Abstract
Rheumatoid arthritis (RA) and osteoarthritis (OA) are the two most prevalent joint diseases. A such, they are important causes of pain and disability in a substantial proportion of the human population. A common characteristic of these diseases is the erosion of articular cartilage and consequently joint dysfunction. Melatonin has been proposed as a link between circadian rhythms and joint diseases including RA and OA. This hormone exerts a diversity of regulatory actions through binding to specific receptors and intracellular targets as well as having receptor‐independent actions as a free radical scavenger. Cytoprotective effects of melatonin involve a myriad of prominent receptor‐mediated pathways/molecules associated with inflammation, of which the role of omnipresent NF‐κB signalling is crucial. Likewise, disturbance of circadian timekeeping is closely involved in the aetiology of inflammatory arthritis. Melatonin is shown to stimulate cartilage destruction/regeneration through direct/indirect modulation of the expression of the main circadian clock genes, such as BMAL, CRY and/or DEC2. In the current article, we review the effects of melatonin on RA and OA, focusing on its ability to regulate inflammatory pathways and circadian rhythms. We also review the possible protective effects of melatonin on RA and OA pathogenesis.
Linked Articles
This article is part of a themed section on Recent Developments in Research of Melatonin and its Potential Therapeutic Applications. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.16/issuetoc
Abbreviations
- AA
adjuvant‐induced arthritis
- Acan
aggrecan
- ARNTL2
aryl hydrocarbon receptor nuclear translocator‐like
- BMAL1
brain and muscle aryl hydrocarbon receptor nuclear translocator‐like 1
- CIA
collagen‐induced arthritis
- CLOCK
circadian locomotor output cycles kaput
- Col2a1
collagen 2A1
- Cry 1
cryptochrome 1
- DEC1
differentially expressed in chondrocytes 2
- iNOS
inducible NOS
- LOX
lipoxygenase
- MSCs
mesenchymal stem cells
- NPAS2
neuronal PAS domain‐containing protein 2
- OA
osteoarthritis
- Per 1
period 1
- RA
rheumatoid arthritis
- REV‐ERBs
reverse‐eritroblastosis viruses
- RORa
RAR‐related orphan receptor A
- RORs
retinoid‐related orphan receptors
- Sirt1
sirtuine 1
- SMAD2/3
mothers against decapentaplegic‐homologue 2/3
- Sox9
sex‐determining region‐related protein 9
Introduction
Rheumatoid arthritis (RA) and osteoarthritis (OA) are the two most prevalent diseases of the joints. They are also major causes of pain and disability in a substantial proportion of the human population (Pap and Korb‐Pap, 2015). In spite of different etiologies of these disorders, a common characteristic is the erosion of articular cartilage and consequently joint dysfunction (Haas and Straub, 2012). Recent studies have provided evidence for a functional circadian clock in cartilage tissue, which drives downstream expression of clock controlled genes (Kouri et al., 2013). The circadian clock regulates endochondral ossification, a bone synthesis process from a cartilaginous template. Chondrocyte proliferation and the rate of change in the growth plates show a circadian variation, as well (Takarada et al., 2012). Moreover, increased rates of calcium and phospho‐mineralization have been detected during the dark phase of the light‐dark cycle (Gossan et al., 2015).
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224, a circadian‐controlled product secreted by the pineal gland, has been suggested as a potential link between circadian rhythms and joint diseases, including RA and OA (Yoshida et al., 2014a,b; Berenbaum and Meng, 2016). Melatonin and its derivatives are wide‐ranging free radical detoxifiers and antioxidants, because of their potential scavenging of free radicals of oxygen species (ROS) and nitrogen species (RNS) and to boost the expression and activity of glutathione and antioxidant enzymes (Reiter et al., 2016). Through these events and their receptor‐mediated actions, melatonin and its metabolites modulate a variety of molecular signalling pathways including proliferation, apoptosis, metastasis and inflammation, in a wide range of pathophysiological situations (Hosseinzadeh et al., 2016). Here, we review the effects of melatonin on RA and OA, focusing on its ability to regulate the inflammatory pathways and the circadian rhythms. We also review the possibilities of protective effects of melatonin on RA and OA pathogenesis.
Melatonin in regulation of inflammatory pathways
The circadian multi‐tasking hormone, melatonin, is mainly synthesized by the pineal gland and also a wide range of other tissues (Reiter et al., 2016). Melatonin is evolutionarily conserved and exerts many different regulatory actions through binding to specific receptors and intracellular targets (Reiter et al., 2010). Depending upon a specific cell or species, melatonin activates a variety of different second messenger cascades after its binding to the high‐affinity G‐protein‐coupled melatonin receptors, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=287/http://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=MT2&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database, on the plasma membrane of the target cells (Majidinia et al., 2017). MT1 receptors are expressed throughout the body but predominantly in the brain. In suprachiasmatic nucleus, both MT1 and MT2 receptors are involved in regulating circadian rhythms (Wiesenberg et al., 1998). Also, high‐affinity nuclear binding sites for melatonin are members of the nuclear receptor family. These may be involved in the antiproliferative activity of melatonin on cancer cells and its immunomodulatory actions (Hosseinzadeh et al., 2016). Besides plasma membrane and nuclear receptors, receptor‐independent signalling accounts for radical scavenging activities of melatonin (Carlberg, 2000). Melatonin is known to elicit both pro and anti‐inflammatory effects depending on the cellular state (Radogna et al., 2010). Cytoprotective effects of melatonin involve several prominent pathways/molecules associated with inflammation, such as the transcription factor NF‐κB (15, 16), http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=316 (Chuffa et al., 2015), MAPK (Lin et al., 2016), inflammatory mediators of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2391 (AA) pathway including http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=269 and lipoxygenase (LOX) (Steinhilber et al., 1995; Li et al., 2000; Lu et al., 2016), long non‐coding RNAs (Akhtar et al., 2016), (Mishra et al., 2011), hypoxia inducible factor(HIF)‐1α (Park et al., 2009; Jahanban‐Esfahlan et al., 2017) and nuclear erythroid 2‐related factor 2 (Nrf2) (Negi et al., 2011). With a closer look, it appears that NF‐κB signalling is central to all these inflammation‐related pathways, and more importantly, the wide‐spectrum antioxidant and anti‐inflammatory effects of melatonin eventually lead to the blockade of NF‐κB signalling (Figure 1). Melatonin protects cells from the devastating effects of oxidative stress, through modulation of NF‐κB pathway, as well (Reiter et al., 2016).
Figure 1.
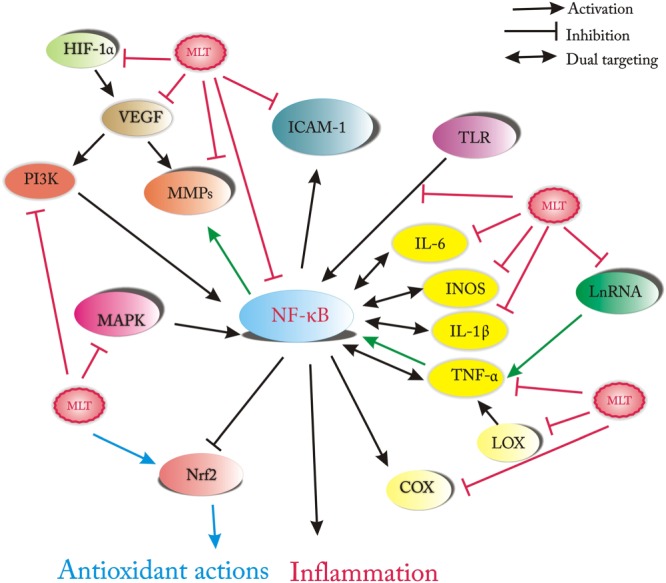
Cellular insults such as ROS, LPS, cytokines (e.g. TNF‐α), several lncRNAs and signalling pathways (e.g. MAPKs, hypoxia inducible factor‐1α and LOX) induce activation of NFkB signalling, which in turn leads to the expression of downstream genes involved in the inflammatory responses, including pro‐inflammatory cytokines (IL1β, IL‐6, TNF‐α), iNOS, adhesion molecules, COX‐2 and MMPs. The anti‐inflammatory effects of melatonin involve inhibiting a wide range of these inflammatory molecules, and the final result is to mitigate inflammation through blockade of NF‐kB signalling.
NF‐κB is an omnipresent oxidative stress‐sensitive transcription factor, which has a primary role in cell survival, immune and inflammatory responses. These actions involve a variety of genes essential in cellular responses (Hoesel and Schmid, 2013). The classic form of NF‐κB is a dimer, consisting of a p50 subunit and a trans‐activating subunit p65 including RelA, RelB and c‐Rel. The dormant form of NF‐κB appears in the cytoplasm of resting cells, forming a complex with its inhibitors, the IκBs (IκBα and IκBβ) (Gilmore, 2006). Upon stimulation, NF‐κB is activated through phosphorylation and degradation of the IκBs by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=578. After its liberation, NF‐κB translocates to the nucleus and binds to enhancer elements within the promoters of responsive genes. NF‐κB/DNA complex recruit coactivators (e.g. p300) and RNA polymerase to activate transcription of target genes (Hoffmann et al., 2006). As shown in Figure 1, NF‐κB activates in response to different inducers, such as ROS, bacterial http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5019 and several cytokines, and induces the expression of genes involved in the inflammatory responses, including pro‐inflammatory cytokines (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250), adhesion molecules, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376&familyId=269&familyType=ENZYME and MMPs (Li and Verma, 2002).
It should be interesting to note that melatonin can be synthesized by the macrophages present in the arthritis lesions. Indeed, NF‐κB is capable of inducing the synthesis of melatonin by immune‐competent cells. NF‐κB activation triggers the synthesis of melatonin in macrophages in the regulatory phase and also in chronic inflammatory processes. This is a long‐term response as it is mediated by the cRel NF‐κB subunit, induced by the p50/RelA NF‐κB (Muxel et al., 2016). The same subunit is relevant for RA and other autoimmune responses. p50/cRel and cRel/cRel interact with Aa‐nat promoter inducing melatonin synthesis, and on the other hand, CD40/cRel is the signalling pathway for RA and p50/cRel induces antibodies and co‐stimulatory molecules (Gregersen and Olsson, 2009; Criswell, 2010). The fact that immune competent cells are able to produce melatonin is important information for understanding the disease and for evaluating the use of melatonin.
Melatonin actions in RA
While the anti‐inflammatory and cytoprotective effects of melatonin are well described in many diseases (Carrillo‐Vico et al., 2013), there are conflicting data regarding beneficial or worsening effects of melatonin in RA patients. A report by Hansson et al. (1990) claimed that constant darkness, by disturbing the rhythm of production of melatonin, promoted autoimmunity to type II collagen and augmented the onset of collagen‐induced arthritis (CIA) in DBA/1 mice. The effect of constant darkness (which causes the melatonin rhythm to run) and constant light (which eliminates pineal melatonin production) was investigated on the disease course of an experimental autoimmune model, type II CIA in DBA/1 female mice. Their results indicated that under constant darkness, mice tend to develop more severe CIA and show a higher level of serum anti‐collagen antibodies than mice maintained under a regular photoperiod or constant light (Hansson et al., 1990). Furthermore, pinealectomy reversed the effects of constant darkness (Hansson et al., 1993). Daily injections of melatonin to DBA/1 mice enhance T cell priming and may aggravate CIA development (Hansson et al., 1992). The dual effect of melatonin as a pro‐inflammatory agent and antioxidant has also been seen in CIA models in rats. Jimenez‐Caliani et al. (2005) claimed that melatonin increased anti‐collagen antibodies, IL‐1β and IL‐6 levels in the serum and joints of arthritic rats, while it lowered oxidative markers nitrite/nitrate and lipid peroxidation in serum but not in joints. Pinealectomy abolished the effects of melatonin and reduced oxidative stress as well as concentrations of cytokines and antibodies in joint but increased the level of serum oxidative markers.
The beneficial anti‐inflammatory effects of melatonin have been reported in a model of adjuvant‐induced arthritis (AA) in rat (Chen and Wei, 2002). In this study, administration of the prophylactic and therapeutic doses of melatonin repressed the inflammatory response and produced enhanced proliferation of thymocytes and secretion of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4985 in AA rats. In addition, melatonin decreased the elevated level of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2352 induced by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5190. In AA rats, the drop in thymocyte proliferation correlated highly with the decrease in the levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1614 in the thymocytes, and melatonin strikingly augmented the level of Met‐Enk, which were hindered by a Ca2+ channel antagonist, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2514. The anti‐inflammatory and immunoregulatory actions of melatonin involved a G protein‐adenyl cyclase‐cAMP transmembrane signal and Met‐Enk release by thymocytes (Chen and Wei, 2002).
The incidence and severity of RA tend to be exacerbated at high latitudes, such as Estonia (Cutolo et al., 2005a). RA patients at these latitudes may experience a more prolonged elevation of melatonin and TNF‐α during the winter months. However, there is no significant difference between the serum level of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2868 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 in Estonian rheumatoid patients and controls from lower latitudes (Cutolo et al., 2005b). RA symptoms are reported to exacerbate in the early morning as well, when the level of pro‐inflammatory cytokines are high and cortisol concentration is low (Sulli et al., 2002). In contrast, West et al. measured significantly lower levels of morning plasma melatonin in RA patients (West and Oosthuizen, 1992) and an increased nocturnal production of melatonin induced by Freund's adjuvant in an experimental model of arthritis (Cano et al., 2002). Synovial macrophages from RA patients elicit high‐affinity binding sites for melatonin in vitro and secrete high levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4977 upon melatonin treatment (Cutolo et al., 1999). Additionally, the amount of melatonin is relatively high in synovial fluid from RA individuals (Maestroni et al., 2002). Conversely, melatonin activates the ERK‐stimulated cyclin‐dependent kinase inhibitors P21 and P27 to hamper the excessive proliferation of RA‐derived fibroblast‐like synoviocytes (Nah et al., 2009). Clearly, the relationship between melatonin and RA requires further definition.
Melatonin actions in OA
In contrast to the controversial effects of melatonin on the prognosis of RA, the cytoprotective and therapeutic potential of melatonin is well established in preclinical and clinical OA settings. Lim et al. (2012) disclosed that the cytoprotective and anti‐inflammatory effects of melatonin in hydrogen peroxide (H2O2)‐stimulated CHON‐001 human chondrocyte cell line and in a rabbit model of OA are mediated through a http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2707 (Sirt1) pathway. Sirt1 is a NAD+‐dependent protein deacetylase that controls many physiological pathways, including circadian rhythms in peripheral tissues (Yang et al., 2016). Melatonin inhibited H2O2‐stimulated cytotoxicity, iNOS and COX‐2 protein, and mRNA expression as it dampened the synthesis of NO and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1883 and blocked H2O2‐induced TNF‐α, IL‐1β and IL‐8 release. In addition, melatonin treatment significantly inhibited H2O2‐induced phosphorylation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=673/http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=518 and MAPK, as well as activation of NF‐κB. Intra‐articular injection of melatonin significantly reduced cartilage destruction in a rabbit model of OA. The effects of melatonin were reversed by sirtinol and Sirt1 siRNA, which blocks Sirt1 activity and down‐regulates its expression respectively. Based on these findings, the authors suggested that the anti‐inflammatory and cytoprotective effects of melatonin in human chondrocytes involve the dynamic action of the Sirt1 pathway (Lim et al., 2012). Hong et al. established an osteoarthritic chondrocyte model characterized by high levels of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1637, shift of cytosolic MMP‐13 into the nucleus, metabolic disruption of collagen 2A1 (COL2A1), proteoglycan loss, atypical distribution and excessive Ca2+ deposition (Hong and Hong, 2015). Administration of melatonin (1 nM) restored aberrant levels of COL2A1 and blocked the cytonuclear translocation of MMP‐13. Moreover, melatonin increased the amount of proteoglycan, which was optimally distributed in chondrocytes. Accordingly, melatonin reduced the amount of cells with Ca2+ crystals. These results suggest the potential of melatonin to turn off the catabolic switch through the regulation of MMP‐13 localization (Hong and Hong, 2015). Pei et al. (2009) reported that melatonin promotes matrix synthesis by articular chondrocytes in vitro. Melatonin also elevated the expression of chondrogenic marker genes such as sex‐determining region‐related protein 9 (Sox9), collagen II and aggrecan (Acan) while down‐regulating collagen X, a hypertrophic marker. These findings document that melatonin not only up‐regulates chondrogenic differentiation but also may reduce osteogenic differentiation. Melatonin enhances internal TGF‐β1 expression in treated chondrocytes suggesting that it enhances cartilage matrix synthesis through activation of the TGF‐β signalling pathway (Pei et al., 2009).
Mesenchymal stem cells (MSCs) can differentiate into multiple lineages, including chondrocytes (Zhou et al., 2015). In injured or diseased joints, elevated oxidative stress in the inflamed micro‐environment hinders the chondrogenic differentiation of MSCs and leads to degradation of neocartilage (Wang et al., 2015). Liu et al. (2014) showed that melatonin successfully restored the inhibitory effect of IL‐1β and TNF‐α on MSC chondrogenesis, which presumably correlated with melatonin's ability to scavenge free radicals, suppress MMPs levels and preserve SOD activity.
Circadian clock, melatonin and joint disease
Suprachiasmatic nuclei in the CNS and clocks in peripheral tissues regulate key aspects of physiology by regulating circadian rhythms in tissue‐specific sets of downstream genes. These circadian clocks regulate many aspects of the immune system, and their disruption contributes to the onset of various inflammatory‐related disease states, as well as joint disease (Takahashi et al., 2008).
Clock genes constitute auto‐regulatory feedback loops with heterodimers formed between brain and muscle aryl hydrocarbon receptor nuclear translocator‐like (ARNTL) 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK) genes. They serve as the main positive transcription factors binding to the E‐box cis‐regulatory enhancer elements, which are found within target gene promoters or enhancers (Buttgereit et al., 2015). An important downstream transcriptional target for BMAL1/CLOCK encodes for Per (Period 1 and 2) and cryptochrome 1 (Cry 1 and 2), the negative feedback proteins, which by accumulating in the nucleus decrease expression of BMAL1/CLOCK complex and so Per/Cry expression, as well (Gibbs and Ray, 2013). Less studied clock molecules, DEC1 and DEC2, compete with BMAL1/CLOCK for E‐box binding (Kouri et al., 2013). Also, ARNTL2/ NPAS2 are functional paralogues of BMAL1/CLOCK respectively. Additional downstream targets of CLOCK/BMAL1 are transcriptional activators of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=88 and repressors such as http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=87 (Roenneberg and Merrow, 2005). Albumin D site‐binding protein is an extra BMAL/CLOCK downstream gene, and its expression is under the control of both BMAL and CRY1 (Stratmann et al., 2010). This core clock machinery regulating circadian time exists in virtually all cells of the body, as well as in the joint chondrocytes (Takarada et al., 2012; Gossan et al., 2013; Honda et al., 2013; Guo et al., 2015).
Melatonin in modulation of circadian clock and inflammation in RA
Disturbance in circadian timekeeping is closely involved in the aetiology of RA (Kouri et al., 2013; Yoshida et al., 2014a,b). Patients with RA exhibit disturbances in the hypothalamic–pituitary–adrenal axis, which is reflected in the altered circadian rhythm of circulating serum level of melatonin, IL‐6, cortisol and in chronic fatigue (Cutolo et al., 2005a; Haas and Straub, 2012). These changes are intertwined with the abnormal expression of several clock genes BMAL, CLOCK, ARNTL2, DEC2 and NPAS2 (Figure 2) (Gibbs and Ray, 2013; Kouri et al., 2013). Interestingly, DEC2, which is selectively expressed in Th2 immune cells, increases the expression of IL‐1β and is abundantly present in the synovial membranes of RA. TNF‐α up‐regulates DEC2 at both mRNA and protein level in a NF‐κB‐dependent manner in cultured human synovial fibroblasts (Olkkonen et al., 2015) and not only DEC2 but also ARNTL2 and NPAS2 are up‐regulated by TNF‐α. The effects of TNF‐α on DEC2 expression are mediated via NF‐κB in HEK293 cells and primary human fibroblasts. The NF‐κB‐mediated effects on DEC2 are manifested by up‐regulation of IL‐1β (Olkkonen et al., 2015).
Figure 2.
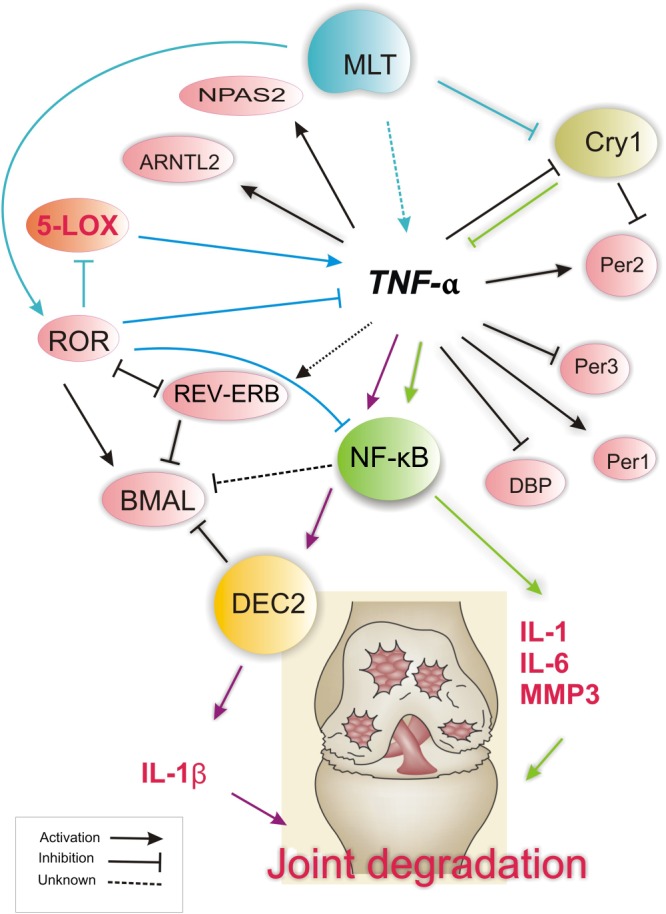
Implications of melatonin in the regulation of clock gene expression in joints during RA. Disruption of circadian clock gene expression is mainly under the influence of TNF‐α. This pro‐inflammatory cytokine is produced at high levels by fibroblast‐like synoviocytes. The unfavourable effects of clock gene disruption in RA is reflected in the suppression of the negative arm action, of which the loss of CRY expression results in pronounced up‐regulation of TNF‐α, IL‐1β and IL‐6 and enhanced severity of RA. Another established link is the up‐regulation of DEC2 via TNF‐α, which subsequently leads to an elevated expression of IL‐1β, hence enhancing the pro‐inflammatory and destructive effects of TNF‐α on the RA joint. High levels of melatonin in RA patients may contribute to clock disturbance at least through three means: (i) modulating pro‐inflammatory states through increasing the level of pro‐inflammatory cytokines, including TNF‐α; (ii) attenuating clock gene cryptochrome1 expression, through the inhibitory mechanism of melatonin; (iii) modulating ROR activation. ROR acts as a major negative regulator of inflammation and is central to both BMAL and melatonin actions. ROR inhibition by REV‐ERB may contribute to BMAL suppression and exacerbate RA. Though the role of BMAL1 clock gene is central to regulating the circadian oscillations and RA joints show abnormal cytoplasmic localization of BMAL in response to TNF‐α induction, the extent of BMAL1 disruption in RA state along with other core clocks remains to be determined.
Narasimamurthy et al. (2012) found another clock gene with impaired expression in RA. The authors showed that the ablation of the core clock gene, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2876, leads to a constitutive elevation of pro‐inflammatory cytokines in a cell‐autonomous manner. http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2876&familyId=916&familyType=OTHER is known to bind to adenylyl cyclase and limit cAMP production, suggesting that the absence of Cry protein(s) might decrease the inhibition of cAMP production, resulting in elevated cAMP and increased PKA activation, subsequently leading to NF‐κB activation through phosphorylation of p65 at S276 (Narasimamurthy et al., 2012). Melatonin is known to attenuate Cry1 gene expression in RA, leading to elevation of type II collagen antibodies and increasing the severity of CIA. In this context, Bang et al. (2012) demonstrated the marked down‐regulation of both mRNA and protein levels of Cry1 in CIA after melatonin treatment, compared with those in the control and CIA‐only group. Melatonin therapy also increased paw thickness. In addition, there was an increased infiltration of inflammatory cells, synovial hyperplasia and the destruction of articular cartilage and bone by melatonin. The concentration of anti‐type II collagen antibodies along with elevated serum concentrations of TNF‐α and IL‐6 in CIA plus melatonin mice was significantly higher than those in the control and CIA‐only animals. Melatonin had no effect on transcription of two other important clock genes, BMAL and Per1 in CIA mice. These results suggest that the clock gene Cry1 may be involved in the exacerbation of melatonin‐mediated arthritis in mice with anti‐type II collagen antibody‐induced arthritis (Bang et al., 2012).
Another likely link can be made between melatonin, clock gene ROR and BMAL. As discussed earlier, melatonin actions involve three pathways: (i) a G‐protein‐mediated membrane signalling; (ii) a nuclear signalling; and (iii) a receptorless radical scavenging action. Nuclear signalling involves the clock gene transcription factor RZR/ROR complex (Carlberg, 2000). Melatonin elicits anti‐allergic and anti‐inflammatory actions by inhibiting http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1385&familyId=271&familyType=ENZYME through transcriptional activation of RZR/ROR (Steinhilber et al., 1995; Carlberg, 2000). The LOX pathway is a main contribution to the RA inflammatory state, and synovial fluid in RA patients shows high levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2487 – a major product of 5‐LOX. LTB4 is a potent pro‐inflammatory chemotactic agent and is reputed as an important mediator of joint inflammation in RA. Importantly, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1385 inhibitors block TNF‐α‐induced NF‐κB signalling in rheumatoid fibroblasts in vitro and a murine paw oedema model (Lin et al., 2014). What is more, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=598&familyId=88&familyType=NHR inhibits NF‐κB‐mediated inflammation through activation of its major inhibitor, IκBα. Ectopic expression of RORa is also reported to inhibit TNF‐α‐induced IL‐6, IL‐8 and COX‐2 expression in human smooth muscle cells. Thus, RORa agonists as well as 5‐LOX inhibitors are potential targets in the treatment of RA (Lin et al., 2014; Muxel et al., 2016). Clock gene ROR is indispensable for activity of both melatonin and BMAL. ROR actions could be antagonized with REV‐ERB, the expression of which significantly increased in RA synovium compared with OA synovium (Kouri et al., 2013).
It is established that Cry‐null mice develop maximal exacerbation of joint swelling and up‐regulation of essential mediators of arthritis including TNF‐α, IL‐1β and IL‐6 and MMP‐3 (Hashiramoto et al., 2010). Given the fact that the stimulation of membrane melatonin receptors inhibits adenylyl cyclase activity, as the CRY transcription factor does, opens the question that how melatonin counteracts the CRY anti‐inflammatory activity in RA joints (Carlberg, 2000).
Melatonin in modulation of circadian clock and inflammation in OA
Recent evidence strongly suggests that the chronic, low‐grade inflammation, which develops with age or autoimmune conditions, is involved in both early and late stages of cartilage degeneration during the development of OA (Goldring and Otero, 2011). In aged, injured and OA joints, levels of pro‐inflammatory and catabolic cytokines, for example, IL‐1 and TNF‐α, are elevated in both animal models and in humans, with signs of synovitis (van den Berg, 2001; Goldring and Otero, 2011). Pro‐inflammatory cytokines/proteins, such as IL‐6, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1677, and MMPs, not only elicit catabolic effects and destroy matrix but also suppress the anabolic pathways by inhibiting Sox9 and Col2a1 expression, further impairing tissue repair (Figure 3) (Guo et al., 2015; Berenbaum and Meng, 2016). Guo et al. (2015) demonstrated that exposure to catabolic cytokines IL‐1β, but not TNF‐α, severely disrupted circadian gene expression rhythm in cartilage. The effect of IL‐1β on the cartilage clock was partly through functional interference with the core CLOCK/BMAL1 complex, mediated via an NFкB‐dependent pathway. IL‐1β but not TNF‐α disrupts the rhythmic expression of circadian clock genes in cartilage. Interestingly, an unexpected lack of effect of TNF‐α in regulating circadian pathways and NF‐κB signalling has been demonstrated in normal cartilage explants (Guo et al., 2015).
Figure 3.
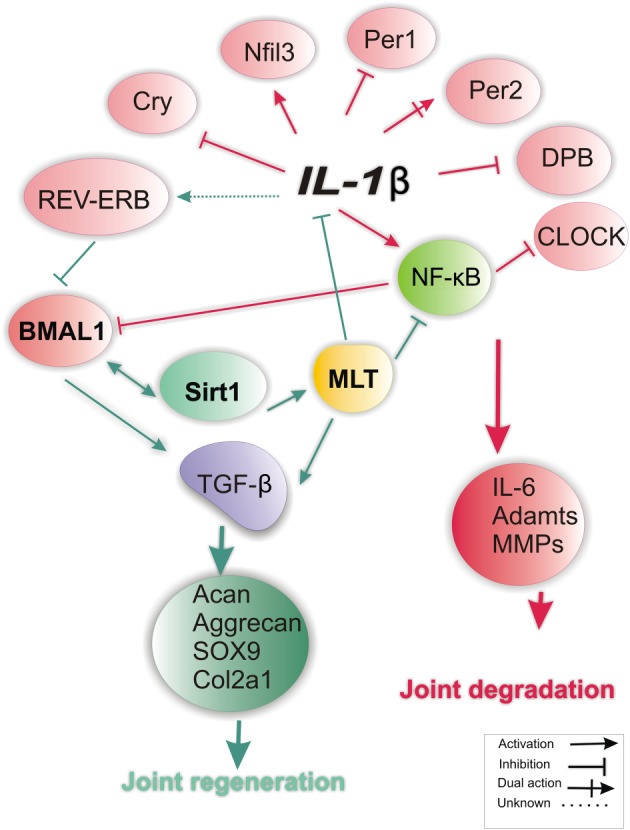
Interplay between melatonin and clock genes in joint during OA. Among pro‐inflammatory factors implicated in OA, IL‐1β accounts for disrupted expression of various clock genes. The role of BMAL1 is dominant in maintaining chondrocyte homeostasis in OA. Here, high levels of IL‐1β in the OA joint disturb the expression of most clock genes, leading to the suppression/reduction of BMAL‐1 expression, of which the loss of Sirt1 expression is central. Loss of BMAL due to inhibition of Sirt1 or via other pathways (up‐regulation of REV‐ERB‐α) can lead to the activation of MMPs, hence promote joint degradation. Melatonin is implicated in clock gene expression at least via two ways: (1) direct inhibitory actions on the release of pro‐inflammatory cytokines (TNF‐α, IL‐ 1β, IL‐8); and (2) blockade of NFkB signalling, which leads to the inhibition of matrix degrading enzymes (MMPs and Adamts4). Notably, circadian gene expression of Sirt1 is a prerequisite for BMAL activation and for the anti‐inflammatory effects of melatonin in the OA joints. Both melatonin and BMAL exploit a common pathway (TGF‐β signalling) to enhance matrix synthesis by articular chondrocytes.
It was recently documented that the expression of the core clock gene BMAL1 is disrupted in human OA cartilage and in aged mouse cartilage. Targeted BMAL1 ablation in mouse chondrocytes abolishes the circadian rhythms and promotes advanced destruction of the articular cartilage (van den Berg, 2001). BMAL1 modulate the circadian expression of many anabolic, catabolic and apoptotic genes that implicate in cartilage homeostasis. BMAL1 loss results in reduced levels of phosphorylated SMAD2/3, NFATC2, Sox9, Acan and Col2a1, but increased levels of phosphorylated SMAD1/5 (Dudek et al., 2016).
Yang et al. (2016) measured both mRNA and protein levels of circadian genes, NAD oxidase (NAD(+)) and Sirt1, in human knee articular cartilage. The results showed that in OA cartilage, the levels of both BMAL1 and NAD(+) decreased significantly, which resulted in the inhibition of nicotinamide phosphoribosyl transferase activity and Sirt1 expression. Furthermore, the knockdown of BMAL1 reduced the level of NAD(+) and exacerbated OA‐like gene expression changes during stimulation with IL‐1β. The overexpression of BMAL1 rescued the gene alterations stimulated by IL‐1β, which was consistent with the inhibitory effect of REV‐ERBα. Sirt1 siRNA treatment not only reduced the protein levels of BMAL1 and moderately elevated the levels of Per2 and REV‐ERBα but also further aggravated the survival of cells and the expression of cartilage matrix‐degrading enzymes induced by IL‐1β. Up‐regulation of Sirt1 reversed the IL‐1β‐induced metabolic imbalance of chondrocytes, suggesting that BMAL1 is a key clock gene implicated in Sirt1‐stimulated cartilage homeostasis (Yang et al., 2016). Notably, anti‐inflammatory and cytoprotective effect of melatonin in OA and MSCs also depends upon Sirt1 activity (Lim et al., 2012; Zhou et al., 2015). It appears that Sirt1 activation is central to the anti‐inflammatory and chondrocyte matrix regeneration potential of BMAL and melatonin (Lim et al., 2012; Zhou et al., 2015; Yang et al., 2016). Needless to say, additional studies are requiredto shed additional light on the role of the circadian hormone melatonin in the regulation of clock genes in OA.
Conclusions
Circadian expression of the pleiotropic hormone melatonin regulates a broad spectrum of the critical biological processes, including inflammation. The anti‐inflammatory actions of melatonin involve a myriad of inflammation‐wired molecules and pathways, to directly or indirectly block NF‐κB signalling and terminate the inflammatory responses. High levels of melatonin exert favourable effects on the prognosis of most inflammation–related, self‐destructive diseases, except RA. High levels of melatonin could lessen the clinical manifestation of OA, although in the context of its sister joint disease, RA, the results are somewhat conflicting. On the other hand, new data point to the deregulation of certain circadian clock genes in the pathology of the joint inflammatory diseases. Disturbed circadian rhythm is associated with disrupted expression of pro‐inflammatory cytokines, as well as deregulated expression of melatonin. It appears that restoring circadian rhythm may open new avenues to alleviate the symptoms of both inflammatory joint diseases, especially in RA patients, where an unfavourable prognosis is predicted by an unbalanced expression of the circadian hormone melatonin.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c,d,e).
Conflict of interest
The authors declare no conflicts of interest.
Jahanban‐Esfahlan, R. , Mehrzadi, S. , Reiter, R. J. , Seidi, K. , Majidinia, M. , Baghi, H. B. , Khatami, N. , Yousefi, B. , and Sadeghpour, A. (2018) Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis: involvement of circadian clock genes. British Journal of Pharmacology, 175: 3230–3238. 10.1111/bph.13898.
Contributor Information
Bahman Yousefi, Email: yousefib@tbzmed.ac.ir.
Alireza Sadeghpour, Email: sadeghpour46@yahoo.com.
References
- Akhtar N, Singh AK, Ahmed S (2016). MicroRNA‐17 suppresses TNF‐alpha signaling by interfering with TRAF2 and cIAP2 association in rheumatoid arthritis synovial fibroblasts. J Immunol 197: 2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5734–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang J, Chang HW, Jung HR, Cho CH, Hur JA, Lee SI et al. (2012). Melatonin attenuates clock gene cryptochrome1, which may aggravate mouse anti‐type II collagen antibody‐induced arthritis. Rheumatol Int 32: 379–385. [DOI] [PubMed] [Google Scholar]
- Berenbaum F, Meng Q‐J (2016). The brain‐joint axis in osteoarthritis: nerves, circadian clocks and beyond. Nat Rev Rheumatol 12: 508–516. [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Smolen JS, Coogan AN, Cajochen C (2015). Clocking in: chronobiology in rheumatoid arthritis. Nat Rev Rheumatol 11: 349–356. [DOI] [PubMed] [Google Scholar]
- Cano P, Cardinali DP, Chacon F, Reyes Toso CF, Esquifino AI (2002). Nighttime changes in norepinephrine and melatonin content and serotonin turnover in pineal glands of young and old rats injected with Freund's adjuvant. Neuro Endocrinol Lett 23: 49–53. [PubMed] [Google Scholar]
- Carlberg C (2000). Gene regulation by melatonin. Ann N Y Acad Sci 917: 387–396. [DOI] [PubMed] [Google Scholar]
- Carrillo‐Vico A, Lardone PJ, Alvarez‐Sanchez N, Rodriguez‐Rodriguez A, Guerrero JM (2013). Melatonin: buffering the immune system. Int J Mol Sci 14: 8638–8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wei W (2002). Effects and mechanisms of melatonin on inflammatory and immune responses of adjuvant arthritis rat. Int Immunopharmacol 2: 1443–1449. [DOI] [PubMed] [Google Scholar]
- Chuffa LG, Fioruci‐Fontanelli BA, Mendes LO, Ferreira Seiva FR, Martinez M, Favaro WJ et al. (2015). Melatonin attenuates the TLR4‐mediated inflammatory response through MyD88‐ and TRIF‐dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 15: 34. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Criswell LA (2010). Gene discovery in rheumatoid arthritis highlights the CD40/NF‐kappaB signaling pathway in disease pathogenesis. Immunol Rev 233: 55–61. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Maestroni GJ, Otsa K, Aakre O, Villaggio B, Capellino S et al. (2005a). Circadian melatonin and cortisol levels in rheumatoid arthritis patients in winter time: a north and south Europe comparison. Ann Rheum Dis 64: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Otsa K, Aakre O, Sulli A (2005b). Nocturnal hormones and clinical rhythms in rheumatoid arthritis. Ann N Y Acad Sci 1051: 372–381. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Villaggio B, Candido F, Valenti S, Giusti M, Felli L et al. (1999). Melatonin influences interleukin‐12 and nitric oxide production by primary cultures of rheumatoid synovial macrophages and THP‐1 cells. Ann N Y Acad Sci 876: 246–254. [DOI] [PubMed] [Google Scholar]
- Dudek M, Gossan N, Yang N, Im HJ, Ruckshanthi JP, Yoshitane H et al. (2016). The chondrocyte clock gene BMAL1 controls cartilage homeostasis and integrity. J Clin Invest 126: 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JE, Ray DW (2013). The role of the circadian clock in rheumatoid arthritis. Arthritis Res Ther 15: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore TD (2006). Introduction to NF‐kappaB: players, pathways, perspectives. Oncogene 25: 6680–6684. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Otero M (2011). Inflammation in osteoarthritis. Curr Opin Rheumatol 23: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossan N, Boot‐Handford R, Meng Q‐J (2015). Ageing and osteoarthritis: a circadian rhythm connection. Biogerontology 16: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossan N, Zeef L, Hensman J, Hughes A, Bateman JF, Rowley L et al. (2013). The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum 65: 2334–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen PK, Olsson LM (2009). Recent advances in the genetics of autoimmune disease. Annu Rev Immunol 27: 363–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Yang N, Borysiewicz E, Dudek M, Williams JL, Li J et al. (2015). Catabolic cytokines disrupt the circadian clock and the expression of clock‐controlled genes in cartilage via an NFкB‐dependent pathway. Osteoarthritis Cartilage 23: 1981–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S, Straub RH (2012). Disruption of rhythms of molecular clocks in primary synovial fibroblasts of patients with osteoarthritis and rheumatoid arthritis, role of IL‐1beta/TNF. Arthritis Res Ther 14: R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson I, Holmdahl R, Mattsson R (1990). Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen‐induced arthritis in DBA/1 mice. J Neuroimmunol 27: 79–84. [DOI] [PubMed] [Google Scholar]
- Hansson I, Holmdahl R, Mattsson R (1992). The pineal hormone melatonin exaggerates development of collagen‐induced arthritis in mice. J Neuroimmunol 39: 23–30. [DOI] [PubMed] [Google Scholar]
- Hansson I, Holmdahl R, Mattsson R (1993). Pinealectomy ameliorates collagen II‐induced arthritis in mice. Clin Exp Immunol 92: 432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiramoto A, Yamane T, Tsumiyama K, Yoshida K, Komai K, Yamada H et al. (2010). Mammalian clock gene Cryptochrome regulates arthritis via proinflammatory cytokine TNF‐alpha. J Immunol 184: 1560–1565. [DOI] [PubMed] [Google Scholar]
- Hoesel B, Schmid JA (2013). The complexity of NF‐κB signaling in inflammation and cancer. Mol Cancer 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Natoli G, Ghosh G (2006). Transcriptional regulation via the NF‐kappaB signaling module. Oncogene 25: 6706–6716. [DOI] [PubMed] [Google Scholar]
- Honda KK, Kawamoto T, Ueda HR, Nakashima A, Ueshima T, Yamada RG et al. (2013). Different circadian expression of major matrix‐related genes in various types of cartilage: modulation by light‐dark conditions. J Biochem 154: 373–381. [DOI] [PubMed] [Google Scholar]
- Hong Y, Hong Y (2015). Anti‐arthritic effects of melatonin through blockade of cytonuclear shift of MMP‐13. FASEB 29: 4–30. [Google Scholar]
- Hosseinzadeh A, Kamrava SK, Joghataei MT, Darabi R, Shakeri‐Zadeh A, Shahriari M et al. (2016). Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res 61: 411–425. [DOI] [PubMed] [Google Scholar]
- Jahanban‐Esfahlan R, de la Guardia M, Ahmadi D, Yousefi B (2017). Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J Cell Physiol 10.1002/jcp.25859. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Caliani AJ, Jimenez‐Jorge S, Molinero P, Guerrero JM, Fernandez‐Santos JM, Martin‐Lacave I et al. (2005). Dual effect of melatonin as proinflammatory and antioxidant in collagen‐induced arthritis in rats. J Pineal Res 38: 93–99. [DOI] [PubMed] [Google Scholar]
- Kouri VP, Olkkonen J, Kaivosoja E, Ainola M, Juhila J, Hovatta I et al. (2013). Circadian timekeeping is disturbed in rheumatoid arthritis at molecular level. PLoS One 8: e54049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang H, Akbar M, Kim HY (2000). Negative regulation of cytosolic phospholipase A(2) by melatonin in the rat pineal gland. Biochem J 351: 709–716. [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM (2002). NF‐kappaB regulation in the immune system. Nat Rev Immunol 2: 725–734. [DOI] [PubMed] [Google Scholar]
- Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG, Chun YH et al. (2012). Cytoprotective and anti‐inflammatory effects of melatonin in hydrogen peroxide‐stimulated CHON‐001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J Pineal Res 53: 225–237. [DOI] [PubMed] [Google Scholar]
- Lin HC, Lin TH, Wu MY, Chiu YC, Tang CH, Hour MJ et al. (2014). 5‐Lipoxygenase inhibitors attenuate TNF‐alpha‐induced inflammation in human synovial fibroblasts. PLoS One 9 e107890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Lee LM, Lee WJ, Chu CY, Tan P, Yang YC et al. (2016). Melatonin inhibits MMP‐9 transactivation and renal cell carcinoma metastasis by suppressing Akt‐MAPKs pathway and NF‐kappaB DNA‐binding activity. J Pineal Res 60: 277–290. [DOI] [PubMed] [Google Scholar]
- Liu X, Xu Y, Chen S, Tan Z, Xiong K, Li Y et al. (2014). Rescue of proinflammatory cytokine‐inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic Biol Med 68: 234–246. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Fu L, Tang Z, Zhang C, Qin L, Wang J et al. (2016). Melatonin inhibits AP‐2beta/hTERT, NF‐kappaB/COX‐2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget 7: 2985–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJ, Sulli A, Pizzorni C, Villaggio B, Cutolo M (2002). Melatonin in rheumatoid arthritis: synovial macrophages show melatonin receptors. Ann N Y Acad Sci 966: 271–275. [DOI] [PubMed] [Google Scholar]
- Majidinia M, Sadeghpour A, Mehrzadi S, Reiter RJ, Khatami N, Yousefi B (2017). Melatonin: a pleiotropic molecule that modulates DNA damage response and repair pathways. J Pineal Res 10.1111/jpi.12416. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Mishra A, Paul S, Swarnakar S (2011). Downregulation of matrix metalloproteinase‐9 by melatonin during prevention of alcohol‐induced liver injury in mice. Biochimie 93: 854–866. [DOI] [PubMed] [Google Scholar]
- Muxel SM, Laranjeira‐Silva MF, Carvalho‐Sousa CE, Floeter‐Winter LM, Markus RP (2016). The RelA/cRel nuclear factor‐kappaB (NF‐kappaB) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J Pineal Res 60: 394–404. [DOI] [PubMed] [Google Scholar]
- Nah SS, Won HJ, Park HJ, Ha E, Chung JH, Cho HY et al. (2009). Melatonin inhibits human fibroblast‐like synoviocyte proliferation via extracellular signal‐regulated protein kinase/P21(CIP1)/P27(KIP1) pathways. J Pineal Res 47: 70–74. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM (2012). Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A 109: 12662–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi G, Kumar A, Sharma SS (2011). Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF‐kappaB and Nrf2 cascades. J Pineal Res 50: 124–131. [DOI] [PubMed] [Google Scholar]
- Olkkonen J, Kouri VP, Hynninen J, Konttinen YT, Mandelin J (2015). Differentially expressed in chondrocytes 2 (DEC2) increases the expression of IL‐1beta and is abundantly present in synovial membrane in rheumatoid arthritis. PLoS One 10 e0145279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap T, Korb‐Pap A (2015). Cartilage damage in osteoarthritis and rheumatoid arthritis‐two unequal siblings. Nat Rev Rheumatol 11: 606–615. [DOI] [PubMed] [Google Scholar]
- Park JW, Hwang MS, Suh SI, Baek WK (2009). Melatonin down‐regulates HIF‐1 alpha expression through inhibition of protein translation in prostate cancer cells. J Pineal Res 46: 415–421. [DOI] [PubMed] [Google Scholar]
- Pei M, He F, Wei L, Rawson A (2009). Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes. J Pineal Res 46: 181–187. [DOI] [PubMed] [Google Scholar]
- Radogna F, Diederich M, Ghibelli L (2010). Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol 80: 1844–1852. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L (2016). Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61: 253–278. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Fuentes‐Broto L (2010). Melatonin: a multitasking molecule. Prog Brain Res 181: 127–151. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M (2005). Circadian clocks – the fall and rise of physiology. Nat Rev Mol Cell Biol 6: 965–971. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhilber D, Brungs M, Werz O, Wiesenberg I, Danielsson C, Kahlen JP et al. (1995). The nuclear receptor for melatonin represses 5‐lipoxygenase gene expression in human B lymphocytes. J Biol Chem 270: 7037–7040. [DOI] [PubMed] [Google Scholar]
- Stratmann M, Stadler F, Tamanini F, van der Horst GT, Ripperger JA (2010). Flexible phase adjustment of circadian albumin D site‐binding protein (DBP) gene expression by CRYPTOCHROME1. Genes Dev 24: 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli A, Maestroni GJ, Villaggio B, Hertens E, Craviotto C, Pizzorni C et al. (2002). Melatonin serum levels in rheumatoid arthritis. Ann N Y Acad Sci 966: 276–283. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL (2008). The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada T, Kodama A, Hotta S, Mieda M, Shimba S, Hinoi E et al. (2012). Clock genes influence gene expression in growth plate and endochondral ossification in mice. J Biol Chem 287: 36081–36095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg WB (2001). Uncoupling of inflammatory and destructive mechanisms in arthritis. Semin Arthritis Rheum 30: 7–16. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhou H, Du Z, Chen X, Zhu F, Wang Z et al. (2015). Cytoprotective effect of melatonin against hypoxia/serum deprivation‐induced cell death of bone marrow mesenchymal stem cells in vitro. Eur J Pharmacol 748: 157–165. [DOI] [PubMed] [Google Scholar]
- West SK, Oosthuizen JM (1992). Melatonin levels are decreased in rheumatoid arthritis. J Basic Clin Physiol Pharmacol 3: 33–40. [DOI] [PubMed] [Google Scholar]
- Wiesenberg I, Missbach M, Carlberg C (1998). The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci 12: 143–150. [PubMed] [Google Scholar]
- Yang W, Kang X, Liu J, Li H, Ma Z, Jin X et al. (2016). Clock gene BMAL1 modulates human cartilage gene expression by crosstalk with Sirt1. Endocrinology 157: 3096–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hashimoto T, Sakai Y, Hashiramoto A (2014a). Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. J Immunol Res 2014: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hashimoto T, Sakai Y, Hashiramoto A (2014b). Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. J Immunol Res 2014: 282495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan G et al. (2015). Melatonin reverses H2 O2 ‐induced premature senescence in mesenchymal stem cells via the SIRT1‐dependent pathway. J Pineal Res 59: 190–205. [DOI] [PMC free article] [PubMed] [Google Scholar]


