Abstract
Melatonin, N‐acetyl‐5‐methoxytryptamine, an evolutionally old molecule, is produced by the pineal gland in vertebrates, and it binds with high affinity to melatonin receptors, which are members of the GPCR family. Among the multiple effects attributed to melatonin, we will focus here on those that are dependent on the activation of the two mammalian MT1 and MT2 melatonin receptors. We briefly summarize the latest developments on synthetic melatonin receptor ligands, including multi‐target‐directed ligands, and the characterization of signalling‐biased ligands. We discuss signalling pathways activated by melatonin receptors that appear to be highly cell‐ and tissue‐dependent, emphasizing the impact of system bias on the functional outcome. Different proteins have been demonstrated to interact with melatonin receptors, and thus, we postulate that part of this system bias has its molecular basis in differences of the expression of receptor‐associated proteins including heterodimerization partners. Finally, bias at the level of the receptor, by the expression of genetic receptor variants, will be discussed to show how a modified receptor function can have an effect on the risk for common diseases like type 2 diabetes in humans.
Linked Articles
This article is part of a themed section on Recent Developments in Research of Melatonin and its Potential Therapeutic Applications. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.16/issuetoc
Abbreviations
- 4P‐PDOT
4‐phenyl‐2‐propionamidotetralin
- AD
Alzheimer's disease
- ASD
autism spectrum disorder
- miRNA
microRNA
- MUPP1
multi‐PDZ domain protein 1
- PGC‐1α
PPAR‐γ coactivator
- RGS
regulator of G‐protein signalling
- SCN
suprachiasmatic nucleus
- SIRT
sirtuin histone deacetylase
- T2D
type 2 diabetes
Introduction
The http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39 family is one of the GPCR subfamilies (Jockers et al., 2016). In higher vertebrates, the melatonin receptor family is composed of two members, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=287&familyId=39&familyType=GPCR and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=288&familyId=39&familyType=GPCR (Reppert et al., 1994; 1995a), which have high affinity for the natural ligand http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224. Human MT1 and MT2 receptors are composed of 350 and 362 amino acids and show an overall sequence homology of 55% and 70% in the transmembrane domain (Oishi and Jockers, 2016). Based on the high‐sequence homology (50%) with MT1 and MT2, the melatonin‐related receptor, also called http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=107, was classified as another member of the melatonin receptor family (Reppert et al., 1996a). However, GPR50 does not bind to melatonin or any other known ligand and, thus, is still considered an orphan receptor (Reppert et al., 1996a; Ngo et al., 2016). Interestingly, GPR50 has been proposed to be the mammalian orthologue of Mel1c, a high‐affinity melatonin receptor found in non‐mammalian vertebrates (Dufourny et al., 2008). The main focus of this review will be on the function of MT1 and MT2 receptors, whereas GPR50 will be considered as a regulatory protein that associates with melatonin receptors.
The natural ligand of MT1 and MT2 receptors is melatonin. Melatonin is an evolutionally very old molecule, which is synthesized in many organisms such as bacteria, protists, fungi, macroalgae, plants and animals. In vertebrates, melatonin is the main hormone produced by the pineal gland and follows a circadian pattern, synchronized to the dark phase of the environmental light/dark cycle. In humans, MT1 and MT2 receptors are expressed in brain and several peripheral organs (Table 1). Because both receptors are mainly coupled to Gαi/o proteins, decreased intracellular levels of the second messenger cAMP is the most commonly reported signalling pathway triggered by melatonin. Melatonin receptors regulate circadian rhythms, sleep, seasonal reproduction, immune functions, retinal physiology and glucose homeostasis (Dubocovich et al., 2010; Jockers et al., 2016). The establishment of MT1 (Liu et al., 1997) and MT2 knockout mice (Jin et al., 2003) has contributed to reveal these various physiological functions and to attribute them to one or the other receptor type. Recent reviews have focused on the description of these mouse models, the relevance to human disease and physiology, and therapeutic potential (Tosini et al., 2014; Zlotos et al., 2014; Jockers et al., 2016; Liu et al., 2016). In this review, we will focus on novel pharmacological aspects of melatonin receptors, discuss their signalling diversity in the context of system bias and start to unravel the molecular basis for system bias by defining melatonin receptor‐associated protein partners. The last section will put the discovery of multiple rare variants of melatonin receptors and associated modification of receptor function in the context of disease risk.
Table 1.
Distribution of MT1 and MT2 melatonin receptors expressed in human tissues
| Tissue | Technique | Reference | ||
|---|---|---|---|---|
| hMT1 | Brain | Cerebellum | RT‐PCR | Mazzucchelli et al. (1996) |
| In situ hybridization | Al‐Ghoul et al. (1998) | |||
| Occipital cortex | RT‐PCR | Mazzucchelli et al., (1996) | ||
| Parietal cortex | RT‐PCR | Mazzucchelli et al. (1996) | ||
| Temporal cortex | RT‐PCR | Mazzucchelli et al. (1996) | ||
| Thalamus | RT‐PCR | Mazzucchelli et al. (1996) | ||
| Frontal cortex | RT‐PCR | Mazzucchelli et al. (1996) | ||
| Hippocampus | RT‐PCR | Mazzucchelli et al. (1996) | ||
| Immunohistochemistry | Savaskan et al. (2001) | |||
| SCN | In situ hybridization | Weaver and Reppert (1996) | ||
| Peripheral tissues | Retina | Immunocytochemistry | Savaskan et al. (2001) | |
| Savaskan et al. (2002) | ||||
| Scher et al. (2002) | ||||
| Scher et al. (2003) | ||||
| Brown and white adipose tissue | RT‐PCR | Brydon et al. (2001) | ||
| Fetal kidney | RT‐PCR | Drew et al. (1998) | ||
| Coronary artery | RT‐PCR | Ekmekcioglu et al. (2001a,b) | ||
| Granulosa cells | RT‐PCR | Soares et al. (2003) | ||
| Niles et al. (1999) | ||||
| Myometrium | RT‐PCR, in‐situ hybridization | Schlabritz‐Loutsevitch et al. (2003) | ||
| Pancreatic alpha and beta cells | RNA sequencing | Blodgett et al. (2015) | ||
| Testis | RT‐PCR | Rossi et al. (2014) | ||
| hMT2 | Brain | Cerebellum | In situ hybridization | Al‐Ghoul et al. (1998) |
| Hippocampus | Immunocytochemistry | Savaskan et al. (2005) | ||
| SCN | Immunocytochemistry | Wu et al. (2013) | ||
| Peripheral tissues | Retina | RT‐PCR | Reppert et al. (1995a) | |
| Immunohistochemistry | Savaskan et al. (2007) | |||
| Brown and white adipose tissue | RT‐PCR | Brydon et al. (2001) | ||
| Fetal kidney | RT‐PCR | Drew et al. (1998) | ||
| Granulosa cells | RT‐PCR | Soares et al. (2003) | ||
| Niles et al. (1999) | ||||
| Placental tissues | RT‐PCR and Western blot | Lanoix et al. (2006) | ||
| Myometrium | In‐situ hybridization | Schlabritz‐Loutsevitch et al. (2003) | ||
| RT‐PCR | Sharkey et al. (2009) | |||
| Pancreatic alpha and beta cells | RNA sequencing | Blodgett et al. (2015) | ||
| Testis | RT‐PCR | Rossi et al. (2014) |
Melatonin receptor pharmacology
State of the art on melatonin receptor ligands
The pharmacological characterization of melatonin receptors started in the late 80s, when melatonin binding sites were detected by using the radiolabelled ligand http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1344 (Dubocovich, 1988). The cloning and expression of recombinant melatonin receptors in the 90s allowed further advances in the pharmacological and functional characterization of MT1 and MT2 receptors (Reppert et al., 1994, 1995a,b,1996b). In some studies, tritiated melatonin (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1357) is also used as a radioligand. However, its low specific activity hampered its broader use despite the fact that it is structurally closer to melatonin than 2‐[125I]‐iodomelatonin. Of note, a recent study comparing the pharmacology of melatonin receptors in 2‐[125I]‐iodomelatonin and [3H]‐melatonin binding experiments revealed that these radioligands do not behave identically. Whereas [3H]‐melatonin detects two melatonin binding sites in both MT1 and MT2 receptors, 2‐[125I]‐iodomelatonin detects only one binding site (Legros et al., 2014). Using G‐protein uncoupling agents, the authors concluded that the two binding sites detected by [3H]‐melatonin represent two different receptor populations, that is, in their activated and inactivated state, while 2‐[125I]‐iodomelatonin binds only to activated receptors. Novel iodinated radioligands like http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7779 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7771, which are the first MT2‐selective radioligands, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7770, an analogue of 2‐[125I]‐iodomelatonin, were recently added to the tool box (Legros et al., 2013; 2016). Although these are promising ligands, it must be noted that they did not detect melatonin binding sites in some brain areas known to express melatonin receptors, like the SCN, pointing to the need for further improvements of these tools.
Considerable efforts are currently being made to develop fluorescently labelled melatonin receptor ligands. These are promising compounds for investigating the pharmacology and localization of melatonin receptors; they can replace radioligand binding assays and be used to visualize receptors in imaging assays respectively. The ligands described so far include the 7‐azamelatonin analogue (Wu et al., 2007), coumarin‐based compounds (de la Fuente Revenga et al., 2015) and bodipy‐fused analogues (Thireau et al., 2014; Viault et al., 2016; Gbahou et al., 2017). Some of these compounds bind with good affinity (nM range) and non‐selectively to MT1 and MT2 receptors, but data on their full pharmacological and functional properties are only fragmentary.
Competition binding experiments, mainly with 2‐[125I]‐iodomelatonin, contributed largely to the identification of melatonin receptor‐selective ligands as recently reviewed (Zlotos et al., 2014; Jockers et al., 2016). The competitive non‐selective antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1363 is still the most used pharmacological tool for characterizing membrane receptor‐mediated effects, while the MT2‐selective antagonist 4‐phenyl‐2‐propionamidotetralin (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1358) is the main pharmacological tool used to discriminate between MT1‐ and MT2‐mediated effects (IUPHAR database). Reliable MT1‐selective ligands are still unavailable. Recently, pharmacoinformatic approaches have revealed an intriguing class of compounds that are able to bind melatonin receptors with μM affinity: carbamate‐derived insecticides (carbaryl and carbofuran) (Popovska‐Gorevski et al., 2017). In fact, previous observations indicated that these compounds affect the function of the pineal gland, resulting in altered levels of nocturnal plasma melatonin (Attia et al., 1991).
Therapeutic applications of melatonin receptor ligands and multi‐target‐directed ligands
The following melatonin receptor ligands are currently available as marketed drugs for the treatment of conditions linked to circadian dysfunction: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1356 [previously named TAK‐375, commercialized as Rozerem® to treat insomnia (Uchikawa et al., 2002; Erman et al., 2006)], http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=198 [previously named S20098, commercialized as Valdoxan® to treat depression (de Bodinat et al., 2010; Guardiola‐Lemaitre et al., 2014)] and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7393 (VEC‐162) [previously named BMS‐214778, commercialized as Hetlioz® to treat sleep and circadian disturbances (Vachharajani et al., 2003; Rajaratnam et al., 2009)]. A recent review summarized the clinical and preclinical effects of these currently marketed drugs targeting melatonin receptors (Liu et al., 2016).
Several recent studies have proposed treatments based on so‐called multi‐target‐directed ligands (Talevi, 2015). Molecules designed to display at least two complementary functions are attractive as (i) they take into account the fact that diseases are often complex multifactorial conditions and, as such, better results are expected; (ii) they abolish the risk of drug interaction, in which case they can replace the co‐administration of two different drugs; and (iii) they facilitate pharmacokinetic aspects, compared with the co‐administration condition. Melatonin‐derived multi‐target‐directed ligands were developed mainly for the treatment of Alzheimer's disease (AD) in an attempt to combine the beneficial effects of melatonin as a neuroprotective, antioxidant and anti‐amyloidogenic agent with other neuroprotective molecules or with classical anti‐cholinesterase inhibitors currently used as the sole treatment for AD. Examples of these hybrid compounds are tacrine–melatonin (Spuch et al., 2010; Zawadzka et al., 2013); melatonin–N,N‐dibenzyl(N‐methyl)amine hybrid ITH91/IQM157 (Buendia et al., 2015a); (−)‐meptazinol–melatonin hybrids (Cheng et al., 2015); curcumin–melatonin (Chojnacki et al., 2014; Gerenu et al., 2015); melatonin–sulforaphane hybrid ITH12674 (Egea et al., 2015) and donepezil–melatonin hybrids (Wang et al., 2016). In vitro and in vivo characterization studies confirmed that many of these molecules show the expected combined effects, validating the multi‐target‐directed ligand approach. The melatonin agonist piromelatine (N‐(2‐[5‐methoxy‐1H‐indol‐3‐yl]ethyl)‐4‐oxo‐4H‐pyran‐2‐carboxamide, or Neu‐P11) (Tian et al., 2010), tested in phase I clinical trial to treat insomnia and currently under phase II clinical trial to treat AD (Neurim Pharmaceuticals Ltd, 2011a,b, 2017), can also be classified as multi‐target‐directed ligand, as it acts also as an agonist at the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3 receptors (He et al., 2013; Liu et al., 2014). Similarly, the anti‐depressive melatonin analogue agomelatine (Valdoxan®) is also a multi‐target ligand, with agonistic properties at MT1 and MT2 receptors and antagonistic properties at the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=8 receptor (Millan et al., 2011).
Biased melatonin receptor ligands
Both melatonin receptors are mainly coupled to the Gαi/o proteins and, thus, melatonin classically signals through dampening of the cAMP/PKA pathway. Additional intracellular cascades that are commonly measured include the melatonin‐induced activation of MEK/ERK kinases and the recruitment of β‐arrestins (Jockers et al., 2016). Studies on biased ligands for melatonin receptors, that is, ligands preferentially modulating one pathway over another as compared with melatonin, are still in their infancy. The first melatonin receptor ligand characterized as a biased ligand was recently described and named ICOA‐9 (Gbahou et al., 2017). ICOA‐9 shows preferential signalling for the Gi/o/cAMP pathway over the β‐arrestin2 and ERK pathways following human MT1 receptor activation. Furthermore, the clinically active antidepressant agomelatine shows functional properties on MT2/5‐HT2C heteromers that are biased towards the Gi/cAMP pathway and, thus, distinct from those of melatonin and 5‐HT2C‐specific antagonists (see next section for more details). Molecular modelling together with molecular docking studies could provide clues about the structural requirements for biased melatonin receptor ligands, similar to the modelling‐assisted study that helped to design ligands with MT1 versus MT2 receptor selectivity and MT2‐specific antagonistic properties (Pala et al., 2013; Spadoni et al., 2015).
System bias of melatonin receptor pharmacology
The above‐mentioned multi‐target drugs add a new twist to the system bias of melatonin receptor pharmacology, since it implies a context‐dependent efficacy of the ligand depending on the presence or absence of heteromeric receptor complexes. Interestingly, agomelatine not only targets MT1, MT2 and 5‐HT2C receptors separately but also the heteromeric complex comprising MT2 and 5‐HT2C receptors (Kamal et al., 2015), which introduces a previously underappreciated system bias. In cells expressing only MT2 receptors, melatonin has no effect on the inositol phosphate (IP) signalling pathway, while it behaves as an agonist at this pathway in the presence of the 5‐HT2C receptor. Similarly, agomelatine has no effect on this pathway in cells expressing only MT2 receptors, but it antagonizes melatonin‐induced IP production in cells co‐expressing MT2 and 5‐HT2C receptors (Kamal et al., 2015). In addition, whereas melatonin shows agonistic properties on the cAMP and IP pathway, agomelatine is an agonist of the cAMP pathway but a neutral antagonist of the IP pathway compared with melatonin in cells expressing the MT2/5‐HT2C heteromer. Conversely, luzindole and 4P‐PDOT, which are competitive antagonists of MT1 and MT2 receptors in the absence of the 5‐HT2C receptor, behave as agonists of the cAMP pathway and full or partial agonists, respectively, of the IP pathway in cells expressing the MT2/5‐HT2C heteromer.
Evidence for the existence of system bias in melatonin receptor pharmacology also comes from studies showing that ligand efficacy depends on the cell context, on the receptor expression levels in a given tissue and on their active or inactive state (Legros et al., 2014). For example, luzindole and 4P‐PDOT (at high concentrations) are usually competitive melatonin receptor antagonists, but behave as inverse agonists at MT1 receptors in the presence of constitutively activated receptors, as shown in rat artery cells expressing endogenous MT1 receptors (Ersahin et al., 2002). Constitutive activation was also observed for MT1 and MT2 receptors in transfected CHO and Neuro2A cell lines, and MT2‐specific inverse agonists (UCM 549 and UCM 724) effectively decreased the constitutive activity of MT2 receptors (Devavry et al., 2012). The pharmacological properties of the competitive melatonin receptor antagonist 4P‐PDOT seem to be even more complex, as not only antagonistic and inverse agonistic effects have been described, as mentioned before, but also partial agonistic activity is also reported for native and recombinant MT2 receptors (Nonno et al., 1999; Ayoub et al., 2002; Dubocovich et al., 2003). The impact of the cell context on the pharmacological properties of melatonin receptors is further confirmed by the study of Logez et al. (2014). They observed a marked decrease in ligand binding affinities of recombinant human MT1 receptors when expressed in the eukaryotic microorganism Pichia pastoris compared with MT1 receptors expressed in CHO cells. Intriguingly, after purifying MT1 receptors from P. pastoris, the pharmacological profile of the receptor resembled that observed in CHO membranes (Logez et al., 2014). The authors suspect that differences in the membrane lipid composition, most likely the cholesterol content, between these two cell types are at the origin of the differences observed in the pharmacological profiles.
Collectively, cell context‐dependent receptor expression, receptor heterodimerization and context‐ and ligand‐dependent bias are among the main factors underlying system bias and are proven to be relevant to the elucidation of the pharmacology of melatonin receptors.
Melatonin receptor signalling
Common aspects of melatonin receptor signalling
As yet, the intrinsic affinity of melatonin receptors for different G proteins has not been systematically determined. The G‐protein coupling profile also depends on the relative expression levels of the different G proteins in a given cellular context, which accounts for the system bias of melatonin receptor pharmacology. In most experimental systems, both MT1 and MT2 receptors are mainly coupled to Gi proteins, thus leading to inhibition of AC activity (Figure 1). A more detailed analysis in HEK293 cells shows that MT1 receptors co‐immunoprecipitate preferentially with Gαi2 and Gαi3 proteins and to a lesser extent to Gq/11, while no coupling to Gαi1, Gαz, Gαo, Gα12 or Gαs was detected (Brydon et al., 1999b). An illustrative example of system bias is the Gα16 protein, which is exclusively expressed in haematopoietic cells (Amatruda et al., 1991). Co‐transfection of MT1 or MT2 receptors together with Gα16 in COS‐7 cells leads to the potentiation of melatonin signalling through the JNK pathway, indicating coupling of both melatonin receptors to Gα16 (Chan et al., 2002). In addition to the frequently observed modulation of cAMP levels by melatonin receptors, modulation of diacylglycerol, inositol trisphosphate and Ca2+ levels have been observed in a cell context‐dependent manner (Brydon et al., 1999a,b). Tissues and cells in which Gq/11 coupling to endogenously expressed melatonin receptors have been observed include the myometrium (Steffens et al., 2003), prostate epithelial cells (Shiu et al., 2010), pancreatic cells (Bähr et al., 2012) and human mesenchymal stem cells (Lee et al., 2014), in addition to non‐mammalian cells (Hotta et al., 2000) and cells expressing recombinant receptors (MacKenzie et al., 2002).
Figure 1.
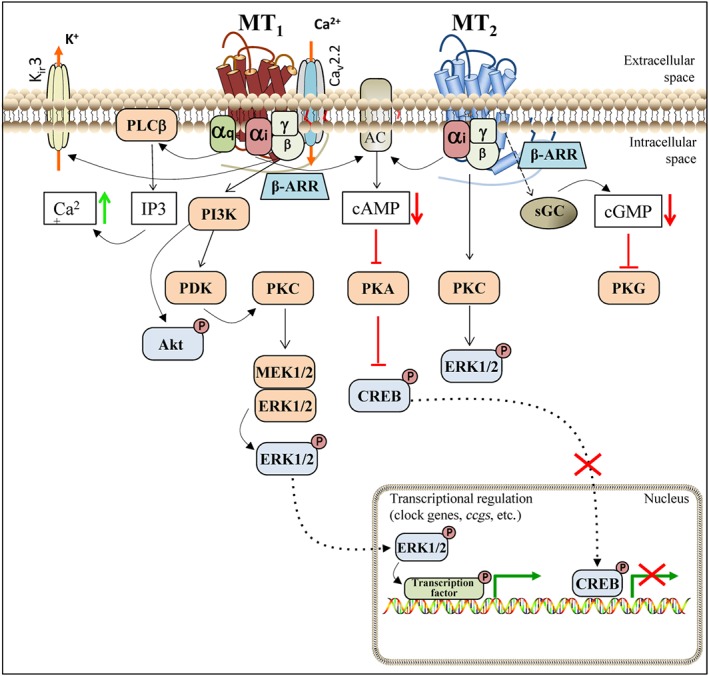
Melatonin receptor signalling pathways. Melatonin activation of MT1 receptors triggers Gαi activation, decreasing the levels of the secondary messanger cAMP, and Gβγ‐dependent activation of PI3K/Akt, PKC and ERK pathways. MT1 coupling to Gq leads to PLC activation and increase in intracellular Ca2+. Melatonin‐induced modulation of neuronal action potential is mediated by MT1‐dependent activation of the potassium and calcium ion channels (Kir3 and Cav2.2). The physical interaction of MT1 receptors with Cav2.2 channels tonically inhibits Cav2.2‐mediated calcium entry through Gβγ subunits. Melatonin activation of MT2 receptors triggers Gαi‐dependent cAMP and ERK signalling pathways and inhibits cGMP levels. Melatonin induced β‐arrestin recruitment to both MT1 and MT2 receptors, but β‐arrestin‐dependent down‐streaming signalling is not yet reported. See text for details. β‐ARR, β‐arrestin; Cav2.2, voltage‐gated calcium channel; ccgs, clock‐controlled genes; CREB, cAMP‐responsive element binding; Kir3, G protein‐coupled inwardly rectifying potassium channel; sGC, soluble GC.
Melatonin can also regulate ion channels, and multiple pathways seem to be involved. Melatonin modulates muscle contractile responses in arteries (Geary et al., 1998; Masana et al., 2002) and in the myometrium (Steffens et al., 2003) by regulating the activity of large‐conductance Ca2+‐activated K+ (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=380, also known as KCa1.1) channels. In the myometrium, the modulation of BKCa channels by melatonin was shown to be dependent on the activation of both Gq/PLC/Ca2+ and Gi/cAMP/PKA pathways. Interestingly, the final outcome of the effect of melatonin on BKCa activity depends on the physiological context, as opposite effects are observed in myocytes obtained from pregnant and non‐pregnant rats (Steffens et al., 2003). At the transcriptional level, melatonin signalling typically inhibits the transcriptional factor cAMP‐responsive element binding (CREB) and activates the transcription of genes under the control of the ERK pathway. Up to now, the major difference between MT1 and MT2 receptors at the signalling level concerns their ability to inhibit cGMP production, which is only observed in MT2‐transfected cells (Petit et al., 1999). This effect was confirmed in human non‐pigmented ciliary epithelial cells expressing endogenous MT2 receptors (Dortch‐Carnes and Tosini, 2013). The general signalling pathways triggered by melatonin receptors are depicted in Figure 1, but the impact of system bias should be kept in mind. We next present further melatonin functions for which at least some of the signalling intermediates have been described, thus expanding the molecular components of melatonin receptor signalling.
Diversity of melatonin's effects and signalling cascades – further evidence of system bias
The regulation of circadian rhythms by melatonin has been extensively studied (Dubocovich et al., 2010). Melatonin has been shown to affect the firing rate of hypothalamic suprachiasmatic nucleus (SCN) neurons, which constitutes the master clock in mammals. This effect is mediated by both receptors in a Gi‐dependent, but cAMP‐independent manner. For MT1 receptors it involves the activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=74, like Kir3 (Nelson et al., 1996; van den Top et al., 2001; Hablitz et al., 2015), while the MT2‐induced phase advance in neuronal activity involves the PKC signalling pathway (McArthur et al., 1997; Hunt et al., 2001). In the presence of pituitary AC activating peptide (PACAP)‐induced cAMP production, both receptors can modulate neuronal activity by the Gi/cAMP pathway (Jin et al., 2003). Melatonin regulation of clock gene expression has been reported in the SCN and both MT1 and MT2 receptors are involved in this effect (Pfeffer et al., 2012; Nagy et al., 2015; Kandalepas et al., 2016). In the striatum, melatonin has been reported to modulate clock gene expression through MT1 receptors in a Gi‐dependent manner (Imbesi et al., 2009). In cerebellar Purkinje cells, the neuronal firing rate is modulated by MT1 receptors through inhibition of http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=80 (Cav2.1) in a Gi/Gβγ/PI3K/PKCδ signalling‐dependent manner (Zhang et al., 2015). Together with the SCN, the hypophyseal pars tuberalis is the main target of melatonin involved in its synchronizing effects. Melatonin transduces photic information through MT1 receptors by regulating the expression of mPer1, mCry1, Clock and Bmal1 genes through a heterologous repressive/sensitization of the cAMP pathway that requires not only MT1 receptors but also the adenosine A2B receptor (von Ball et al., 2002; Dardente et al., 2003; von Gall et al., 2005; Wood and Loudon, 2014). The regulator of G‐protein signalling (RGS)4 (Dupre et al., 2011) and the basic helix‐loop‐helix Per‐Arnt‐Sim domain transcription factor NPAS4 are also suggested to participate in this signalling cascade (West et al., 2013). Although the circadian machinery is present in every cell, the effect of melatonin on rhythmic clock gene expression in other tissues is not clear and seems to be cell type‐dependent (Muhlbauer et al., 2009; Owino et al., 2016). The precise mechanism underlying the effect of melatonin on clock genes is not well defined and might involve transcriptional and post‐translational modulation of clock proteins (reviewed by Vriend and Reiter, 2015).
Many studies have demonstrated that melatonin plays an important role in regulating different aspects of retinal physiology. The clock machinery of the retina is responsive to melatonin, and both receptors are involved, but the precise signalling pathway has not, as yet, been elucidated. In MT1 receptor knockout mice, the rhythmic expression of Per1 was not abolished, but the phase was significantly affected (Dinet et al., 2007). Significant changes in the pattern of expression of other clock genes, as well as clock‐controlled genes (ccgs, like cfos) were also observed in melatonin receptor knockout mice (Hiragaki et al., 2014; Kunst et al., 2015). Melatonin controls the retinal light sensitivity at night (Baba et al., 2013), an effect that was shown to depend on MT1/MT2 heteromers (further discussed in the Melatonin receptor oligomers section), which preferentially activate the Gq/PLC/Ca2+ pathway (Baba et al., 2013), while regulation of photoreceptor viability is believed to depend on the survival‐related Akt/FOXO1 signalling pathway (Gianesini et al., 2016).
In addition to retinal cells, melatonin also modulates the viability of neurons under physiological and pathological conditions. The neuroprotective and anti‐apoptotic properties of endogenous and exogenous melatonin have been extensively investigated, and different signalling pathways underlie these effects. In neural stem cells, the effect of melatonin on cell survival, maturation and differentiation is melatonin receptor‐dependent, as it is prevented by the competitive melatonin receptor antagonist luzindole (Ramirez‐Rodriguez et al., 2009; Tocharus et al., 2014; Chu et al., 2016; Ortiz‐Lopez et al., 2016). In pluripotent stem cells melatonin‐induced neural differentiation involves the PI3K/Akt pathway and is also blocked by luzindole (Shu et al., 2016). However, prolonged exposure of embryonic stem cells to melatonin favours the pluripotency state of the cells in a MT1‐dependent manner that involves a synergism between the effect of the PI3K/Akt and ERK pathways that results in the up‐regulation of the glucose transporter GLUT1 (Wu et al., 2017). In an in vivo rat model of neuro‐inflammation, endogenous melatonin protects cerebellar neurons from LPS toxicity, while neuronal death is observed in the presence of the competitive melatonin receptor antagonist luzindole (Pinato et al., 2015). Similarly, Wang et al. (2011) observed that neurons were more vulnerable to cell death in the presence of luzindole and in MT1‐silenced cells. In ischaemia/reperfusion models, the protective effect of melatonin relies on its anti‐apoptotic and antioxidant effects, as it is known to up‐regulate several antioxidant enzymes, including SOD1 and glutathione peroxidase (Parada et al., 2014; O'Neal‐Moffitt et al., 2015; Ramos et al., 2017). In cerebral ischaemia, the protective effect of melatonin and agomelatine was linked to activation of the nuclear factor erythroid‐related factor 2 (Nrf2), which regulates the expression of antioxidant enzymes (Ding et al., 2014; Chumboatong et al., 2017). In in vitro and in vivo ischaemic models, the multi‐target‐directed 5‐HT and melatonin receptor Neu‐P11 ligand promoted neuronal survival through the activation of PI3K/Akt, ERK and JAK2 pathways (Buendia et al., 2015b) (Figure 2).
Figure 2.
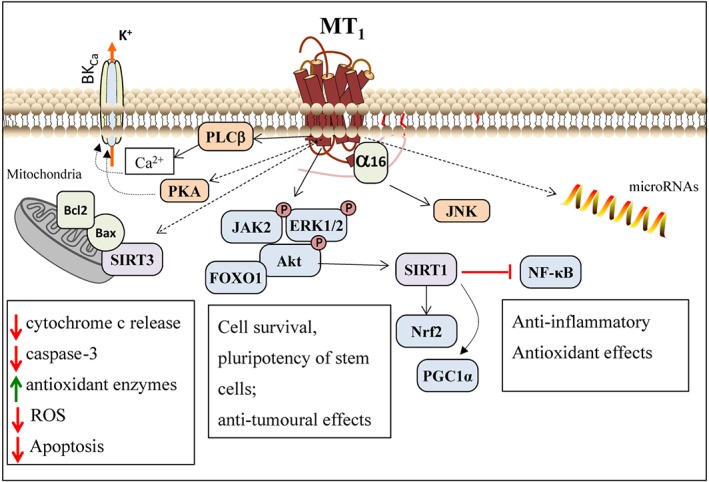
Extended, context‐specific melatonin receptor signalling pathways. Depending on the cell type or the presence of cell stressors, melatonin can activate additional melatonin receptor‐dependent signalling cascades. These pathways have been reported mainly for MT1 receptors, but the participation of MT2 receptors cannot be excluded. Melatonin modulation of mitochondrial function is reported under oxidative stress condition and in neurodegenerative diseases. Proposed signalling pathways involve the regulation of the activity and/or translocation of Bcl2/Bax and SIRT proteins. Activation of JAK2, ERK and the Akt/FOXO1 complex are suggested to mediate melatonin‐induced cell survival and to modulate pluripotency/differentiation of stem cells, while melatonin‐induced inhibition of these pathways is reported in cancer cells. MT1‐dependent activation of SIRT1 might underlie melatonin anti‐inflammatory and anti‐oxidative effects through regulation of transcription factors like Nrf2, PGC1α and NF‐κB. MT1‐coupling to G16 protein occurs in haematopoietic cells and triggers the JNK pathway. In several cell types, including cancer cells, melatonin is reported to modulate the expression of different miRNAs. See text for details. SIRT, sirtuin.
A number of observations suggest that the antioxidant and anti‐apoptotic effects of melatonin depend largely on mitochondrial function and dynamics (Tan et al., 2016). Melatonin has been reported to modulate the expression levels and localization of Bax and Bcl‐2 proteins and to inhibit the release of cytochrome c and the activation of caspase‐3 (Radogna et al., 2008; Wang et al., 2009; Luchetti et al., 2010). The JAK2/STAT3 pathway is suspected to mediate melatonin‐induced Bax/Bcl‐2 translocation in cardiomyocytes (Yang et al., 2013), while ERK activation and p38 MAPK inhibition were proposed to mediate the anti‐apoptotic effect of melatonin in monocytes (Luchetti et al., 2009). Additional mitochondria‐associated signalling cascades activated by melatonin include activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=848 (SIRTs) (reviewed by Mayo et al., 2017) through AMPK/SIRT3/SOD2 and SIRT1/PPAR‐γ coactivator (PGC‐1α), as shown in hepatocytes (Guo et al., 2014; Chen et al., 2015; Pi et al., 2015). Of note, the transcription factor PGC‐1α is also regulated by MT1 receptors in the retina (Kunst et al., 2015). Melatonin signalling through SIRT might also underlie the well‐known anti‐inflammatory action of melatonin through inhibition of the inflammatory transcription factor NF‐κB (Tajes et al., 2009; Zhao et al., 2017). In mouse models of neurodegenerative diseases, such as AD, amyotrophic lateral sclerosis and Huntington's disease, the neuroprotective effect of melatonin was linked to MT1‐dependent modulation of mitochondrial function (Dragicevic et al., 2011; Wang et al., 2011; Zhang et al., 2013). Interestingly, the inhibitory effect of melatonin on cytochrome c release could be reproduced in purified brain mitochondria, presumably through mitochondrial MT1 receptors, as suggested by the authors (Wang et al., 2011). Recently, further evidence for the intriguing mitochondrial localization of MT1 receptors was obtained by using the first cell‐impermeable melatonin receptor agonist, which allowed us to discriminate between MT1‐triggered Gi/cAMP signalling at the cell surface and in mitochondria (Gbahou et al., 2017). Whether the neuroprotective effect of melatonin linked to mitochondrial function is due to mitochondrial MT1 signalling is currently under investigation.
In addition to the above‐mentioned examples, the impact of system bias on melatonin receptor signalling can also be nicely exemplified in the cancer field, where the repertoire of signalling pathways modulated by melatonin is highly context‐specific. In general, melatonin is reported to display anti‐tumoural properties, inhibiting proliferation and inducing apoptosis. In breast cancer models, the anti‐tumoural effect of melatonin appears to involve mainly MT1 receptors through inhibition of the phosphorylation of signalling molecules such as AKT, ERK and PKC (Hill et al., 2011; 2015). In these cells, melatonin has also been reported to activate the p53 DNA‐protective pathway in a receptor‐dependent manner (Santoro et al., 2013), while in ovarian cancer, melatonin inhibits Akt, p38 MAPK and mTOR signalling (Ferreira et al., 2014).
New players contributing to system bias of melatonin receptor function
MicroRNAs
Additional complexity to the study of melatonin receptors signalling emerges when considering the growing number of reports pointing to the role of microRNAs (miRNA) in mediating the effects of melatonin. In prostate cancer cells, the anti‐angiogenic effect of melatonin was linked to an up‐regulation of miRNA3195 and miRNA374b (Sohn et al., 2015). MiR‐24 is a miRNA often up‐regulated in several types of cancer, and melatonin was able to down‐regulate it in a luzindole‐sensitive manner (Mori et al., 2016). Regulation of miRNAs by melatonin has been also demonstrated in hepatocytes (Kim et al., 2015; 2017) and neurons (Carloni et al., 2016). Interestingly, miRNAs can also regulate the expression of MT1 receptors (Zhu et al., 2014). It has recently been reported that the expression of miRNAs can vary in a daily pattern (Marcola et al., 2016), which introduces an additional bias regarding the time when data are collected. Because miRNAs are highly context‐dependent, it is likely that they greatly contribute to the system bias on melatonin receptors signalling. Altogether, an extended melatonin receptor signalling network is emerging from these studies in the light of the system bias concept (Figure 2).
Melatonin receptor oligomers
The work from Ayoub et al. (2002) was the first to propose that, similar to other GPCRs, melatonin receptors could exist as homomers and/or heteromers. By BRET experiments using transfected HEK293 cells, we demonstrated that melatonin receptors form homomers and heteromers in living cells, with prevalence for the formation of the heteromers (Ayoub et al., 2002; 2004). Melatonin activation did not have any apparent effect on the oligomerization state of the receptors. Importantly, the heteromer showed a distinct pharmacological profile in BRET experiments compared with MT2 homomers, with the competitive antagonist luzindole being 100 times more potent on heteromers (Ayoub et al., 2004). Both binding sites seem to be preserved in the heteromer when inspected individually and are not subject to negative cooperativity (Ayoub et al., 2004). Although many GPCRs have a natural tendency to oligomerize (Ferre et al., 2014), such oligomers appear to be less abundant in tissues at natural expression levels, and their physiological relevance has been proven only in some cases. MT1/MT2 heteromers were detected in retinal photoreceptor cells and were shown to be key players in improving retinal light sensitivity at night (Baba et al., 2013). The in vitro characterization of the heteromer revealed that the PKC signalling pathway is potentiated by melatonin under this condition and, indeed, the in vivo effect is also PKC‐dependent (Baba et al., 2013). Inasmuch as many tissues express both melatonin receptors, including the SCN, the involvement of MT1/MT2 heteromers in mediating melatonin's effects deserves further investigation. Interestingly, different melatonin signalling patterns are detected in cerebellar granular cells expressing endogenous MT1 and MT2 receptors. In this cellular context, melatonin at a low nM concentration inhibits rather than stimulates ERK and Akt pathways, while a stimulatory response is detected if either receptor is silenced. Conversely, melatonin decreases forskolin‐simulated cAMP production only in cells expressing both MT1 and MT2 receptors, while no effect is detected in cerebellar granular cells expressing only one type of melatonin receptor (Imbesi et al., 2008). It is likely that the responsiveness of these cells to melatonin relies on the MT1/MT2 heteromer.
The orphan receptor of the melatonin receptor family, GPR50, has also been demonstrated to engage in oligomeric complexes with MT1 and MT2 receptors (Levoye et al., 2006). Contrary to what was observed for MT1/MT2 heteromers, in this case, the dimer formation markedly altered ligand binding and signalling properties of MT1, but not of MT2 receptors, as melatonin binding and Gi protein and ß‐arrestin coupling of MT1 receptors are lost in the MT1/GPR50 heterodimer. The negative modulation of MT1 receptors by GPR50 was confirmed in hCMEC/D3 cells expressing both receptors endogenously, as melatonin signalling was observed only after GPR50 silencing (Levoye et al., 2006). The physiological relevance of the MT1/GPR50 heterodimer, as well as the regulatory factors of their association and dissociation in vivo, remain to be elucidated. Nevertheless, the fact that GPR50 is expressed in the pituitary gland, in several hypothalamic nuclei and in the median eminence, which are main loci of melatonin's action, as well as in other central areas (Batailler et al., 2012), implies that a GPR50‐dependent regulation of melatonin receptors signalling might be physiologically relevant.
As mentioned before, the pharmacological properties of agomelatine lead to the investigation of the existence of heteromers comprising melatonin and 5‐HT receptors. In transfected HEK293 cells, it was observed that both MT1 and MT2 receptors are able to associate with the 5‐HT2c receptor in a heteromeric complex (Kamal et al., 2015). The pharmacology of MT2 receptors seems to be altered in the heteromer, as melatonin activates not only Gi‐dependent signalling but also Gq/PLC signalling, which is not observed in cells expressing the MT2 receptor alone (Kamal et al., 2015). We proposed a transactivation model in which the melatonin‐activated MT2 receptor is able to allosterically transactivate 5‐HT2c‐dependent Gq signalling. These heteromers were also targeted by agomelatine suggesting that MT2/5‐HT2c heteromers might participate in the antidepressant effect of this drug. The biased pharmacology of melatonin receptor dimers is shown in Figure 3.
Figure 3.

Signalling of melatonin receptor homomers and heteromers. Melatonin activation of MT1/MT1 homomers triggers intracellular signalling predominantly through the Gi pathway over the Gq pathway, while MT2/MT2 homomers signal exclusively through Gi proteins. In the MT1/MT2 heteromer, melatonin signalling is biased towards Gq activation over Gi. When dimerizing with GPR50, the MT1 receptor loses its ability to bind melatonin, to trigger Gi signalling and to recruit β‐arrestin (β‐ARR). Melatonin activation of the MT2/5‐HT2c heteromer triggers 5‐HT2C receptor dependent Gq signalling through a MT2 receptor transactivation mechanism. See text for details. MLT, melatonin.
Melatonin receptor‐associated protein complexes
Formation of receptor heteromers is only one of the possible ways that GPCRs have to shape their cellular micro‐environment to ultimately determine the signalling outcome. Other proteins that might be constitutive or agonist‐induced components of these receptor‐associated complexes have also been identified for melatonin receptors. By combining different proteomic and genomic approaches and different biological resources expressing endogenous melatonin receptors, an interactome of MT1 and MT2 receptors composed of 366 individual proteins was built (Figure 4) (Daulat et al., 2007; Benleulmi‐Chaachoua et al., 2016). This represents one of the most complete and diverse GPCR interactomes currently available. Out of the 366 interactors, only 52 were identical between the two receptors. Many of these common interactors belong to the family of small G proteins (Rab and Rho GTPases), heterotrimeric G proteins, molecular chaperones (calnexin and calreticulin), cytosqueleton components (filamin, myosin, etc.) and ubiquitin ligases. This suggests common functions and highlights the previously underappreciated link between melatonin receptors and small G proteins and cytosqueleton organization that warrants further attention.
Figure 4.
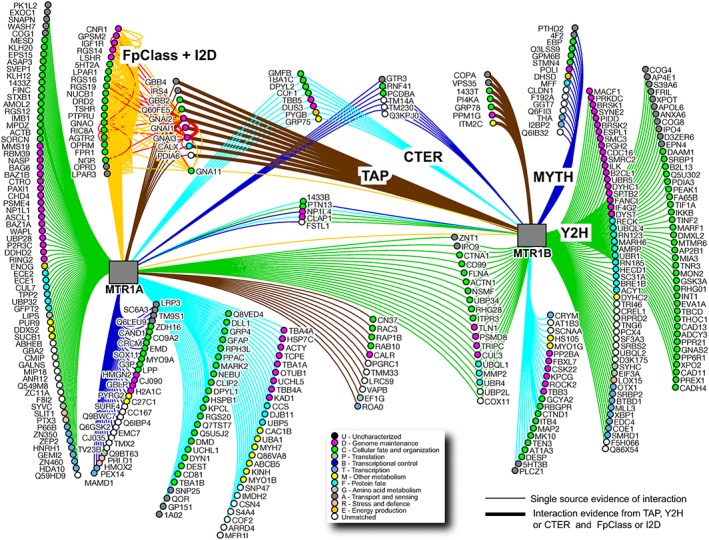
Melatonin receptors interactome. MTR1A (MT1)‐ and MTR1B (MT2)‐interacting proteins were identified in 20 different screens and are clustered based on the different identification methods: dark blue for the MYTH, blue for Cter peptide purification, green for the Y2H and brown for the TAP methods. Thick lines correspond to confirmed protein–protein interactions and node colours refer to predicted gene ontology biological function. See text for details. CTER, carboxyl terminus peptide affinity chromatography; MYTH, membrane yeast two‐hybrid; TAP, tandem affinity purification; Y2H, yeast two‐hybrid. Modified from Benleulmi‐Chaachoua et al. (2016), with permission.
MT1 and MT2 receptors are known to undergo agonist‐dependent and ‐independent internalization (Gerdin et al., 2003; Guillaume et al., 2008). The melatonin receptor interactome contains trafficking proteins such as caveolin, dynamin 1, AP2 and AP4 adaptor proteins that act in concert with GPCRs kinases and ß‐arrestins to promote receptor internalization (Levoye et al., 2006; Bondi et al., 2008; Maurice et al., 2008).
The interactome also revealed that most of the interactions are unique for each receptor (168 and 143 for MT1 and MT2 respectively). This result was unexpected given the fact that previous studies revealed only few functional differences between MT1 and MT2 receptors. These novel results will be a rich source of investigation for the next few years to unravel and understand the functional differences between both receptors. Among the possible new functions are links with several ion transporters/channels like the electroneutral potassium‐chloride co‐transporter 1, the zinc transporters and the electroneutral Na/HCO3 co‐transporter in the MT2 interactome. Another remarkable finding was the exclusive presence of several synaptic proteins in the MT1 interactome such as synapsin, SNAP25 and 47, the voltage‐gated calcium channel Cav2.2, Munc‐18, rabphilin and snapin. The absence of these proteins from the MT2 interactome suggested differences in the subcellular localization of MT1 and MT2 receptors in neurons, a notion that was confirmed in primary hippocampal neurons. The different localization of melatonin receptors in neurons is likely to increase our understanding of the different roles of both receptors in the brain and, at the same, adds a not yet appreciated spatial bias to melatonin receptor function. The interactome also provides some clues as to how the different localization of both receptors is achieved as the MT1, but not the MT2 receptor, interacts with kinesin‐1, which has been shown to transport the Na+ channel to axons (Barry et al., 2014). The interaction of MT1 receptors with the Cav2.2 channel was shown to be of functional importance as in vitro experiments in CHO cells showed that the presence of MT1 receptors tonically inhibits the Cav2.2‐promoted calcium current through Gβγ subunits (Benleulmi‐Chaachoua et al., 2016). Indeed, voltage‐dependent inhibition involving direct binding of Gβγ subunit to the channel (Zamponi and Currie, 2013) is the most widespread mechanism by which GPCRs regulate voltage‐gated calcium channels. Localization of MT1 receptors in the presynaptic membrane suggests its involvement in synaptic functions such as neurotransmission. This conclusion corroborates earlier reports suggesting a possible influence of melatonin on neurotransmitter release and uptake in some brain regions, like the ventral hippocampus, medulla pons, preoptic area and median and posterior hypothalamus but not in others (Cardinali et al., 1975; Zisapel and Laudon, 1982). This new input from the interactome analysis will renew the interest in the possible role of MT1 receptors in neurotransmitter regulation.
Among the best‐characterized interactors of the MT1 receptor is RGS20 (Maurice et al., 2010) and the multi‐PDZ domain protein, MUPP1 (Guillaume et al., 2008). Whereas RGS20 regulates the speed of Gi‐protein signalling of MT1 receptors by accelerating the activation kinetics of Kir3 channels, the expression of MUPP1 is obligatory for MT1 receptors to inhibit cAMP production by a yet poorly defined mechanism. Altogether, these data demonstrate the tremendous influence of melatonin receptor‐associated proteins on their function and underline the necessity to define the interactome of melatonin receptors in a given cell type to fully understand the functional outcome of melatonin stimulation in a given tissue.
Melatonin receptor variants
Receptor variants introduce a bias at the level of the receptor that contributes to inter‐individual differences in receptor function, which are suspected to contribute to the risk of common diseases. Genetic variation may also have important consequences on drug action. This variation may occur at the level of different ethnic groups (typically for frequent variants) or at the level of individuals (typically for rare or very rare variants). Consequently, information of the existence of receptor variants within cohorts for clinical trials helps to stratify and homogenize the cohorts to decrease their genetic variability and to help improve the outcome for clinical trials.
Recent large‐scale sequencing studies revealed considerable inter‐individual genetic variability in GPCR coding genes. In humans, an average of 32 non‐synonymous variants has been estimated to exist per GPCR in a sample of 10 000 individuals (Nelson et al., 2012; Karamitri and Jockers, 2014). In the case of melatonin receptors, 8 and 42 non‐synonymous variants have been demonstrated to occur in the MTNR1A and MTNR1B genes coding for the MT1 and MT2 receptor respectively (Jockers et al., 2016). These numbers are likely to increase with the increased number of sequenced human genomes and targeted exon sequencing. Based on reports indicating that an alteration in melatonin synthesis is associated with autism spectrum disorders (ASDs), non‐synonymous variants have been identified in the MTNR1A and MTNR1B genes in 300 ASD patients and a matched control population. No significant difference in the prevalence of these variants was found indicating that they do not contribute to ASD risk (Chaste et al., 2010). A similar conclusion was reached after sequencing of MTNR1A and MTNR1B in individuals with attention deficit hyperactivity disorder (ADHD; Chaste et al., 2011). From a pharmacological point of view, it is important to note that the majority of the 16 non‐synonymous variants identified in these studies showed altered receptor function with some of them showing a biased signalling profile compared with the wild‐type receptor (Chaste et al., 2010). This was the first report to show that variability of melatonin action does exist at the receptor level.
Inspired by genome‐wide association studies revealing a robust association of the minor allele of the common rs10830963 variant located in the intron of MTNR1B with increased type 2 diabetes (T2D) risk (Bouatia‐Naji et al., 2009; Lyssenko et al., 2009), the two exons of the MTNR1B gene were sequenced in 7632 individuals including 2186 individuals with T2D (Bonnefond et al., 2012). Forty non‐synonymous MT2 variants were identified including 38 rare and the two common variants. Those variants with a loss‐of‐function phenotype, but not the functionally neutral ones, were associated with increased T2D risk, establishing for the first time a link between melatonin receptor function and the risk for a common disease such as T2D. Subsequent studies that attempted to understand the functional basis of the association of the common rs10830963 variant with T2D risk indicated that risk allele carriers express two to four times more MTNR1B mRNA in their human pancreatic islets (van de Bunt et al., 2015). Taken together with other experimental findings, a model was proposed based on the assumption that MT2 protein levels are increased, exaggerating the putative inhibitory effect of melatonin on pancreatic insulin production in risk allele carriers (Tuomi et al., 2016). Obviously, this hypothesis contrasts with the conclusion drawn from rare loss‐of‐function variants, namely, that defective melatonin receptor function is associated with T2D risk. This apparent controversy is discussed in several recent commentaries (Bonnefond et al., 2016; Mulder, 2017). Drawing the right conclusions will be of relevance for human health, as it will be important to know whether melatonin supplementation (as practised by millions of people in the world) is beneficial or detrimental in terms of glucose homeostasis and T2D risk. From a pharmacological point of view, functional evidence for the expression of MT2 receptors in human pancreatic beta cells is weak. The MT2 receptor has not been detected at the protein level, and mRNA expression is only detectable in less than 5% of the cells at very low (close to background) levels (Segerstolpe et al., 2016; Thomsen et al., 2016). Evidence for the inhibitory effect of melatonin on cAMP levels and insulin production is substantial in rodents at the cellular and animal level (Peschke et al., 2007); however, conflicting results are reported in human cells with some studies even showing a stimulation/potentiation of insulin production by melatonin (Ramracheya et al., 2008; Costes et al., 2015). It is important to note that the physiological effect of melatonin on metabolism is expected to be fundamentally different as diurnal humans are day active, whereas nocturnal rodents are night active, even though melatonin is always secreted during the night. In the light of this inconclusive evidence, further hypotheses have to be explored to understand the effect of melatonin on glucose homeostasis in humans. This includes the search for further functions of melatonin in pancreatic beta cells, such as the recently reported stimulatory role of melatonin on human beta‐cell survival (Costes et al., 2015). Whether these effects are indeed mediated through melatonin receptors or through novel pharmacological units, such as heteromeric complexes, has to be investigated. Melatonin target tissues, other than pancreatic beta cells, like the brain or the liver and adipose tissues will have to be considered. Furthermore, focusing on the inhibitory effect of melatonin receptors on the cAMP pathway might also be too restrictive and other G protein‐ and β‐arrestin‐dependent signalling events might be more relevant.
In conclusion, the currently available data show that melatonin receptor variants exist in the human population and that they are of relevance for major common diseases. However, we are only at the beginning of our understanding of the full impact of such variants on human health. An expansion of future studies towards the MTNR1A gene and other diseases like sleep and circadian rhythm disorders represents an interesting field of future research.
Conclusion and perspectives
Multiple functions have been attributed to melatonin receptors that are transmitted by the activation of a large diversity of signalling pathways. Current knowledge clearly indicates that the signalling profile of melatonin receptors is highly cell‐ and tissue‐dependent, arguing for the existence of system bias as an important determinant of the functional outcome of melatonin receptor signalling. This highly complex arrangement makes it difficult to transpose functional properties described in one cell context into another. It also implies that the exogenous expression of recombinant receptors might be only of limited predictive value for the signalling properties of endogenous melatonin receptors in a given tissue. Interesting areas of future research are the detailed investigation of the intriguing localization of melatonin receptors in intracellular compartments such as mitochondria, the widespread formation of melatonin receptor heteromers and the development of novel generations of multi‐target‐directed ligands. New radioactive and fluorescently labelled tracer molecules are likely to detect further activation states of melatonin receptors that will be highly informative in defining new melatonin receptor complexes. Finally, the generation of biased ligands for melatonin receptors is still in its infancy but warrants further attention given the huge expectation of these compounds for therapeutic application in terms of signalling specificity and reduced side effects.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Dr M. Caron (Duke University, North Carolina) for inspiring discussions. This work was supported by the Agence Nationale de la Recherche (ANR 2011‐BSV1‐012‐01 ‘MLT2D’, ANR‐2011‐META ‘MELA‐BETES’ and ANR‐12‐RPIB‐0016 ‘MED‐HET‐REC‐2’), the Fondation de la Recherche Médicale (Equipe FRM DEQ20130326503), Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS) and the ‘Who am I?’ laboratory of excellence no. ANR‐11‐LABX‐0071 funded by the French Government through its ‘Investments for the Future’ programme operated by the French National Research Agency (ANR) under grant no. ANR‐11‐IDEX‐0005‐01 (to R.J.).
Cecon, E. , Oishi, A. , and Jockers, R. (2018) Melatonin receptors: molecular pharmacology and signalling in the context of system bias. British Journal of Pharmacology, 175: 3263–3280. 10.1111/bph.13950.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Ghoul WM, Herman MD, Dubocovich ML (1998). Melatonin receptor subtype expression in human cerebellum. Neuroreport 9: 4063–4068. [DOI] [PubMed] [Google Scholar]
- Amatruda TT, Steele DA, Slepak VZ, Simon MI (1991). G alpha 16, a G protein alpha subunit specifically expressed in hematopoietic cells. Proc Natl Acad Sci U S A 88: 5587–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia AM, Reiter RJ, Withyachumnarnkul B, Mostafa MH, Soliman SA, El‐Sebae AK (1991). Chronic administration of sublethal doses of carbaryl increases pineal N‐acetyltransferase and hydroxyindole‐O‐methyltransferase activities and serum melatonin levels. J Pineal Res 10: 49–54. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Couturier C, Lucas‐Meunier E, Angers S, Fossier P, Bouvier M et al (2002). Monitoring of ligand‐independent dimerization and ligand‐induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem 277: 21522–21528. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Levoye A, Delagrange P, Jockers R (2004). Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol 66: 312–321. [DOI] [PubMed] [Google Scholar]
- Baba K, Benleulmi‐Chaachoua A, Journe AS, Kamal M, Guillaume JL, Dussaud S et al (2013). Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal 6: ra89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähr I, Mühlbauer E, Albrecht E, Peschke E (2012). Evidence of the receptor‐mediated influence of melatonin on pancreatic glucagon secretion via the Gαq protein‐coupled and PI3K signaling pathways. J Pineal Res 53: 390–398. [DOI] [PubMed] [Google Scholar]
- Barry J, Gu Y, Jukkola P, O'neill B, Gu H, Mohler PJ et al (2014). Ankyrin‐G directly binds to kinesin‐1 to transport voltage‐gated Na(+) channels into axons. Dev Cell 28: 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batailler M, Mullier A, Sidibe A, Delagrange P, Prevot V, Jockers R et al (2012). Neuroanatomical distribution of the orphan GPR50 receptor in adult sheep and rodent brains. J Neuroendocrinol 24: 798–808. [DOI] [PubMed] [Google Scholar]
- Benleulmi‐Chaachoua A, Chen L, Sokolina K, Wong V, Jurisica I, Emerit MB et al (2016). Protein interactome mining defines melatonin MT1 receptors as integral component of presynaptic protein complexes of neurons. J Pineal Res 60: 95–108. [DOI] [PubMed] [Google Scholar]
- Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, Kennedy NJ et al (2015). Novel observations from next‐generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 64: 3172–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CD, Mckeon RM, Bennett JM, Ignatius PF, Brydon L, Jockers R et al (2008). MT1 melatonin receptor internalization underlies melatonin‐induced morphologic changes in Chinese hamster ovary cells and these processes are dependent on Gi proteins, MEK 1/2 and microtubule modulation. J Pineal Res 44: 288–298. [DOI] [PubMed] [Google Scholar]
- Bonnefond A, Clement N, Fawcett K, Yengo L, Vaillant E, Guillaume JL et al (2012). Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet 44: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond A, Karamitri A, Jockers R, Froguel P (2016). The difficult journey from genome‐wide association studies to pathophysiology: the melatonin receptor 1B (MT2) paradigm. Cell Metab 24: 345–347. [DOI] [PubMed] [Google Scholar]
- Bouatia‐Naji N, Bonnefond A, Cavalcanti‐Proenca C, Sparso T, Holmkvist J, Marchand M et al (2009). A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 41: 89–94. [DOI] [PubMed] [Google Scholar]
- Brydon L, Petit L, Decoppet P, Barrett P, Morgan PJ, Strosberg AD et al (1999a). Polymorphism and signalling of melatonin receptors. Reprod Nutr Develop 39: 315–324. [DOI] [PubMed] [Google Scholar]
- Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R (2001). Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology 142: 4264–4271. [DOI] [PubMed] [Google Scholar]
- Brydon L, Roka F, Petit L, Decoppet P, Tissot M, Barrett P et al (1999b). Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol Endocrinol 13: 2025–2038. [DOI] [PubMed] [Google Scholar]
- Buendia I, Egea J, Parada E, Navarro E, Leon R, Rodriguez‐Franco MI et al (2015a). The melatonin‐N,N‐dibenzyl(N‐methyl)amine hybrid ITH91/IQM157 affords neuroprotection in an in vitro Alzheimer's model via hemo‐oxygenase‐1 induction. ACS Chem Nerosci 6: 288–296. [DOI] [PubMed] [Google Scholar]
- Buendia I, Gomez‐Rangel V, Gonzalez‐Lafuente L, Parada E, Leon R, Gameiro I et al (2015b). Neuroprotective mechanism of the novel melatonin derivative Neu‐P11 in brain ischemia related models. Neuropharmacology 99: 187–195. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Nagle CA, Freire F, Rosner JM (1975). Effects of melatonin on neurotransmitter uptake and release by synaptosome‐rich homogenates of the rat hypothalamus. Neuroendocrinology 18: 72–85. [DOI] [PubMed] [Google Scholar]
- Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, Longini M et al (2016). Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR‐34a/silent information regulator 1 pathway. J Pineal Res 61: 370–380. [DOI] [PubMed] [Google Scholar]
- Chan AS, Lai FP, Lo RK, Voyno‐Yasenetskaya TA, Stanbridge EJ, Wong YH (2002). Melatonin MT1 and MT2 receptors stimulate c‐Jun N‐terminal kinase via pertussis toxin‐sensitive and ‐insensitive G proteins. Cellular Signal 14: 249–257. [DOI] [PubMed] [Google Scholar]
- Chaste P, Clement N, Botros HG, Guillaume JL, Konyukh M, Pagan C et al (2011). Genetic variations of the melatonin pathway in patients with attention‐deficit and hyperactivity disorders. J Pineal Res 51: 394–399. [DOI] [PubMed] [Google Scholar]
- Chaste P, Clement N, Mercati O, Guillaume JL, Delorme R, Botros HG et al (2010). Identification of pathway‐biased and deleterious melatonin receptor mutants in autism spectrum disorders and in the general population. PLoS One 5: e11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Qing W, Sun M, Lv L, Guo D, Jiang Y (2015). Melatonin protects hepatocytes against bile acid‐induced mitochondrial oxidative stress via the AMPK‐SIRT3‐SOD2 pathway. Free Radic Res 49: 1275–1284. [DOI] [PubMed] [Google Scholar]
- Cheng S, Zheng W, Gong P, Zhou Q, Xie Q, Yu L et al (2015). (−)‐Meptazinol‐melatonin hybrids as novel dual inhibitors of cholinesterases and amyloid‐beta aggregation with high antioxidant potency for Alzheimer's therapy. Bioorg Med Chem 23: 3110–3118. [DOI] [PubMed] [Google Scholar]
- Chojnacki JE, Liu K, Yan X, Toldo S, Selden T, Estrada M et al (2014). Discovery of 5‐(4‐hydroxyphenyl)‐3‐oxo‐pentanoic acid [2‐(5‐methoxy‐1H‐indol‐3‐yl)‐ethyl]‐amide as a neuroprotectant for Alzheimer's disease by hybridization of curcumin and melatonin. ACS Chem Nerosci 5: 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Tu Y, Chen J, Tan D, Liu X, Pi R (2016). Effects of melatonin and its analogues on neural stem cells. Mol Cell Endocrinol 420: 169–179. [DOI] [PubMed] [Google Scholar]
- Chumboatong W, Thummayot S, Govitrapong P, Tocharus C, Jittiwat J, Tocharus J (2017). Neuroprotection of agomelatine against cerebral ischemia/reperfusion injury through an antiapoptotic pathway in rat. Neurochem Int 102: 114–122. [DOI] [PubMed] [Google Scholar]
- Costes S, Boss M, Thomas AP, Matveyenko AV (2015). Activation of melatonin signaling promotes beta‐cell survival and function. Mol Endocrinol 29: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardente H, Menet JS, Poirel VJ, Streicher D, Gauer F, Vivien‐Roels B et al (2003). Melatonin induces Cry1 expression in the pars tuberalis of the rat. Brain Res Mol Brain Res 114: 101–106. [DOI] [PubMed] [Google Scholar]
- Daulat AM, Maurice P, Froment C, Guillaume JL, Broussard C, Monsarrat B et al (2007). Purification and identification of G protein‐coupled receptor protein complexes under native conditions. Mol Cell Proteomics 6: 835–844. [DOI] [PubMed] [Google Scholar]
- de Bodinat C, Guardiola‐Lemaitre B, Mocaer E, Renard P, Munoz C, Millan MJ (2010). Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov 9: 628–642. [DOI] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Herrera‐Arozamena C, Fernandez‐Saez N, Barco G, Garcia‐Orue I, Sugden D et al (2015). New coumarin‐based fluorescent melatonin ligands. Design, synthesis and pharmacological characterization. Eur J Med Chem 103: 370–373. [DOI] [PubMed] [Google Scholar]
- Devavry S, Legros C, Brasseur C, Delagrange P, Spadoni G, Cohen W et al (2012). Description of the constitutive activity of cloned human melatonin receptors hMT(1) and hMT(2) and discovery of inverse agonists. J Pineal Res 53: 29–37. [DOI] [PubMed] [Google Scholar]
- Dinet V, Ansari N, Torres‐Farfan C, Korf HW (2007). Clock gene expression in the retina of melatonin‐proficient (C3H) and melatonin‐deficient (C57BL) mice. J Pineal Res 42: 83–91. [DOI] [PubMed] [Google Scholar]
- Ding K, Wang H, Xu J, Li T, Zhang L, Ding Y et al (2014). Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: the Nrf2‐ARE signaling pathway as a potential mechanism. Free Radic Biol Med 73: 1–11. [DOI] [PubMed] [Google Scholar]
- Dortch‐Carnes J, Tosini G (2013). Melatonin receptor agonist‐induced reduction of SNP‐released nitric oxide and cGMP production in isolated human non‐pigmented ciliary epithelial cells. Exp Eye Res 107: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic N, Copes N, O'neal‐Moffitt G, Jin J, Buzzeo R, Mamcarz M et al (2011). Melatonin treatment restores mitochondrial function in Alzheimer's mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res 51: 75–86. [DOI] [PubMed] [Google Scholar]
- Drew JE, Williams LM, Hannah LT, Barrett P, Abramovich DR (1998). Melatonin receptors in the human fetal kidney: 2‐[125I]iodomelatonin binding sites correlated with expression of Mel1a and Mel1b receptor genes. J Endocrinol 156: 261–267. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML (1988). Pharmacology and function of melatonin receptors. FASEB J 2: 2765–2773. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J (2010). International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein‐coupled melatonin receptors. Pharmacol Rev 62: 343–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML, Rivera‐Bermudez MA, Gerdin MJ, Masana MI (2003). Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci 8: d1093–d1108. [DOI] [PubMed] [Google Scholar]
- Dufourny L, Levasseur A, Migaud M, Callebaut I, Pontarotti P, Malpaux B et al (2008). GPR50 is the mammalian ortholog of Mel1c: evidence of rapid evolution in mammals. BMC Evol Biol 8: 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre SM, Dardente H, Birnie MJ, Loudon AS, Lincoln GA, Hazlerigg DG (2011). Evidence for RGS4 modulation of melatonin and thyrotrophin signalling pathways in the pars tuberalis. J Neuroendocrinol 23: 725–732. [DOI] [PubMed] [Google Scholar]
- Egea J, Buendia I, Parada E, Navarro E, Rada P, Cuadrado A et al (2015). Melatonin‐sulforaphane hybrid ITH12674 induces neuroprotection in oxidative stress conditions by a ‘drug‐prodrug’ mechanism of action. Br J Pharmacol 172: 1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekmekcioglu C, Haslmayer P, Philipp C, Mehrabi MR, Glogar HD, Grimm M et al (2001a). Expression of the MT1 melatonin receptor subtype in human coronary arteries. J Recept Signal Transduct Res 21: 85–91. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu C, Haslmayer P, Philipp C, Mehrabi MR, Glogar HD, Grimm M et al (2001b). 24h variation in the expression of the mt1 melatonin receptor subtype in coronary arteries derived from patients with coronary heart disease. Chronobiol Int 18: 973–985. [DOI] [PubMed] [Google Scholar]
- Erman M, Seiden D, Zammit G, Sainati S, Zhang J (2006). An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia. Sleep Med 7: 17–24. [DOI] [PubMed] [Google Scholar]
- Ersahin C, Masana MI, Dubocovich ML (2002). Constitutively active melatonin MT(1) receptors in male rat caudal arteries. Eur J Pharmacol 439: 171–172. [DOI] [PubMed] [Google Scholar]
- Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ et al (2014). G protein‐coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66: 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira GM, Martinez M, Camargo IC, Domeniconi RF, Martinez FE, Chuffa LG (2014). Melatonin attenuates Her‐2, p38 MAPK, p‐AKT, and mTOR levels in ovarian carcinoma of ethanol‐preferring rats. J Cancer 5: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbahou F, Cecon E, Viault G, Gerbier R, Jean‐Alphonse F, Karamitri A et al (2017). Design and validation of the first cell‐impermeant melatonin receptor agonist. Br J Pharmacol 174: 2409–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary GG, Duckles SP, Krause DN (1998). Effect of melatonin in the rat tail artery: role of K+ channels and endothelial factors. Br J Pharmacol 123: 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdin MJ, Masana MI, Ren D, Miller RJ, Dubocovich ML (2003). Short‐term exposure to melatonin differentially affects the functional sensitivity and trafficking of the hMT(1) and hMT(2) melatonin receptors. J Pharmacol Exp Ther 304: 931–939. [DOI] [PubMed] [Google Scholar]
- Gerenu G, Liu K, Chojnacki JE, Saathoff JM, Martinez‐Martin P, Perry G et al (2015). Curcumin/melatonin hybrid 5‐(4‐hydroxy‐phenyl)‐3‐oxo‐pentanoic acid [2‐(5‐methoxy‐1H‐indol‐3‐yl)‐ethyl]‐amide ameliorates AD‐like pathology in the APP/PS1 mouse model. ACS Chem Nerosci 6: 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianesini C, Hiragaki S, Laurent V, Hicks D, Tosini G (2016). Cone viability is affected by disruption of melatonin receptors signaling. Invest Ophthalmol Vis Sci 57: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola‐Lemaitre B, De Bodinat C, Delagrange P, Millan MJ, Munoz C, Mocaer E (2014). Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol 171: 3604–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume JL, Daulat AM, Maurice P, Levoye A, Migaud M, Brydon L et al (2008). The PDZ protein mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J Biol Chem 283: 16762–16771. [DOI] [PubMed] [Google Scholar]
- Guo P, Pi H, Xu S, Zhang L, Li Y, Li M et al (2014). Melatonin improves mitochondrial function by promoting MT1/SIRT1/PGC‐1 alpha‐dependent mitochondrial biogenesis in cadmium‐induced hepatotoxicity in vitro. Toxicol Sci 142: 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz LM, Molzof HE, Abrahamsson KE, Cooper JM, Prosser RA, Gamble KL (2015). GIRK channels mediate the nonphotic effects of exogenous melatonin. J Neurosci 35: 14957–14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Ouyang X, Zhou S, Yin W, Tang C, Laudon M et al (2013). A novel melatonin agonist Neu‐P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer' disease. Horm Behav 64: 1–7. [DOI] [PubMed] [Google Scholar]
- Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L et al (2015). Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer 22: R183–R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SM, Blask DE, Xiang S, Yuan L, Mao L, Dauchy RT et al (2011). Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia 16: 235–245. [DOI] [PubMed] [Google Scholar]
- Hiragaki S, Baba K, Coulson E, Kunst S, Spessert R, Tosini G (2014). Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One 9: e106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta CT, Gazarini ML, Beraldo FH, Varotti FP, Lopes C, Markus RP et al (2000). Calcium‐dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat Cell Biol 2: 466–468. [DOI] [PubMed] [Google Scholar]
- Hunt AE, Alghoul WM, Gillette MU, Dubocovich ML (2001). Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Amer J Physiol Cell Physiol 280: C110–C118. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Arslan AD, Yildiz S, Sharma R, Gavin D, Tun N et al (2009). The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal ‘clock’ gene expression in striatal neurons in vitro. J Pineal Res 46: 87–94. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Uz T, Dzitoyeva S, Giusti P, Manev H (2008). Melatonin signaling in mouse cerebellar granule cells with variable native MT1 and MT2 melatonin receptors. Brain Res 1227: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPHAR/BPS Guide to Pharmacology . [Online] Available at http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39 (accessed 30 March 2017).
- Jin X, Von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM et al (2003). Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol 23: 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G et al (2016). Update on melatonin receptors. IUPHAR review. Br J Pharmacol 173: 2702–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M, Gbahou F, Guillaume JL, Daulat AM, Benleulmi‐Chaachoua A, Luka M et al (2015). Convergence of melatonin and serotonin (5‐HT) signaling at MT2/5‐HT2C receptor heteromers. J Biol Chem 290: 11537–11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalepas PC, Mitchell JW, Gillette MU (2016). Melatonin signal transduction pathways require e‐box‐mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLoS One 11: e0157824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitri A, Jockers R (2014). Exon sequencing of G protein‐coupled receptor genes and perspectives for disease treatment In: Stefens CW. (ed). Methods in Pharmacology and Toxicology Series, G Protein‐coupled Receptor Genetics: Research and Methods in the Post‐genomic Era. Springer Science: New York. [Google Scholar]
- Kim SJ, Kang HS, Lee JH, Park JH, Jung CH, Bae JH et al (2015). Melatonin ameliorates ER stress‐mediated hepatic steatosis through miR‐23a in the liver. Biochem Biophys Res Commun 458: 462–469. [DOI] [PubMed] [Google Scholar]
- Kim YD, Hwang SL, Lee EJ, Kim HM, Chung MJ, Elfadl AK et al (2017). Melatonin ameliorates alcohol‐induced bile acid synthesis by enhancing miR‐497 expression. J Pineal Res 62 10.1111/jpi.12386. [DOI] [PubMed] [Google Scholar]
- Kunst S, Wolloscheck T, Kelleher DK, Wolfrum U, Sargsyan SA, Iuvone PM et al (2015). Pgc‐1alpha and Nr4a1 are target genes of circadian melatonin and dopamine release in murine retina. Invest Ophthalmol Vis Sci 56: 6084–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix D, Ouellette R, Vaillancourt C (2006). Expression of melatoninergic receptors in human placental choriocarcinoma cell lines. Hum Reprod 21: 1981–1989. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jung YH, Oh SY, Yun SP, Han HJ (2014). Melatonin enhances the human mesenchymal stem cells motility via melatonin receptor 2 coupling with Gαq in skin wound healing. J Pineal Res 57: 393–407. [DOI] [PubMed] [Google Scholar]
- Legros C, Brasseur C, Delagrange P, Ducrot P, Nosjean O, Boutin JA (2016). Alternative radioligands for investigating the molecular pharmacology of melatonin receptors. J Pharmacol Exp Ther 356: 681–692. [DOI] [PubMed] [Google Scholar]
- Legros C, Devavry S, Caignard S, Tessier C, Delagrange P, Ouvry C et al (2014). Melatonin MT1 and MT2 receptors display different molecular pharmacologies only in the G‐protein coupled state. Br J Pharmacol 171: 186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros C, Matthey U, Grelak T, Pedragona‐Moreau S, Hassler W, Yous S et al (2013). New radioligands for describing the molecular pharmacology of MT1 and MT2 melatonin receptors. Int J Mol Sci 14: 8948–8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P et al (2006). The orphan GPR50 receptor specifically inhibits MT(1) melatonin receptor function through heterodimerization. EMBO J 25: 3012–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK et al (1997). Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19: 91–102. [DOI] [PubMed] [Google Scholar]
- Liu J, Clough SJ, Hutchinson AJ, Adamah‐Biassi EB, Popovska‐Gorevski M, Dubocovich ML (2016). MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol 56: 361–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Yin D, Chen L, Qu WM, Chen CR, Laudon M et al (2014). Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology (Berl) 231: 3973–3985. [DOI] [PubMed] [Google Scholar]
- Logez C, Berger S, Legros C, Banères JL, Cohen W, Delagrange P et al (2014). Recombinant human melatonin receptor MT1 isolated in mixed detergents shows pharmacology similar to that in mammalian cell membranes. PLoS One 9: e100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti F, Betti M, Canonico B, Arcangeletti M, Ferri P, Galli F et al (2009). ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB‐stressed U937 cells. Free Radic Biol Med 46: 339–351. [DOI] [PubMed] [Google Scholar]
- Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M et al (2010). Melatonin signaling and cell protection function. FASEB J 24: 3603–3624. [DOI] [PubMed] [Google Scholar]
- Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P et al (2009). Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie RS, Melan MA, Passey DK, Witt‐Enderby PA (2002). Dual coupling of MT(1) and MT(2) melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem Pharmacol 63: 587–595. [DOI] [PubMed] [Google Scholar]
- Marcola M, Lopes‐Ramos CM, Pereira EP, Cecon E, Fernandes PA, Tamura EK et al (2016). Light/dark environmental cycle imposes a daily profile in the expression of microRNAs in rat CD133(+) cells. J Cell Physiol 231: 1953–1963. [DOI] [PubMed] [Google Scholar]
- Masana MI, Doolen S, Ersahin C, Al‐Ghoul WM, Duckles SP, Dubocovich ML et al (2002). MT(2) melatonin receptors are present and functional in rat caudal artery. J Pharmacol Exp Ther 302: 1295–1302. [DOI] [PubMed] [Google Scholar]
- Maurice P, Daulat AM, Broussard C, Mozo J, Clary G, Hotellier F et al (2008). A generic approach for the purification of signaling complexes that specifically interact with the carboxy‐terminal domain of G protein‐coupled receptors. Mol Cell Proteomics 7: 1556–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice P, Daulat AM, Turecek R, Ivankova‐Susankova K, Zamponi F, Kamal M et al (2010). Molecular organization and dynamics of the melatonin MT receptor/RGS20/G(i) protein complex reveal asymmetry of receptor dimers for RGS and G(i) coupling. EMBO J 29: 3646–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo JC, Sainz RM, Gonzalez Menendez P, Cepas V, Tan DX, Reiter RJ (2017). Melatonin and sirtuins: a “not‐so unexpected” relationship. J Pineal Res 62. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM (1996). The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res 39: 117–126. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Hunt AE, Gillette MU (1997). Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology 138: 627–634. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Kamal M, Jockers R, Chanrion B, Labasque M et al (2011). The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5‐HT2C receptors. Int J Neuropsychopharmacol 14: 768–783. [DOI] [PubMed] [Google Scholar]
- Mori F, Ferraiuolo M, Santoro R, Sacconi A, Goeman F, Pallocca M et al (2016). Multitargeting activity of miR‐24 inhibits long‐term melatonin anticancer effects. Oncotarget 7: 20532–20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlbauer E, Gross E, Labucay K, Wolgast S, Peschke E (2009). Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol 606: 61–71. [DOI] [PubMed] [Google Scholar]
- Mulder H (2017). Melatonin signalling and type 2 diabetes risk: too little, too much or just right? Diabetologia 60: 826–829. [DOI] [PubMed] [Google Scholar]
- Nagy AD, Iwamoto A, Kawai M, Goda R, Matsuo H, Otsuka T et al (2015). Melatonin adjusts the expression pattern of clock genes in the suprachiasmatic nucleus and induces antidepressant‐like effect in a mouse model of seasonal affective disorder. Chronobiol Int 32: 447–457. [DOI] [PubMed] [Google Scholar]
- Nelson CS, Marino JL, Allen CN (1996). Melatonin receptors activate heteromeric G‐protein coupled Kir3 channels. Neuroreport 7: 717–720. [DOI] [PubMed] [Google Scholar]
- Nelson MR, Wegmann D, Ehm MG, Kessner D, St Jean P, Verzilli C et al (2012). An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science 337: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurim Pharmaceuticals Ltd (2011a). Safety, tolerability, pharmacokinetics and pharmacodynamics of Neu‐P11 in subjects with primary insomnia. In: https://clinicaltrials.gov Bethesda (MD): National Library of Medicine (US). Available at https://clinicaltrials.gov/ct2/show/NCT01114126?term=Neu-P11&rank=2 (accessed 13/3/2017).
- Neurim Pharmaceuticals Ltd (2011b). Sleep laboratory study to investigate the safety and efficacy of Neu‐P11 in primary insomnia patients. In: https://clinicaltrials.gov Bethesda (MD): National Library of Medicine (US). Available at https://clinicaltrials.gov/ct2/show/NCT01489969?term=Neu-P11&rank=3 (accessed 13/3/2017).
- Neurim Pharmaceuticals Ltd (2017). Safety and efficacy of piromelatine in mild Alzheimer's disease patients (ReCOGNITION). In: https://clinicaltrials.gov Bethesda (MD): National Library of Medicine (US). 2017. Available at https://clinicaltrials.gov/ct2/show/NCT02615002?term=Neu-P11&rank=4 (accessed 13/3/2017).
- Ngo T, Kufareva I, Coleman JLJ, Graham RM, Abagyan R, Smith NJ (2016). Identifying ligands at orphan GPCRs: current status using structure‐based approaches. Br J Pharmacol 173: 2934–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles LP, Wang JX, Shen L, Lobb DK, Younglai EV (1999). Melatonin receptor mRNA expression in human granulosa cells. Mol Cell Endocrinol 156: 107–110. [DOI] [PubMed] [Google Scholar]
- Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM (1999). Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt(1)‐antagonists. Brit J Pharmacol 127: 1288–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi A, Jockers R (2016). Melatonin receptor MT1 and MT2 In: Encyclopedia of Signaling Molecules. Ed Springer: New York, pp. 1–6. 10.1007/978-1-4614-6438-9_101751-1. [DOI] [Google Scholar]
- O'Neal‐Moffitt G, Delic V, Bradshaw PC, Olcese J (2015). Prophylactic melatonin significantly reduces Alzheimer's neuropathology and associated cognitive deficits independent of antioxidant pathways in AbetaPP(swe)/PS1 mice. Mol Neurodegener 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz‐Lopez L, Perez‐Beltran C, Ramirez‐Rodriguez G (2016). Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness‐like behavior in adult mice. Neuropharmacology 103: 211–221. [DOI] [PubMed] [Google Scholar]
- Owino S, Contreras‐Alcantara S, Baba K, Tosini G (2016). Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS One 11: e0148214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala D, Lodola A, Bedini A, Spadoni G, Rivara S (2013). Homology models of melatonin receptors: challenges and recent advances. Int J Mol Sci 14: 8093–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada E, Buendia I, Leon R, Negredo P, Romero A, Cuadrado A et al (2014). Neuroprotective effect of melatonin against ischemia is partially mediated by alpha‐7 nicotinic receptor modulation and HO‐1 overexpression. J Pineal Res 56: 204–212. [DOI] [PubMed] [Google Scholar]
- Peschke E, Stumpf I, Bazwinsky I, Litvak L, Dralle H, Muhlbauer E (2007). Melatonin and type 2 diabetes – a possible link? J Pineal Res 42: 350–358. [DOI] [PubMed] [Google Scholar]
- Petit L, Lacroix I, Decoppet P, Strosberg AD, Jockers R (1999). Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′‐5′‐monophosphate pathway. Biochem Pharmacol 58: 633–639. [DOI] [PubMed] [Google Scholar]
- Pfeffer M, Rauch A, Korf HW, Von Gall C (2012). The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light‐induced phase advances by acting upon MT2 receptors. Chronobiol Int 29: 415–429. [DOI] [PubMed] [Google Scholar]
- Pi H, Xu S, Reiter RJ, Guo P, Zhang L, Li Y et al (2015). SIRT3‐SOD2‐mROS‐dependent autophagy in cadmium‐induced hepatotoxicity and salvage by melatonin. Autophagy 11: 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinato L, Da Silveira Cruz‐Machado S, Franco DG, Campos LM, Cecon E, Fernandes PA et al (2015). Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct Funct 220: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovska‐Gorevski M, Dubocovich ML, Rajnarayanan RV (2017). Carbamate insecticides target human melatonin receptors. Chem Res Toxicol 30: 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radogna F, Cristofanon S, Paternoster L, D'alessio M, De Nicola M, Cerella C et al (2008). Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl‐2. J Pineal Res 44: 316–325. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G et al (2009). Melatonin agonist tasimelteon (VEC‐162) for transient insomnia after sleep‐time shift: two randomised controlled multicentre trials. Lancet 373: 482–491. [DOI] [PubMed] [Google Scholar]
- Ramirez‐Rodriguez G, Klempin F, Babu H, Benitez‐King G, Kempermann G (2009). Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 34: 2180–2191. [DOI] [PubMed] [Google Scholar]
- Ramos E, Patino P, Reiter RJ, Gil‐Martin E, Marco‐Contelles J, Parada E et al (2017). Ischemic brain injury: new insights on the protective role of melatonin. Free Radic Biol Med 104: 32–53. [DOI] [PubMed] [Google Scholar]
- Ramracheya RD, Muller DS, Squires PE, Brereton H, Sugden D et al (2008). Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res 44: 273–279. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF (1995a). Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A 92: 8734–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Cassone VM, Godson C, Kolakowski LF (1995b). Melatonin receptors are for the birds: molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron 15: 1003–1015. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T (1994). Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13: 1177–1185. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T, Mahle CD, Kolakowski LF Jr (1996a). Cloning of a melatonin‐related receptor from human pituitary. FEBS Lett 386: 219–224. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Godson C (1996b). Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci 17: 100–102. [DOI] [PubMed] [Google Scholar]
- Rossi SP, Windschuettl S, Matzkin ME, Terradas C, Ponzio R, Puigdomenech E et al (2014). Melatonin in testes of infertile men: evidence for anti‐proliferative and anti‐oxidant effects on local macrophage and mast cell populations. Andrology 2: 436–449. [DOI] [PubMed] [Google Scholar]
- Santoro R, Mori F, Marani M, Grasso G, Cambria MA, Blandino G et al (2013). Blockage of melatonin receptors impairs p53‐mediated prevention of DNA damage accumulation. Carcinogenesis 34: 1051–1061. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F et al (2005). Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease. J Pineal Res 38: 10–16. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Jockers R, Ayoub M, Angeloni D, Fraschini F, Flammer J et al (2007). The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer's disease. Curr Alzheimer Res 4: 47–51. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Olivieri G, Brydon L, Jockers R, Krauchi K, Wirz JA et al (2001). Cerebrovascular melatonin MT1‐receptor alterations in patients with Alzheimer's disease. Neurosci Lett 308: 9–12. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Wirz‐Justice A, Olivieri G, Pache M, Krauchi K, Brydon L et al (2002). Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem 50: 519–526. [DOI] [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H (2002). MT1 melatonin receptor in the human retina: expression and localization. Investigative Ophthalmology And Visual Science 43: 889–897. [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H (2003). All amacrine cells express the MT1 melatonin receptor in human and macaque retina. Exp Eye Res 77: 375–382. [DOI] [PubMed] [Google Scholar]
- Schlabritz‐Loutsevitch N, Hellner N, Middendorf R, Muller D, Olcese J (2003). The human myometrium as a target for melatonin. J Clin Endocrinol Metab 88: 908–913. [DOI] [PubMed] [Google Scholar]
- Segerstolpe A, Palasantza A, Eliasson P, Andersson EM, Andreasson AC, Sun X et al (2016). Single‐cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 24: 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey JT, Puttaramu R, Word RA, Olcese J (2009). Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J Clin Endocrinol Metab 94: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SY, Pang B, Tam CW, Yao KM (2010). Signal transduction of receptor‐mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of Galpha(s) and Galpha(q) proteins. J Pineal Res 49: 301–311. [DOI] [PubMed] [Google Scholar]
- Shu T, Wu T, Pang M, Liu C, Wang X, Wang J et al (2016). Effects and mechanisms of melatonin on neural differentiation of induced pluripotent stem cells. Biochem Biophys Res Commun 474: 566–571. [DOI] [PubMed] [Google Scholar]
- Soares JM Jr, Masana MI, Ersahin C, Dubocovich ML (2003). Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J Pharmacol Exp Ther 306: 694–702. [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Won G, Lee J, Lee S, Kim SH (2015). Upregulation of miRNA3195 and miRNA374b mediates the anti‐angiogenic properties of melatonin in hypoxic PC‐3 prostate cancer cells. J Cancer 6: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni G, Bedini A, Lucarini S, Mari M, Caignard DH, Boutin JA et al (2015). Highly potent and selective MT2 melatonin receptor full agonists from conformational analysis of 1‐benzyl‐2‐acylaminomethyl‐tetrahydroquinolines. J Med Chem 58: 7512–7525. [DOI] [PubMed] [Google Scholar]
- Spuch C, Antequera D, Isabel Fernandez‐Bachiller M, Isabel Rodriguez‐Franco M, Carro E (2010). A new tacrine‐melatonin hybrid reduces amyloid burden and behavioral deficits in a mouse model of Alzheimer's disease. Neurotox Res 17: 421–431. [DOI] [PubMed] [Google Scholar]
- Steffens F, Zhou XB, Sausbier U, Sailer C, Motejlek K, Ruth P et al (2003). Melatonin receptor signaling in pregnant and nonpregnant rat uterine myocytes as probed by large conductance Ca2+‐activated K+ channel activity. Mol Endocrinol 17: 2103–2115. [DOI] [PubMed] [Google Scholar]
- Tajes M, Gutierrez‐Cuesta J, Ortuno‐Sahagun D, Camins A, Pallas M (2009). Anti‐aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res 47: 228–237. [DOI] [PubMed] [Google Scholar]
- Talevi A (2015). Multi‐target pharmacology: possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front Pharmacol 6: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Qin L, Reiter RJ (2016). Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci 17: 2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thireau J, Marteaux J, Delagrange P, Lefoulon F, Dufourny L, Guillaumet G et al (2014). Original design of fluorescent ligands by fusing BODIPY and melatonin neurohormone. ACS Med Chem Lett 5: 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen SK, Ceroni A, Van De Bunt M, Burrows C, Barrett A, Scharfmann R et al (2016). Systematic functional characterization of candidate causal genes for type 2 diabetes risk variants. Diabetes 65: 3805–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian SW, Laudon M, Han L, Gao J, Huang FL, Yang YF et al (2010). Antidepressant‐ and anxiolytic effects of the novel melatonin agonist Neu‐P11 in rodent models. Acta Pharmacol Sin 31: 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocharus C, Puriboriboon Y, Junmanee T, Tocharus J, Ekthuwapranee K, Govitrapong P (2014). Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience 275: 314–321. [DOI] [PubMed] [Google Scholar]
- Tosini G, Owino S, Guillaume JL, Jockers R (2014). Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. Bioessays 36: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi T, Nagorny CL, Singh P, Bennet H, Yu Q, Alenkvist I et al (2016). Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab 23: 1067–1077. [DOI] [PubMed] [Google Scholar]
- Uchikawa O, Fukatsu K, Tokunoh R, Kawada M, Matsumoto K, Imai Y et al (2002). Synthesis of a novel series of tricyclic indan derivatives as melatonin receptor agonists. J Med Chem 45: 4222–4239. [DOI] [PubMed] [Google Scholar]
- Vachharajani NN, Yeleswaram K, Boulton DW (2003). Preclinical pharmacokinetics and metabolism of BMS‐214778, a novel melatonin receptor agonist. J Pharm Sci 92: 760–772. [DOI] [PubMed] [Google Scholar]
- van de Bunt M, Manning Fox JE, Dai X, Barrett A, Grey C, Li L et al (2015). Transcript expression data from human islets links regulatory signals from genome‐wide association studies for type 2 diabetes and glycemic traits to their downstream effectors. PLoS Genet 11: e1005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Buijs RM, Ruijter JM, Delagrange P, Spanswick D, Hermes ML (2001). Melatonin generates an outward potassium current in rat suprachiasmatic nucleus neurones in vitro independent of their circadian rhythm. Neuroscience 107: 99–108. [DOI] [PubMed] [Google Scholar]
- Viault G, Séverine S, Mourvelat S, Lagaraine C, Devavry S, Lefoulon F et al (2016). Design, synthesis and biological evaluation of fluorescent ligands for MT1 and/or MT2 melatonin receptors. RSC Adv 6: 62508–62521. [Google Scholar]
- von Ball C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm‐Draeger PM et al (2002). Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci 5: 234–238. [DOI] [PubMed] [Google Scholar]
- von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW (2005). Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann N Y Acad Sci 1040: 508–511. [DOI] [PubMed] [Google Scholar]
- Vriend J, Reiter RJ (2015). Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res 58: 1–11. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang ZM, Li XM, Li F, Wu JJ, Kong LY et al (2016). Synthesis and evaluation of multi‐target‐directed ligands for the treatment of Alzheimer's disease based on the fusion of donepezil and melatonin. Bioorg Med Chem 24: 4324–4338. [DOI] [PubMed] [Google Scholar]
- Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Sirianni AC, Zhu S et al (2009). Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke 40: 1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sirianni A, Pei Z, Cormier K, Smith K, Jiang J et al (2011). The melatonin MT1 receptor axis modulates mutant Huntingtin‐mediated toxicity. J Neurosci 31: 14496–14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM (1996). The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport 8: 109–112. [DOI] [PubMed] [Google Scholar]
- West A, Dupre SM, Yu L, Paton IR, Miedzinska K, Mcneilly AS et al (2013). Npas4 is activated by melatonin, and drives the clock gene Cry1 in the ovine pars tuberalis. Mol Endocrinol 27: 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Loudon A (2014). Clocks for all seasons: unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. J Endocrinol 222: R39–R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Song C, Zhang J, Zhao J, Fu B, Mao T et al (2017). Melatonin‐mediated upregulation of GLUT1 blocks exit from pluripotency by increasing the uptake of oxidized vitamin C in mouse embryonic stem cells. FASEB J 31: 1731–1743. [DOI] [PubMed] [Google Scholar]
- Wu PW, Cheng YM, Hsieh WT, Wang YH, Wei CY, Chou PT (2007). 7‐Azamelatonin: efficient synthetic routes, excited‐state double proton transfer properties and biomedical implications. ChemMedChem 2: 1071–1075. [DOI] [PubMed] [Google Scholar]
- Wu YH, Ursinus J, Zhou JN, Scheer FA, Ai‐Min B, Jockers R et al (2013). Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord 148: 357–367. [DOI] [PubMed] [Google Scholar]
- Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S et al (2013). JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res 55: 275–286. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Currie KP (2013). Regulation of Ca(V)2 calcium channels by G protein coupled receptors. Biochim Biophys Acta 1828: 1629–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzka A, Lozinska I, Moleda Z, Panasiewicz M, Czarnocki Z (2013). Highly selective inhibition of butyrylcholinesterase by a novel melatonin‐tacrine heterodimers. J Pineal Res 54: 435–441. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cook A, Kim J, Baranov SV, Jiang J, Smith K et al (2013). Melatonin inhibits the caspase‐1/cytochrome c/caspase‐3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 55: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li H, Pu Y, Gong S, Liu C, Jiang X et al (2015). Melatonin‐mediated inhibition of Purkinje neuron P‐type Ca(2)(+) channels in vitro induces neuronal hyperexcitability through the phosphatidylinositol 3‐kinase‐dependent protein kinase C delta pathway. J Pineal Res 58: 321–334. [DOI] [PubMed] [Google Scholar]
- Zhao L, Liu H, Yue L, Zhang J, Li X, Wang B et al (2017). Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF‐κB signaling pathway following subarachnoid hemorrhage in mice. Mol Neurobiol 54: 1612–1621. [DOI] [PubMed] [Google Scholar]
- Zhu HQ, Li Q, Dong LY, Zhou Q, Wang H, Wang Y (2014). MicroRNA‐29b promotes high‐fat diet‐stimulated endothelial permeability and apoptosis in apoE knock‐out mice by down‐regulating MT1 expression. Int J Cardiol 176: 764–770. [DOI] [PubMed] [Google Scholar]
- Zisapel N, Laudon M (1982). Dopamine release induced by electrical field stimulation of rat hypothalamus in vitro: inhibition by melatonin. Biochem Biophys Res Commun 104: 1610–1616. [DOI] [PubMed] [Google Scholar]
- Zlotos DP, Jockers R, Cecon E, Rivara S, Witt‐Enderby PA (2014). MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J Med Chem 57: 3161–3185. [DOI] [PubMed] [Google Scholar]


