice employed in each study (C57BL/6J from Charles River in the present study and C57BL/6 OlaHsd mice from Harlan in the previous one; Ramachandra et al., 2007). Regarding CBD, it seems that lowering the dose (from 30 mg·kg−1, s.c., used in the previous study to 20 mg·kg−1, s.c.) may affect its ability to reduce ethanol intake and motivation in the OEA. Therefore, these results suggest that the association of low doses of CBD plus naltrexone may produce synergistic actions; nevertheless, further studies are needed in order to verify this conclusion.
To clarify the potential neurochemical mechanism underlying the effects of CBD plus naltrexone combination, the gene expressions of Oprm1, TH and 5‐HT1A receptors were measured in the NAc, VTA and DR respectively. Rewarding and reinforcing properties of alcohol are mediated mainly by dopaminergic pathways from the VTA to the NAc (Nestler et al., 1993; Koob et al., 1998). In this respect, several authors described an up‐regulation of TH gene expression in the VTA under acute (Oliva et al., 2008) and chronic ethanol administration (Ortiz et al., 1995; Lee et al., 2005). Ethanol intake also promotes the release of endogenous opioids (Marinelli et al., 2003). The exact mechanism by which ethanol interacts with the opioid circuitry remains unclear, although it seems that the μ receptor modulates dopamine transmission within the cortico‐mesolimbic system (Thorsell, 2013) and mediates the rewarding properties of ethanol (Bilbao et al., 2015). Indeed, a recently published study showed that rewarding properties are also mediated by serotonergic pathways, which has cell bodies that project from the DR to the NAc (Liu et al., 2014). Additionally, an increase in 5‐HT1A receptor binding has been observed in the raphe nuclei of Rhesus monkeys exposed to chronic ethanol self‐administration (Hillmer et al., 2014). Likewise, exposure to chronic alcohol also seems to cause 5‐HT1A receptor supersensitivity in mice, which in turn contributes to high levels of alcohol drinking (Kelai et al., 2008). In the present study, only the combination of CBD and naltrexone modified the gene expression of Oprm1 (−30%), TH (−50%) and 5‐HT1A receptors (−27%) in the different brain regions analysed. Although CBD significantly reduced TH gene expression in the VTA, it is interesting to highlight that the association of CBD plus naltrexone induced a greater reduction of TH gene expression (−35 vs. −50%, respectively). Contrary to what our group previously reported (Navarrete et al., 2014), naltrexone (0.7 mg·kg−1, p.o.) alone failed to reduce the relative gene expression of TH.
Besides, the administration of CBD alone or in combination with naltrexone induced a significant reduction of 5‐HT1A receptor gene expression in the DR. However, no effect was induced by naltrexone when given alone. Therefore, the down‐regulation of 5‐HT1A receptor gene expression observed in the CBD + naltrexone group appears to be mediated by CBD. These results are in agreement with previous studies demonstrating that CBD can act as an allosteric modulator of 5‐HT1A receptors (Russo et al., 2005; Campos et al., 2012). Since the chronic exposure to an agonist induces a reduction in the gene expression of its target (Salort et al., 2017; Kim et al., 2018), it seems feasible to hypothesize that a down‐regulation of the 5‐HT1A receptor gene expression would be expected when a 5‐HT1A receptor agonist or allosteric modulator is administered.
Considering the role of the 5‐HT1A receptor in alcohol consumption and the allosteric effects of CBD on these receptors, it is possible that the effects of the association of CBD plus naltrexone may be mediated, at least in part, by 5‐HT1A receptors. To further investigate the relative specificity of the 5‐HT1A receptor in the mechanism underlying the effects of CBD and naltrexone, mice were pretreated with the 5‐HT1A antagonist WAY 100635 before receiving naltrexone and/or CBD in the OEA paradigm. Interestingly, pretreatment with WAY 100635 blocked the effects (reduction of ethanol intake and motivation) induced by CBD and naltrexone without having any effect by itself. Despite this finding, further studies are needed to clarify this mechanism, these results support the involvement of 5‐HT1A receptors in the effects of CBD plus naltrexone.
In summary, the results indicate that combining low doses of CBD plus naltrexone has a greater efficacy at reducing ethanol consumption and the motivation to drink that either drug administered alone. These behavioural effects were accompanied by a more pronounced reduction of TH gene expression in the VTA and a reduction of Oprm1 and 5‐HT1A receptor gene expressions in the NAc and DR respectively. Interestingly, 5‐HT1A receptors appear to play, at least in part, a relevant role in the effects mediated by the combination of CBD and naltrexone. Taken together, these results represent the first step regarding the potential therapeutic use of the combination of CBD plus naltrexone, serving as a reference point for future clinical research. The fact that CBD is currently marketed as a drug for the treatment of spasticity in multiple sclerosis (Sativex®) will further accelerate the development of clinical studies.
Author contributions
J.M. and M.S.G.G. conceived and designed the experiments; A.V.M performed the experiments; A.I.F.S. and A.I.T.S. elaborated the CBD s.c. controlled release formulation. A.V.M. analysed the data and drafted the relevant text; A.V.M., M.S.G.G. and J.M. wrote the manuscript. All authors have read and approved the final version of this manuscript.
Conflict of interest
The authors declare no conflicts of interests.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Data S1 1. Information regarding the weight of mice for experiment 1.
2. Information regarding the weight of mice for experiment 2.
Acknowledgements
This research was supported by ‘Instituto de Salud Carlos III’ (RETICS, RD12/0028/0019 and RD16/0017/0014), ‘Plan Nacional Sobre Drogas’ (PNSD 2016/016) and ‘Ministerio de Economía y Competitividad’ (FIS, PI14/00438) to J.M.; A.V.‐M. is a predoctoral fellow supported by ‘Plan Nacional Sobre Drogas’ (PNSD 2016/016).
Viudez‐Martínez, A. , García‐Gutiérrez, M. S. , Fraguas‐Sánchez, A. I. , Torres‐Suárez, A. I. , and Manzanares, J. (2018) Effects of cannabidiol plus naltrexone on motivation and ethanol consumption. British Journal of Pharmacology, 175: 3369–3378. 10.1111/bph.14380.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR et al (2011). Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 168: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH et al (2015). A pharmacogenetic determinant of mu‐opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol Psychiatry 77: 850–858. [DOI] [PubMed] [Google Scholar]
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR (2015). Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Ferreira FR, Guimaraes FS (2012). Cannabidiol blocks long‐lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J Psychiatr Res 46: 1501–1510. [DOI] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Sonego AB, Guimaraes FS (2016). Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 112: 119–127. [DOI] [PubMed] [Google Scholar]
- Cohen C, Curet O, Perrault G, Sanger DJ (1999). Reduction of oral ethanol self‐administration in rats by monoamine oxidase inhibitors. Pharmacol Biochem Behav 64: 535–539. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, Machado S, Arias‐Carrion O, Crippa JA et al (2014). Antidepressant‐like and anxiolytic‐like effects of cannabidiol: a chemical compound of Cannabis sativa . CNS Neurol Disord Drug Targets 13: 953–960. [DOI] [PubMed] [Google Scholar]
- El‐Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D et al (2010). Antidepressant‐like effect of delta9‐tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav 95: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD (2013). Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol Clin Exp Res 37: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar‐Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin‐Santos R et al (2009). Distinct effects of {delta}9‐tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 66: 95–105. [DOI] [PubMed] [Google Scholar]
- Garcia‐Gutierrez MS, Navarrete F, Aracil A, Bartoll A, Martinez‐Gras I, Lanciego JL et al (2016). Increased vulnerability to ethanol consumption in adolescent maternal separated mice. Addict Biol 21: 847–858. [DOI] [PubMed] [Google Scholar]
- Garcia‐Gutierrez MS, Perez‐Ortiz JM, Gutierrez‐Adan A, Manzanares J (2010). Depression‐resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol 160: 1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Samson HH (1986). The induction of oral ethanol self‐administration by contingent ethanol delivery. Drug Alcohol Depend 16: 361–368. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW (1990). Antianxiety effect of cannabidiol in the elevated plus‐maze. Psychopharmacology (Berl) 100: 558–559. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL (2005). Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol‐induced neurotoxicity. J Pharmacol Exp Ther 314: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer AT, Wooten DW, Tudorascu DL, Barnhart TE, Ahlers EO, Resch LM et al (2014). The effects of chronic alcohol self‐administration on serotonin‐1A receptor binding in nonhuman primates. Drug Alcohol Depend 144: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12: 699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz J, Miernik K, Wachal M, Walczak J, Krumpl G (2013). Naltrexone (50 mg) plus psychotherapy in alcohol‐dependent patients: a meta‐analysis of randomized controlled trials. Am J Drug Alcohol Abuse 39: 144–160. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait‐Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K et al (2003). Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 361: 1677–1685. [DOI] [PubMed] [Google Scholar]
- Kelai S, Hanoun N, Aufrere G, Beauge F, Hamon M, Lanfumey L (2006). Cannabinoid‐serotonin interactions in alcohol‐preferring vs. alcohol‐avoiding mice. J Neurochem 99: 308–320. [DOI] [PubMed] [Google Scholar]
- Kelai S, Renoir T, Chouchana L, Saurini F, Hanoun N, Hamon M et al (2008). Chronic voluntary ethanol intake hypersensitizes 5‐HT (1A) autoreceptors in C57BL/6J mice. J Neurochem 107: 1660–1670. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cho S, Woo JA, Liggett SB (2018). A CREB‐mediated increase in miRNA let‐7f during prolonged beta‐agonist exposure: a novel mechanism of beta2‐adrenergic receptor down‐regulation in airway smooth muscle. FASEB J fj201701278R. 10.1096/fj.201701278R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Han BD, Park JM, Kim MJ, Stromberg MF (2004). Effect of the combination of naltrexone and acamprosate on alcohol intake in mice. Psychiatry Clin Neurosci 58: 30–36. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P et al (1998). Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 22: 3–9. [PubMed] [Google Scholar]
- Lee MR, Leggio L (2014). Combined pharmacotherapies for the management of alcoholism: rationale and evidence to date. CNS Drugs 28: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Park SW, Kim YK, Kim DJ, Jeong J, Myrick H et al (2005). Effects of naltrexone on the ethanol‐induced changes in the rat central dopaminergic system. Alcohol Alcohol 40: 297–301. [DOI] [PubMed] [Google Scholar]
- Lemos JI, Resstel LB, Guimaraes FS (2010). Involvement of the prelimbic prefrontal cortex on cannabidiol‐induced attenuation of contextual conditioned fear in rats. Behav Brain Res 207: 105–111. [DOI] [PubMed] [Google Scholar]
- Levin R, Peres FF, Almeida V, Calzavara MB, Zuardi AW, Hallak JE et al (2014). Effects of cannabinoid drugs on the deficit of prepulse inhibition of startle in an animal model of schizophrenia: the SHR strain. Front Pharmacol 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C et al (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M et al (2014). Dorsal raphe neurons signal reward through 5‐HT and glutamate. Neuron 81: 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−Delta Delta C (T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Long LE, Malone DT, Taylor DA (2006). Cannabidiol reverses MK‐801‐induced disruption of prepulse inhibition in mice. Neuropsychopharmacology 31: 795–803. [DOI] [PubMed] [Google Scholar]
- Manzanares J M‐RJ, Viudez‐Martínez A, Navarron C, Aracil‐Fernandez A, Navarrete F, Garcia‐Gutierrez MS (2016). Cannabidiol a drug lacking reinforcing activity. Society for Neuroscience San Diego (CA, USA) 77.02/AAA18.
- Marinelli PW, Quirion R, Gianoulakis C (2003). A microdialysis profile of beta‐endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 169: 60–67. [DOI] [PubMed] [Google Scholar]
- Martin‐Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S et al (2012). Acute effects of a single, oral dose of d9‐tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des 18: 4966–4979. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R (2002). Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol 42: 11S–19S. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM (1999). Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol 17: 185–194. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimaraes FS (2006). Anxiolytic‐like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry 30: 1466–1471. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Guimaraes FS (2005). Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur J Pharmacol 512: 199–205. [DOI] [PubMed] [Google Scholar]
- Manzanares J, García‐Gutiérrez MS (2017). Is the cannabidiol potentially useful for the treatment of neuropsychiatric and drug‐use disorders. Res Rev Biosci 12: 112. [Google Scholar]
- Navarrete F, Rubio G, Manzanares J (2014). Effects of naltrexone plus topiramate on ethanol self‐administration and tyrosine hydroxylase gene expression changes. Addict Biol 19: 862–873. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hope BT, Widnell KL (1993). Drug addiction: a model for the molecular basis of neural plasticity. Neuron 11: 995–1006. [DOI] [PubMed] [Google Scholar]
- Nicholson ER, Dilley JE, Froehlich JC (2018). Co‐administration of low‐dose naltrexone and bupropion reduces alcohol drinking in alcohol‐preferring (P) rats. Alcohol Clin Exp Res 42: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva JM, Manzanares J (2007). Gene transcription alterations associated with decrease of ethanol intake induced by naltrexone in the brain of Wistar rats. Neuropsychopharmacology 32: 1358–1369. [DOI] [PubMed] [Google Scholar]
- Oliva JM, Ortiz S, Perez‐Rial S, Manzanares J (2008). Time dependent alterations on tyrosine hydroxylase, opioid and cannabinoid CB1 receptor gene expressions after acute ethanol administration in the rat brain. Eur Neuropsychopharmacol 18: 373–382. [DOI] [PubMed] [Google Scholar]
- Orru A, Fujani D, Cassina C, Conti M, Di Clemente A, Cervo L (2012). Operant, oral alcoholic beer self‐administration by C57BL/6J mice: effect of BHF177, a positive allosteric modulator of GABA (B) receptors. Psychopharmacology (Berl) 222: 685–700. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X et al (1995). Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse 21: 289–298. [DOI] [PubMed] [Google Scholar]
- Palkovits M (1983). Punch sampling biopsy technique. Methods Enzymol 103: 368–376. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates. 2nd Edition, Academic Press, San Diego. [Google Scholar]
- Peres FF, Levin R, Almeida V, Zuardi AW, Hallak JE, Crippa JA et al (2016). Cannabidiol, among other cannabinoid drugs, modulates prepulse inhibition of startle in the SHR animal model: implications for schizophrenia pharmacotherapy. Front Pharmacol 7: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra V, Phuc S, Franco AC, Gonzales RA (2007). Ethanol preference is inversely correlated with ethanol‐induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res 31: 1669–1676. [DOI] [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera‐Matas A, Morris CV, Hurd YL (2009). Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue‐induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci 29: 14764–14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Moreira FA, Correa FM, Guimaraes FS (2006). Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res 172: 294–298. [DOI] [PubMed] [Google Scholar]
- Rosner S, Hackl‐Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M (2010). Acamprosate for alcohol dependence. Cochrane Database Syst Rev (9): CD004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK (2005). Agonistic properties of cannabidiol at 5‐HT1a receptors. Neurochem Res 30: 1037–1043. [DOI] [PubMed] [Google Scholar]
- Salort G, Alvaro‐Bartolome M, Garcia‐Sevilla JA (2017). Regulation of cannabinoid CB2 receptor constitutive activity in vivo: repeated treatments with inverse agonists reverse the acute activation of JNK and associated apoptotic signaling in mouse brain. Psychopharmacology (Berl) 234: 925–941. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Lahmek P, Pham H, Aubin HJ (2014). Disulfiram efficacy in the treatment of alcohol dependence: a meta‐analysis. PLoS One 9: e87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A (2013). The mu‐opioid receptor and treatment response to naltrexone. Alcohol Alcohol 48: 402–408. [DOI] [PubMed] [Google Scholar]
- Twardowschy A, Castiblanco‐Urbina MA, Uribe‐Marino A, Biagioni AF, Salgado‐Rohner CJ, Crippa JA et al (2013). The role of 5‐HT1A receptors in the anti‐aversive effects of cannabidiol on panic attack‐like behaviors evoked in the presence of the wild snake Epicrates cenchria crassus (Reptilia, Boidae). J Psychopharmacol 27: 1149–1159. [DOI] [PubMed] [Google Scholar]
- Viudez‐Martinez A, Garcia‐Gutierrez MS, Navarron CM, Morales‐Calero MI, Navarrete F, Torres‐Suarez AI et al (2018). Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict Biol 23: 154–164. [DOI] [PubMed] [Google Scholar]
- Weiss F LA, Wagner G, Deness G, Kerr T, Watry D, Suto N (2016). Transdermal CBD attenuates cocaine intake in rats with addiction‐linked cocaine history. Annual Meeting Society for Neuroscience, San Diego (CA).
- Winton‐Brown TT, Allen P, Bhattacharyya S, Borgwardt SJ, Fusar‐Poli P, Crippa JA et al (2011). Modulation of auditory and visual processing by delta‐9‐tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology 36: 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelati TV, Biojone C, Moreira FA, Guimaraes FS, Joca SR (2010). Antidepressant‐like effects of cannabidiol in mice: possible involvement of 5‐HT1A receptors. Br J Pharmacol 159: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Cheer JF (2016). Beyond the CB1 receptor: is cannabidiol the answer for disorders of motivation? Annu Rev Neurosci 39: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Rodrigues JA, Cunha JM (1991). Effects of cannabidiol in animal models predictive of antipsychotic activity. Psychopharmacology (Berl) 104: 260–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 1. Information regarding the weight of mice for experiment 1.
2. Information regarding the weight of mice for experiment 2.


