Abstract
Melatonin is well known for its circadian production by the pineal gland, and there is a growing body of data showing that it is also produced by many other cells and organs, including immune cells. The chronobiotic role of pineal melatonin, as well as its protective effects in vitro and in vivo, have been extensively explored. However, the interaction between the chronobiotic and defence functions of endogenous melatonin has been little investigated. This review details the current knowledge regarding the coordinated shift in melatonin synthesis from the pineal gland (circadian and monitoring roles) to the regulation of acute immune responses via immune cell production and autocrine effects, producing systemic interactions termed the immune‐pineal axis. An acute inflammatory response drives the transcription factor, NFκB, to switch melatonin synthesis from pinealocytes to macrophages/microglia and, upon acute inflammatory resolution, back to pinealocytes. The potential pathophysiological relevance of immune‐pineal axis dysregulation is highlighted, with both research and clinical implications, across several medical conditions, including host/parasite interaction, neurodegenerative diseases and cancer.
Linked Articles
This article is part of a themed section on Recent Developments in Research of Melatonin and its Potential Therapeutic Applications. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.16/issuetoc
Abbreviations
- AANAT
aralkylamine N‐acetyltransferase
- ASMT
acetylserotonin O‐methyltransferase
- BCG
Bacillus of Calmette‐Guérin
- CYP1B1
cytochrome P4501B1
- GR
glucocorticoid receptor
- PAMPs/DAMPs
pathogen/danger‐associated molecular patterns
- miRNA
microRNAs
- NAS
N‐acetylserotonin (N‐acetyl‐5‐HT)
- TAD
transactivation domain
- TLR
toll‐like receptor
Initial consideration
Mammalian defence mechanisms involve continuous surveillance during homeostatic states and the mounting of stereotypical responses to pathogen/danger‐associated molecular patterns (PAMPs, DAMPs), when appropriate. Surveillance requires monitoring and the detection of danger, to optimize danger/pathogen and cell debris removal. Microbes also induce acquired immune responses, which involve sequential defences. Each of these processes is tightly regulated, as a defect or exacerbation of any aspect of them may result in chronic diseases. Indeed, uncontrolled immune responses underlie the biological basis of a wide array of distinct medical conditions, including cancers, as well as neurodegenerative, chronic and autoimmune disorders.
A growing body of data implicates http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224 in defence responses, including (i) the impact of immune responses on the daily rhythm of melatonin synthesis by the pineal gland and (ii) the roles of endogenous melatonin produced by the pineal gland or by activated immune‐competent cells. This article highlights the biological relevance of endogenous melatonin and the importance of its cellular site of production. The effects of exogenous melatonin and its analogues on inflammatory‐based pathologies have been extensively reviewed elsewhere (Anderson and Maes, 2014; Esteban‐Zubero et al., 2017; Shukla et al., 2017; Zhelev et al., 2017).
In the 1990s, when the anti‐inflammatory effects of melatonin were initially described (Hardeland and Rodriguez, 1995), we showed that the size of the inflammatory lesion in the paw of mice chronically infected with the tuberculosis bacillus (Bacillus of Calmette‐Guérin, BCG) presents a daily rhythm, the nadir being at night. Pinealectomy and adrenalectomy abolished this daily variation, whilst replenishing melatonin in the drinking water restored the rhythm (Lopes et al., 2001). In addition, BCG induced higher neutrophil chemotaxis in mice at the end of the light phase than during the dark phase (Bureau et al., 1986), reinforcing the idea that the nocturnal rise of melatonin exerts a negative control on leukocyte migration. Accordingly, melatonin was later shown to reduce the expression of adhesion molecules in cultured primary endothelial cells (Lotufo et al., 2006), as well as reducing the rolling and adhesion of neutrophils at the endothelial layer of post‐capillary veins (Lotufo et al., 2001). The suppressive effects of melatonin on neutrophil transmigration led to the posing of the question as to whether innate immune responses would be impaired during the night.
The working hypothesis proposed 10 years ago integrates the immunological roles of pineal and extra‐pineal melatonin, which can be coordinated by signalling pathways triggered by PAMPs, DAMPs and cytokines. This bidirectional communication between the pineal gland and the immune system, termed the immune‐pineal axis, provides a framework for understanding the role of melatonin during surveillance, as well as in the mounting and resolution of inflammatory responses (Figure 1) (Markus et al., 2007), and in inflammatory‐related diseases (Antonioli et al., 2012; Levandovski et al., 2013; Papaioannou et al., 2014). The switching of melatonin production from the pineal gland to immune‐competent cells located in damaged tissues and back to the pineal gland is orchestrated by the different effects of the transcription factor nuclear factor κB (NFκB), a classical regulator of inflammatory responses. Understanding the mechanisms of the immune‐pineal axis provides a pharmacological target for the pathological deviations that are evident in a host of medical conditions.
Figure 1.
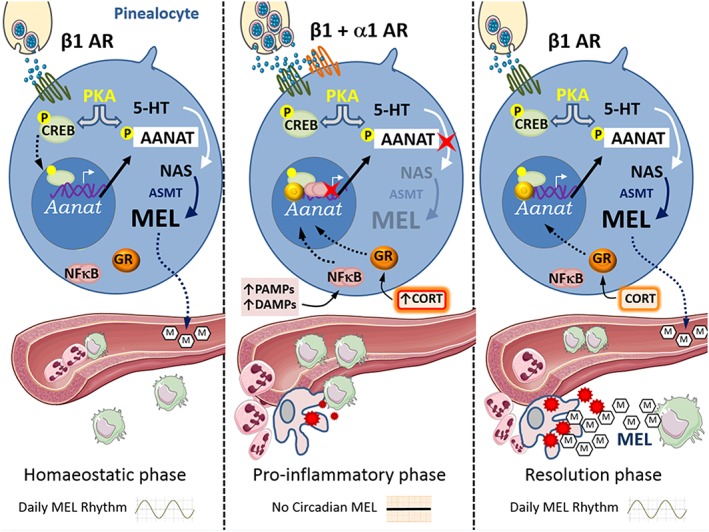
The immune‐pineal axis ‐ backward and forward switch of melatonin synthesis from the pinealocytes to macrophages. Left panel: Homoeostatic condition ‐ the sympathetic output to the pineal gland is activated at night. β1‐adrenoceptors‐mediated PKA activation promotes the phosphorylation of CREB. PCREB migrates to the nucleus and induces the transcription of Aanat. PKA also phosphorylates AANAT, protecting it from ubiquitination and proteasomal degradation. PAANAT converts 5HT to NAS, the direct precursor of melatonin. The activation of melatonin membrane receptors in endothelial cells impairs the expression of adhesion molecules, avoiding spurious migration of leukocytes. Central panel: Pro‐inflammatory phase ‐ resident monocytes detect microbes or lesioned tissue and releases cytokines. In pinealocytes, PAMPs and DAMPs interact with membrane receptors and induce the activation of NFκB. p50/p50 NFκB dimers translocate to the nucleus and bind to the Aanat promoter, reducing its transcriptional activity. In the presence of a high sympathetic output, when both α1‐ and β1‐adrenoceptors are activated, adrenal cortex hormone (corticosteroid/cortisol) blocks pineal melatonin synthesis. The impairment of melatonin production at night allows neutrophils migration thorough the endothelial layer. Activated neutrophils release pro‐inflammatory cytokines and cytotoxic mediators recruiting monocytes that differentiate in macrophages and initiate tissue clearing. Right panel: Resolution phase ‐ macrophages‐synthesized melatonin acts in an autocrine/paracrine manner inducing the expression of membrane molecules, such as dectin‐1, that potentiate phagocytosis. This synthesis of melatonin is mediated by the NFκB dimer cRel/RelA, which promotes the transcription of Aanat. In the pineal gland, as the sympathetic tonus is reduced, GR activation favours Aanat transcription and allows the recovery of the nocturnal melatonin output.
Surveillance
Circulating melatonin
In healthy conditions, leukocytes circulate without transposing the endothelial barrier, whereas innate immune responses trigger a fast migration of leukocytes to the lesion site. Interestingly, the adhesiveness of neutrophils to rat cultured endothelial cells inversely correlates with the blood concentration of melatonin at the hour of harvesting, suggesting a long‐lasting priming of endothelial cells by endogenous melatonin (Tamura et al., 2010; Marçola et al., 2013). Accordingly, endothelial cells harvested at night have a decreased expression of adhesion molecules, such as PECAM‐1 and ICAM‐1, and lower nuclear levels of NFκB versus those harvested during the day (Marçola et al., 2013). Moreover, the expression of 19 genes that code pro‐inflammatory proteins is down‐regulated in endothelial cells at night, whereas the expression of CD180, which inhibits toll‐like receptor 4 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1754), is up‐regulated (Divanovic et al., 2005; Karper et al., 2013; Marçola et al., 2013).
Over the past decade, the epigenetic, non‐coding RNAs, microRNAs (miRNAs), have emerged as important regulators of coordinated gene patterning responses, including those of the immune response (Baltimore et al., 2008; Iliopoulos et al., 2009). Circadian variation of miRNA expression has reciprocal interactions with wider daily rhythm machinery (Shende et al., 2014). Interestingly, the effects of melatonin on memory and neural loss (Wang et al., 2013) and on breast tumour response (Lee et al., 2013) are mediated by the differential expression of miRNAs. In physiological conditions, next‐generation deep sequencing has indicated two distinct miRNA expression profiles in day‐ and night‐time endothelial cells (Marçola et al., 2016). Night‐time cells show a more complex biological network that is strongly controlled by five miRNAs, whilst only two miRNAs are overexpressed in daytime cells. The miRNA profile of night‐time cells negatively regulates the inflammatory response and cell maturation, whilst in daytime cells, a pluripotent phenotype is favoured. The miR‐146a is highly expressed at night (Marçola et al., 2016) and reduces the expression of Traf6 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2042 genes, which decrease the expression of adhesion molecules and leukocyte migration (Taganov et al., 2006; Cheng et al., 2013). As such, some of the night‐time immune regulatory effects of melatonin may be mediated, in part, via its impact on the patterning of miRNA expression.
The daily mobilization and proliferation of bone marrow stem and progenitor cells, and therefore immunocompetent cell generation, also highlights the circadian impact on the immune system. Circulating bloodstream haematopoietic stem cells have a morning peak, driven by sympathetic nervous system‐mediated stromal cell activation (Schildger et al., 1991; Kollet et al., 2012). Rhythmic proliferation of colony forming units for granulocytes and macrophages is disrupted in pinealectomized mice and re‐established by treatment with melatonin (Haldar et al., 1992). In addition, bone marrow cells can synthesize melatonin, which may act locally in an autocrine/paracrine manner (Conti et al., 2000). Taken together, these data indicate that bone marrow is another component of the immune‐pineal axis, where pineal melatonin may have a role in surveillance. However, further investigation is required as to melatonin's role in haematopoiesis. Indeed, unlike the blood–endothelial barrier, data are lacking as to the effects of melatonin and circadian rhythm control on bone marrow endothelial cells, which also play an important role in stem cell migration.
Thus, the daily melatonin rhythm contributes to maintaining endothelial cells in a non‐reactive phenotype at night by regulating central mechanisms involved in the transcription of genes related to cell migration and acute inflammatory responses. This, therefore, suggests that when the mounting of an inflammatory response is required, the pineal gland, directly or indirectly, should be able to rapidly detect PAMPs and DAMPs and stop melatonin synthesis.
Pineal gland
Classically, the synthesis of pineal melatonin involves the conversion of 5‐HT (serotonin) to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5451) by the phosphorylated form of aralkylamine N‐acetyltransferase (P‐AANAT) (AANAT, EC 2.3.1.87), and the methylation of NAS to melatonin (N‐acetyl‐5‐methoxy tryptamine) by the enzyme acetylserotonin O‐methyltransferase (ASMT, EC.2.1.1.4) (reviewed by Simonneaux and Ribelayga, 2003). The transcription of Aanat and phosphorylation of AANAT are regulated on a daily basis, whilst the activity of ASMT is regulated in a seasonal manner (Ribelayga et al., 2000; Pawlak et al., 2009). Phosphorylation of AANAT by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=284 is mediated by sympathetic stimulation of pinealocytes http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=28. In vitro studies showed that activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=4 potentiates the effects of β1‐adrenoceptor stimulation; however, inhibition of β1‐adrenoceptors completely abolishes the pineal melatonin synthesis induced by sympathetic nerve terminal stimulation (Mortani Barbosa et al., 2000).
In nocturnal animals, the transcription of the Aanat gene is also regulated on a daily basis by PKA phosphorylation of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2734#OtherNames, which binds to response elements localized in the Aanat promoter and first intron (Simonneaux and Ribelayga, 2003). Sympathetic input to the pineal gland is driven by the suprachiasmatic nuclei, which are daily synchronized to the environmental light/dark cycle via the retinohypothalamic tract. Sympathetic activation of β1‐adrenoceptors also activates regulator G‐protein signalling 2 (Matsuo et al., 2013) inducing the down regulation of these receptors, which is minimal at late night and restored at the beginning of the dark phase (Pangerl et al., 1990). This mechanism helps to assure that daytime melatonin is independent of circulating catecholamines.
Melatonin is simultaneously delivered to blood and cerebrospinal fluid (Skinner and Malpaux, 1999), with its circadian profile being identical in both substances (Legros et al., 2014; Leston et al., 2015), thereby conveying simultaneous timing information to both the brain and body. The pineal gland is formed of pinealocytes (90%), astrocytes and microglia (Møller and Baeres, 2002). Astrocytes are localized around the pineal stalk encircling pinealocytes, nerve fibres and blood vessels (Møller and Baeres, 2002; Carvalho‐Sousa et al., 2011), and microglia around the vascular tree (Kaur et al., 1997; Jiang‐Shieh et al., 2005). Cytokines released by microglia modulate pinealocytes melatonin synthesis via NFκB activation (Figure 2) (Fernandes et al., 2006; Carvalho‐Sousa et al., 2011; da Silveira Cruz‐Machado et al., 2012). This is the mechanism responsible for the transient suppression of melatonin synthesis by PAMPs and DAMPs. PAMPs and DAMPs suppress the nocturnal melatonin surge by a direct action on pinealocytes or by activating microglia. The NFκB pathway also has a role in regulating the daily melatonin rhythm (Cecon et al., 2010). Under healthy conditions, the nuclear NFκB dimer, p50/p50, increases continuously from the beginning to the end of the light phase, decreasing sharply after lights‐off (Cecon et al., 2010). The saw‐tooth profile is not changed by blocking α1‐adrenoceptors but is inhibited by maintaining the animals under constant light. A high nuclear content of NFκB, both in cultured pineal glands or in vivo, blocks noradrenaline‐induced Aanat transcription (Ferreira et al., 2005; Cecon et al., 2010). The same saw‐tooth profile is observed for genes codifying http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=316 (Tlr1, Tlr2, Tlr3, Tlr4, Tlr6 and Tlr7), http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=301 (Il1r1, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1708 and Tnfrsf1a), adaptors and effector proteins from the NFκB and MAPK families (da Silveira Cruz‐Machado et al., 2017). Conversely, the circadian variation of the high affinity IgE receptor is mediated by noradrenergic input (Ganguly et al., 2007).
Figure 2.
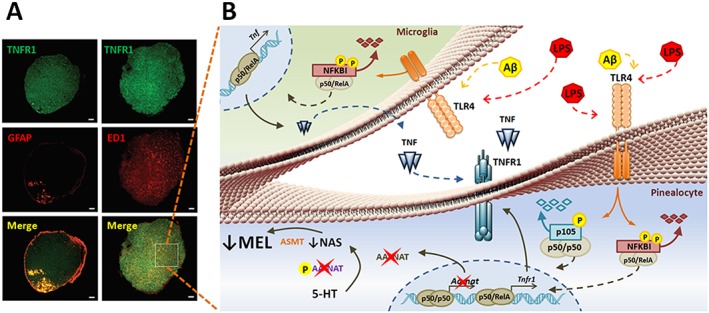
Microglia–pinealocyte interaction in the pineal gland. Left panel: Representative images showing the expression of TNFR1 (green) in rat pineal glands. Astrocytes (GFAP), microglia (ED1) and pinealocytes (non‐labelled cells) express TNFR1. Astrocytes are localized in the stalk, whilst microglia are dispersed along all the gland (Carvalho‐Sousa et al., 2011). Right panel: Microglia–pinealocyte interaction – besides TNFR1, pinealocytes and astrocytes, and microglia also express TLRs (da Silveira Cruz‐Machado et al., 2012). In pinealocytes, activation of TLR4 by a PAMP, as LPS, or a DAMP, as Aβ (amyloid β peptide), inhibits the synthesis of melatonin and induces the synthesis of TNFR1. In microglia, TLR4 leads to the synthesis of TNF, which acting on TNFR1 in the pinealocytes reinforces the synthesis of melatonin. These multiple effects are mediated by specific NFκB dimers, p50/p50 blocks, whereas p50/RelA promotes gene transcription. Figures adapted from Frontiers in Endocrinoly (A) and Plos One (B).
Innate‐immune response
The model of an immune‐pineal axis extended the idea of a bi‐directional communication between chronobiotic and immune function (Skwarlo‐Sonta et al., 2003). The immune‐pineal axis proposes that switching the melatonin source from the pineal gland to immune‐competent cells orchestrates the timing of leukocyte migration and adjusts the monocyte phenotype for each phase of the inflammatory response. The pivotal mechanism that regulates this switch is the NFκB pathway (Markus et al., 2007, 2013). The NFκB family is composed of two proteins (p50 and p52) without, and three proteins (RelA, RelB and cRel) with, a transactivation domain (TAD) (Lawrence and Fong, 2010; Zhang et al., 2017). Dimers containing one TAD subunit induce gene transcription. NFκB dimers are sequestered in the cytoplasm and, upon stimulation, translocate to the nucleus and bind to κB responsive elements. Depending on the subunits forming the NFκB dimers, the same extracellular signal can turn on or turn off melatonin synthesis by interacting with κB elements present in the Aanat promoter and first intron (Markus et al., 2007; Muxel et al., 2012, 2016).
The pineal gland is a circumventricular organ irrigated by a large web of fenestrated vascular ramifications (Duvernoy and Risold, 2007; Matsushima and Reiter, 1975). This localization exposes pinealocytes and associated glial cells to circulating PAMPs, DAMPs and cytokines. Indeed, LPS inhibits melatonin synthesis in rats (Tamura et al., 2010), hamsters (Laranjeira‐Silva et al., 2015) and chicken (Piesiewicz et al., 2012), X‐irradiation in rats (Barfuss et al., 1969), IL‐1β in ewes (Herman et al., 2017) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074 in humans (Pontes et al., 2006).
TLR4, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1870 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1734 trigger the nuclear translocation of p50/RelA NFκB dimers, promoting the transcription of TNF, IL‐1β, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5060 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 in microglia (Tsai et al., 2001a,b; da Silveira Cruz‐Machado et al., 2012). In pinealocytes, NFκB activation exerts a dual effect. The dimer p50/RelA induces the synthesis of TNFR1, whilst p50/p50 blocks the noradrenaline‐induced transcription of Aanat. Therefore, the microglia‐pinealocyte network guarantees a two‐step inhibition in Aanat expression, as the pinealocyte NFκB pathways is initially triggered by TLR4 and then by TNFR1 activation following TNF release from the microglia (Fernandes et al., 2006; da Silveira Cruz‐Machado et al., 2010) (Figure 2). Accordingly, a negative correlation exists between TNF levels and nocturnal melatonin levels, which has been observed in humans after elected surgeries (Pontes et al., 2007; Tatsch‐Dias et al., 2013), as well as in non‐infectious mastitis (Pontes et al., 2006).
Another important component in regulating the transient inhibition of melatonin synthesis by the pineal gland is the release of glucocorticoids from the adrenal cortex, which occurs in association with innate immune and stress responses (Ferreira et al., 2005; Fernandes et al., 2006, 2017; Couto‐Moraes et al., 2009). Simultaneous activation of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=625 (GRs) and both adrenoceptor subtypes (α1 and β1) reduces the synthesis of melatonin, in contrast, following stimulation of β1‐adrenoceptors and GRs melatonin synthesis is induced (Yuwiler and Wetterberg, 1989; Zhao and Touitou, 1993; Couto‐Moraes et al., 2009; Fernandes et al., 2017). Therefore, a high stress response would reduce melatonin synthesis, whilst a lower level of stress would result in its potentiation. In line with this hypothesis, a peritoneal injection of saline promotes a decrease in melatonin synthesis that is blocked by adrenalectomy (Troiani et al., 1988). In contrast, transpineal infusion of corticosterone (Fernandes et al., 2009), increased levels of corticosterone following mild stress (Couto‐Moraes et al., 2009) and chronic inflammation (Lopes et al., 2001) increase nocturnal melatonin output. In addition, the corticosterone peak signals the rest/activity transition at darkness onset, thereby blocking the gene transcriptions that impair the β1‐adrenoceptor‐mediated induction of Aanat, consequently increasing melatonin output (da Silveira Cruz‐Machado et al., 2017). The restoration of circulating melatonin observed in systemic (Tamura et al., 2010) and non‐infectious inflammatory processes (Pontes et al., 2007; Tatsch‐Dias et al., 2013) is associated with a reduction in leukocyte adhesion to the endothelial layer. In summary, glucocorticoids have dual effects on pineal melatonin synthesis, highlighting an important interaction between the hypothalamus‐pituitary axis (HPA) and the immune‐pineal axis. During the mounting of an inflammatory response, HPA axis activation contributes to blocking melatonin synthesis, whilst in the recovery phase, or in chronic inflammatory processes, HPA axis activation can contribute to the restoration of pineal melatonin synthesis and normal circadian function.
The immune‐pineal axis is also implicated in the local synthesis of melatonin at the lesion site. The regulation of leukocyte migration by melatonin relies on melatonin inhibition of the expression of adhesion molecules in the endothelial layer (Tamura et al., 2010). The suppression of nocturnal melatonin synthesis by the pineal gland makes the endothelial cells more reactive to PAMPs and DAMPs, including ATP, from inflammatory sites (Cardoso et al., 2017). Activated macrophages/microglia synthesize melatonin in an NFκB‐dependent manner at the site of the inflammatory response. We have shown that increased AANAT activity is a key event for the synthesis of melatonin by mononuclear and polymorphonuclear phagocytes from the human colostrum and RAW 264.7 macrophages stimulated by TNF, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5019 or zymosan (Pontes et al., 2006; Muxel et al., 2012; Pires‐Lapa et al., 2013). This opposite effect of NFκB on Aanat transcription in pinealocytes and macrophages depends on the type of NFκB dimers activated. In pinealocytes, the homodimer p50/p50, which lacks TAD, is translocated by LPS (da Silveira Cruz‐Machado et al., 2010), whilst in activated macrophages c‐Rel/RelA NFκB dimers bind to κB elements in the Aanat promoter (Carvalho‐Sousa et al., 2011; Muxel et al., 2012; Pires‐Lapa et al., 2013).
In 1988, it was observed that melatonin synthesized by the activation of peripheral blood monocytes was dependent on the synthesis of the enzymes AANAT and ASMT, leading to cell deactivation (Finocchiaro et al., 1988). This was an early indicator of the importance of melatonin synthesis in immunoregulation. Indeed, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4968 induces the synthesis of melatonin by lymphocytes, and melatonin blocks the expression of INFγ (Finocchiaro et al., 1988), whilst inducing the expression of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4985 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=305 (Carrillo‐Vico et al., 2005). This effect is mediated by the high affinity membrane melatonin http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=287 (Jimenez‐Jorge et al., 2007), which sensitizes the synthesis of IL‐2 to the circadian melatonin rhythm (Pontes et al., 2007). In the context of the immune‐pineal axis, melatonin synthesized by macrophages and microglia reduces their reactivity, whilst increases in their phagocytic capacity are classically associated with an M2‐like phenotype. Melatonin synthesized by zymosan‐stimulated human colostrum monocytes induces the expression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2927, increasing their phagocytosis of the fungi particle (Pires‐Lapa et al., 2013). Interestingly, intra‐cerebral injections of LPS in rats increases the synthesis of melatonin in the cerebellum but not in the cortex and hippocampus (Pinato et al., 2015). Reinforcing the idea that the synthesis of melatonin by extra‐pineal tissue is specific, pinealectomy reduces the level of melatonin in the hippocampus and cortex of rats injected with LPS, whilst the concentration in the cerebellum is as high as in sham‐operated or control animals. Furthermore, the percentage of cell death in cerebellum, which was significantly lower than that of the cortex and hippocampus, increased significantly after blockade of MT receptors with luzindole. Therefore, melatonin synthesized in other areas of the brain is able to protect local cells.
Macrophages and microglia are cells able to change their phenotype to fulfil a variety of roles, including participating in host defence, wound healing and immune regulation (Mosser and Edwards, 2008). Melatonin inhibits the expression of cytokines and adhesion molecules, as well as reactive oxygen and nitrogen species (Simonneaux and Ribelayga, 2003). In the context of an immune‐pineal axis, it is important to note that melatonin synthesized following PAMPs/DAMPs stimulation changes the macrophage/microglia phenotype from host defence to wound healing (Yi and Kim, 2017). Therefore, autocrine melatonin is an important aspect of the progression and resolution of the innate immune response. In line with this, melatonin blocks its own synthesis in these cells by inhibiting the NFκB pathway (Gilad et al., 1998), thereby temporally limiting its influence on local inflammatory responses.
The goal of this review is to present current knowledge on the role and the mechanisms involved in the switch of different sources of melatonin in healthy and acute defence responses. We did not intend to extensively cover the mechanisms of action of melatonin as an anti‐inflammatory drug, but shortly, it should be considered that this pleiotropic molecule acts in a wide range of doses (pM to mM) through high affinity GPCRs, binding to key molecules such as calmodulin and also scavenging free radicals (Jockers et al., 2016; Reiter et al., 2017). MT receptor subtypes, MT1 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=288, can be arranged as homo or heterodimers, formed by MT1 plus MT2 receptors, or MT1 receptors plus the orphan GPCR http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=107, the mammal homologue of the mel1c receptors found in the amphibian. Interestingly, the dimerization of the MT1 receptor with GPR50 reduces the affinity of the receptor to melatonin.
Another important consideration is the localization of high‐affinity receptors, which are found not only in the plasma membrane but also in the mitochondrial membrane (Gbahou et al., 2017). The scavenger effect relies on the electron donor property not only of melatonin but also of the metabolites N1‐acetyl‐N2‐formyl‐5‐methoxykynuramine and N1‐acetyl‐5‐methoxykynuramine, which ensures that the anti‐inflammatory function can surmount the ceiling of bimolecular interactions (Reiter et al., 2017). The direct interaction of melatonin with calmodulin provides a mechanism for controlling actin polymerization and, therefore, cell motility, a key property for the effect of defence cells (Benítez‐King, 2006). Finally, receptor‐mediated effects involve the transcription of genes and expression of proteins related to PAMP and DAMP receptors, cell adhesion, inducible enzymes, such as inducible NOS (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376, antioxidant enzymes such as SOD, catalase and glutathione peroxidase (Tamura et al., 2010; Jockers et al., 2016). Thus, melatonin, a highly potent agent against oxidative damage generated by mitochondrial activity (Reiter et al., 2017) and synthesized by immune‐competent cells challenged by bacteria, virus and parasites (Markus et al., 2013), will also act directly on mitochondrial membrane MT receptors (Gbahou et al., 2017) reducing oxidative stress and contributing to changing the pro‐inflammatory response to a regulatory profile.
Uncontrolled immune reactions are evident in a diverse array of medical conditions, including parasitosis, sepsis, neurodegenerative disorders, autoimmune diseases and cancer. Therefore, the fine‐tuned temporal regulation of immune responses is as important as the appropriate mounting of acute reactions. Consequently, mechanisms that allow the restoration of pineal melatonin production are integral components of the function of the immune‐pineal axis under physiological conditions.
Evasion of the immune‐pineal axis
Parasitic diseases, such as malaria and cutaneous leishmaniasis, do not interfere with the daily rhythm of melatonin synthesis and neither activates the cellular arm of the immune‐pineal axis. Leishmania is a parasite that lives inside macrophages and requires an increase in arginine uptake for the production of the polyamines that are essential to parasite survival (de Menezes et al., 2016). The activation of MT1/MT2 receptors inhibits the transcription of the cationic amino acid transporter‐2B gene (Cat2B), reducing the uptake of arginine (Laranjeira‐Silva et al., 2015). Accordingly, blocking MT receptors with luzindole at night increases the infectivity of Leishmania amazonensis, whereas exogenous melatonin reduces infectivity, independently of the hour of the day. Therefore, melatonin emerges as a potential adjuvant treatment for leishmaniasis and could be combined with a pentavalent antimonial to generate a multi‐target, low‐toxicity and low‐cost therapeutic approach.
Plasmodium chabaudi and Plasmodium falciparum merozoites, in contrast, show a daily rhythm of red blood cell infection, which involves the simultaneous appearance of billions of parasites in the blood stream. Interestingly, the development of P. chabaudi and P. falciparum is synchronized by host‐produced melatonin, which may reflect an evolutionary strategy to evade the host immune defence. When animals are either pinealectomized or injected with the antagonist of MT receptors luzindole, the synchronization of the parasitic life cycle is disrupted and parasitaemia is reduced (Hotta et al., 2000). Thus, suppressing melatonin synthesis at night and blocking MT receptors could be an effective strategy for managing malaria.
Lack of immune‐pineal axis recovery
Neuroinflammation
The identification of common molecular mechanisms underlying different neuroinflammatory diseases can generate insights into their pathogenesis and provide the basis for developing novel therapeutic strategies. The sustained activation of the immune‐pineal axis by unresolved inflammatory processes is evident in many medical conditions, including Alzheimer's disease, Autism Spectrum Disorders, Fragile X Syndrome and Parkinson's disease (Skene and Swaab, 2003; Noseda et al., 2014; Mack et al., 2016). In Alzheimer disease (Zhou et al., 2003), Autism and Fragile X Syndrome (Tordjman et al., 2013) pineal function is impaired prior to the detection of the clinical symptoms, suggesting a prognostic role for nocturnal melatonin levels. Additionally, exogenous melatonin has been shown to improve some of the symptoms of these diseases, including preventing chronodisruption and promoting neuroprotection (Mack et al., 2016; Noseda et al., 2014; Tordjman et al., 2013.
Alzheimer's disease, Autism and Fragile X Syndrome are associated with a dysfunction in the processing of amyloid precursor protein (APP; Sokol et al., 2011). In Alzheimer's disease, the increase in cerebrospinal β‐amyloid (Aβ) correlates with Aβ images observed at preclinical stages of the disease (Zhao et al., 2017), and we have recently shown that Aβ interacts with TLRs in rat pineal gland, triggering the synthesis of cytokines and reducing the expression of Aanat and the synthesis of melatonin via activation of the NFκB pathway (Cecon et al., 2015). This opens up the possibility that Aβ interferes in the melatonergic profile in asymptomatic phases of the disease. In addition, Aβ blocks the functional response of MT1 and MT2 receptors but not β1‐adrenoceptors present in endothelial cells, as measured by the phosphorylation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514. Reliable methods of detecting and following the progress of Alzheimer's disease are an important area for preventing disease progression. In addition, the increase in TNF and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2868, considered biomarkers of disease progression, may explain why the melatonin rhythm is not restored (Lehallier et al., 2015). At the present stage, the concept of the immune‐pineal axis could provide a new mechanistic approach for understanding the progression of Alzheimer's disease during the non‐symptomatic phase.
Cancer
Individuals such as night shift workers, who are frequently exposed to light at night and experience biological timing disturbance, have a significantly increased risk of developing breast (Davis et al., 2001; Schernhammer et al., 2001), colon (Schernhammer et al., 2003), prostate (Kubo et al., 2006; Conlon et al., 2007) and endometrial (Viswanathan et al., 2007) cancers. Indeed, pinealectomy and constant light exposure can stimulate tumourigenesis and the growth of breast and hepatocellular carcinomas in rodent models (Tamarkin et al., 1981; Blask et al., 1999, 2003; van den Heiligenberg et al., 1999; Anisimov et al., 2004). Moreover, studies demonstrate that the perfusion of human breast cancer xenografts with melatonin‐rich blood, collected from premenopausal women during the night, inhibits tumour cell proliferation (Blask et al., 2005). The exposure of volunteers to light at night completely abolished the oncostatic effects of blood perfusion, which could be restored by adding physiological concentrations of melatonin to the blood. Melatonin has thus emerged as a direct link between chronodisruption and cancer promotion (Blask, 2009).
It is now well established that inflammatory responses also play a critical role in tumour development and progression (Coussens and Werb, 2002; Grivennikov et al., 2010). Many cancers arise from sites of infection and chronic inflammation (Karin et al., 2006), with the tumour micro‐environment being orchestrated by innate immune cells that cooperate with tumour growth, invasion and migration (Whiteside, 2008). The transcription factor, NFκB, has emerged as a pivotal molecular link between inflammation and cancer (Karin, 2009). Tumour cells produce cytokines and chemokines that attract leukocytes and participate in the feed‐forward loop of NFκB activation (Karin, 2009; Oeckinghaus and Ghosh, 2009). NFκB‐related cytokines, such as IL‐6 and TNF, are present in the tumour micro‐environment and can reach significant levels in the blood of cancer patients (Galizia et al., 2002; Bachelot et al., 2003; Michalaki et al., 2004; Tas et al., 2005; Dalaveris et al., 2009). The IKK–NFκB system can thus have many roles in cancer, including: (i) functioning in malignant cells to promote uncontrolled cell cycle progression and resistance to apoptosis; (ii) modulating innate immune cells, by activating the production of growth factors and cytokines that stimulate tumour growth and angiogenesis; and (iii) acting in the pineal gland to suppress the nocturnal production of melatonin. Indeed, compared to healthy controls, cancer patients present decreased levels of circulating melatonin (Tamarkin et al., 1982; Bartsch et al., 1997; Mazzoccoli et al., 2005; Schernhammer and Hankinson, 2005; 2009), suggesting that the activation of the immune‐pineal axis, along with chronodisruption, participates in the complex association between the melatonergic system and cancer initiation/promotion.
The antitumor effects of melatonin treatment have been widely documented, using both in vivo and in vitro experimental models of different types of malignancies, including breast and prostate cancers, melanomas and gliomas (Cutando et al., 2012). This pleiotropic and multitask indolamine acts through receptor‐dependent and ‐independent mechanisms to inhibit cell cycle progression and cell migration/invasion, to induce apoptosis and cell differentiation, whilst also blocking angiogenesis, activating the immune‐system and preventing chronodisruption (Mediavilla et al., 2010). In clinical oncology, a recent meta‐analysis of randomized controlled trials indicates that melatonin, as an adjuvant therapy, significantly increases tumour remission, improves 1 year survival rate and attenuates radio/chemotherapy‐related side effects, including thrombocytopaenia, neurotoxicity and fatigue (Wang et al., 2012). Melatonin has a low toxicity profile across a wide range of doses, whilst also improving cancer‐related symptoms, such as anorexia, cachexia and sleep disturbances (Reiter et al., 2002; Mahmoud et al., 2005).
As observed during immune‐pineal axis activation, the production of melatonin by extra‐pineal tissues plays an important role in different pathological states. Given the autocrine/paracrine protective effect of melatonin produced by cerebellar glial cells during neuroinflammation (Pinato et al., 2015), we recently investigated the pathophysiological relevance of glioma‐synthesized melatonin. Using human glioma cell lines, we demonstrated that the ability of gliomas to synthesize/accumulate melatonin negatively correlates with their overall malignancy (Kinker et al., 2016). Additionally, the analysis of The Cancer Genome Atlas (TCGA) RNAseq data revealed that grade IV gliomas present a decreased mRNA expression of ASMT, the final enzyme in melatonin biosynthesis, combined with a high expression of cytochrome P4501B1 (CYP1B1), the main enzyme for extra‐hepatic melatonin metabolism. As such, we designed a predictive model of the content of melatonin in the tumour micro‐environment, the ASMT:CYP1B1 expression index, which attempts to combine the rates of melatonin synthesis and metabolism. Importantly, a low ASMT:CYP1B1 value, which suggests decreased melatonin, was associated with poor patient survival and enhanced tumour expression of pro‐proliferation genes.
The melatonergic system has been investigated in a few other types of cancer including pinealomas, melanoma, retinoblastoma and cholangiocarcinoma (Bernard et al., 1995; Slominski et al., 2002; Fukuda et al., 2010; Han et al., 2011). Interestingly, cholangiocarcinoma cells have a reduced expression of AANAT and ASMT, whilst synthesizing significantly less melatonin than non‐malignant cholangiocytes (Han et al., 2011). Additionally, as observed in gliomas, ASMT levels decrease with the grade of pineal parenchymal tumours (Fukuda et al., 2010). The melatonin catabolic enzyme, CYP1B1, in contrast, is widely known as a tumour‐associated antigen and is overexpressed in malignant neoplasms, such as breast, prostate, lung, oesophagus and skin cancers (Murray et al., 1997; Maecker et al., 2003). These findings suggest a prognostic role for the melatonergic system of tumour cells and provide insights into the potential use of ASMT and CYP1B1 as pharmacological targets.
Concluding remarks
The concept of the immune‐pineal axis highlights the importance of switching the source of melatonin production from the pineal gland to extra‐pineal sites and back to pineal gland production. Such changes in melatonin source act to regulate several important processes involved in the recognition and appropriate activation of the immune response. The present article reviews the current state of knowledge regarding the immune‐pineal axis, as well as the problems that emerge when this axis is desynchronized. As such, the putative immunomodulatory roles of melatonin, including endocrine, autocrine and paracrine roles, may occur via effects at different sites, with significant health consequences. At the centre of the process may lie the transcription factor, NFκB, and its binding to κB responsive elements in the Aanat promoter and first intron, which blocks noradrenaline‐induced melatonin synthesis in pinealocytes and induces melatonin synthesis in activated macrophages. Therefore, pathogen‐ or danger‐associated molecular patterns induce a transient shuttle of melatonin production from pinealocytes to activated macrophage/microglia. Finally, the blockade of NFκB activity in pinealocytes and immune cells deactivates the immune‐pineal axis, so as to restore pineal melatonin synthesis. Thus, increased glucocorticoid production or mediators synthesized during the anti‐inflammatory phase play major roles in regulating increased Aanat transcription in pinealocytes. Such phenomena are of vital importance because the rhythmic synthesis of melatonin is well documented to modulate both daily and seasonal variations in immune functions, as well as the daily control of immune surveillance. In addition, pathological conditions with impaired melatonin rhythm may be a consequence of ineffective immune‐pineal axis functioning. The assessment of daily melatonin rhythm, therefore, has relevance for facilitating disease prevention and health promotion in the general population. Therefore, the melatonergic system is an important target for pharmacological interventions in an array of diverse medical conditions that are commonly linked to inflammatory‐related processes.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d,e).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Financial support National Council of Science and Technology, CNPq (305378/2009); São Paulo Research Foundation, FAPESP (2013/13691‐1, 2010/52687‐1, 2014/27287‐0, 2015/04557‐5).
Markus, R. P. , Fernandes, P. A. , Kinker, G. S. , da Silveira Cruz‐Machado, S. , and Marçola, M. (2018) Immune‐pineal axis – acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. British Journal of Pharmacology, 175: 3239–3250. 10.1111/bph.14083.
References
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 174: S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017e). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Maes M (2014). Redox regulation and the autistic spectrum: role of tryptophan catabolites, immuno‐inflammation, autoimmunity and the amygdala. Curr Neuropharmacol 12: 148–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Baturin DA, Popovich IG, Zabezhinski MA, Manton KG, Semenchenko AV et al (2004). Effect of exposure to light‐at‐night on life span and spontaneous carcinogenesis in female CBA mice. Int J Cancer 111: 475–479. [DOI] [PubMed] [Google Scholar]
- Antonioli M, Rybka J, Carvalho LA (2012). Neuroimmune endocrine effects of antidepressants. Neuropsychiatr Dis Treat 8: 65–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelot T, Ray‐Coquard I, Menetrier‐Caux C, Rastkha M, Duc A, Blay JY (2003). Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone‐refractory metastatic breast cancer patients. Br J Cancer 88: 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD (2008). MicroRNAs: new regulators of immune cell development and function. Nat Immunol 9: 839–845. [DOI] [PubMed] [Google Scholar]
- Barfuss DW, Tait GR, Ellis LC (1969). Melatonin synthesis in rat pineal glands inhibited by x‐irradiation. Proc Soc Exp Biol Med 132: 35–36. [DOI] [PubMed] [Google Scholar]
- Bartsch C, Bartsch H, Karenovics A, Franz H, Peiker G, Mecke D (1997). Nocturnal urinary 6‐sulphatoxymelatonin excretion is decreased in primary breast cancer patients compared to age‐matched controls and shows negative correlation with tumor‐size. J Pineal Res 23: 53–58. [DOI] [PubMed] [Google Scholar]
- Benítez‐King G (2006). Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res 40: 1–9. [DOI] [PubMed] [Google Scholar]
- Bernard M, Donohue SJ, Klein DC (1995). Human hydroxyindole‐O‐methyltransferase in pineal gland, retina and Y79 retinoblastoma cells. Brain Res 696: 37–48. [DOI] [PubMed] [Google Scholar]
- Blask DE (2009). Melatonin, sleep disturbance and cancer risk. Sleep Med Rev 13: 257–264. [DOI] [PubMed] [Google Scholar]
- Blask DE, Sauer LA, Dauchy RT, Holowachuk EW, Ruhoff MS, Kopff HS (1999). Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor‐mediated signal transduction events. Cancer Res 59: 4693–4701. [PubMed] [Google Scholar]
- Blask DE, Dauchy RT, Sauer LA, Krause JA, Brainard GC (2003). Growth and fatty acid metabolism of human breast cancer (MCF‐7) xenografts in nude rats: impact of constant light‐induced nocturnal melatonin suppression. Breast Cancer Res Treat 79: 313–320. [DOI] [PubMed] [Google Scholar]
- Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA et al (2005). Melatonin‐depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65: 11174–11184. [DOI] [PubMed] [Google Scholar]
- Bureau JP, Labrecque G, Coupe M, Garrelly L (1986). Influence of BCG administration time on the in‐vivo migration of leukocytes. Chronobiol Int 3: 23–28. [DOI] [PubMed] [Google Scholar]
- Cardoso TC, Pompeu TE, Silva CLM (2017). The P2Y1 receptor‐mediated leukocyte adhesion to endothelial cells is inhibited by melatonin. Purinergic Signal 13: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo‐Vico A, Lardone PJ, Fernandez‐Santos JM, Martin‐Lacave I, Calvo JR, Karasek M et al (2005). Human lymphocyte‐synthesized melatonin is involved in the regulation of the interleukin‐2/interleukin‐2 receptor system. J Clin Endocrinol Metab 90: 992–1000. [DOI] [PubMed] [Google Scholar]
- Carvalho‐Sousa CE, da Silveira Cruz‐Machado S, Tamura EK, Fernandes PA, Pinato L, Muxel SM et al (2011). Molecular basis for defining the pineal gland and pinealocytes as targets for tumor necrosis factor. Front Endocrinol (Lausanne) 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecon E, Fernandes PA, Pinato L, Ferreira ZS, Markus RP (2010). Daily variation of constitutively activated nuclear factor kappa B (NFκB) in rat pineal gland. Chronobiol Int 27: 52–67. [DOI] [PubMed] [Google Scholar]
- Cecon E, Chen M, Marcola M, Fernandes PAC, Jockers R, Markus RP (2015). Amyloid beta peptide directly impairs pineal gland melatonin synthesis and melatonin receptor signaling through the ERK pathway. FASEB J 29: 2566–2582. [DOI] [PubMed] [Google Scholar]
- Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D et al (2013). MicroRNA‐146 represses endothelial activation by inhibiting pro‐inflammatory pathways. EMBO Mol Med 5: 1017–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon M, Lightfoot N, Kreiger N (2007). Rotating shift work and risk of prostate cancer. Epidemiology 18: 182–183. [DOI] [PubMed] [Google Scholar]
- Conti A, Conconi S, Hertens E, Skwarlo‐Sonta K, Markowska M, Maestroni JM (2000). Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res 28: 193–202. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002). Inflammation and cancer. Nature 420: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto‐Moraes R, Palermo‐Neto J, Markus RP (2009). The immune‐pineal axis: stress as a modulator of pineal gland function. Ann N Y Acad Sci 1153: 193–202. [DOI] [PubMed] [Google Scholar]
- Cutando A, Lopez‐Valverde A, Arias‐Santiago S, DE Vicente J, DE Diego RG (2012). Role of melatonin in cancer treatment. Anticancer Res 32: 2747–2753. [PubMed] [Google Scholar]
- Dalaveris E, Kerenidi T, Katsabeki‐Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI et al (2009). VEGF, TNF‐alpha and 8‐isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer 64: 219–225. [DOI] [PubMed] [Google Scholar]
- Davis S, Mirick DK, Stevens RG (2001). Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 93: 1557–1562. [DOI] [PubMed] [Google Scholar]
- Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A et al (2005). Negative regulation of Toll‐like receptor 4 signaling by the Toll‐like receptor homolog RP105. Nat Immunol 6: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Risold PY (2007). The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev 56: 119–147. [DOI] [PubMed] [Google Scholar]
- Esteban‐Zubero E, Lopez‐Pingarron L, Alatorre‐Jimenez MA, Ochoa‐Moneo P, Buisac‐Ramon C, Rivas‐Jimenez M et al (2017). Melatonin's role as a co‐adjuvant treatment in colonic diseases: a review. Life Sci 170: 72–81. [DOI] [PubMed] [Google Scholar]
- Fernandes PA, Tamura EK, D'Argenio‐Garcia L, Muxel SM, Cruz‐Machado SD, Marcola M et al (2017). Dual effect of catecholamines and corticosterone crosstalk on pineal gland melatonin synthesis. Neuroendocrinology 104: 126–134. [DOI] [PubMed] [Google Scholar]
- Fernandes PACM, Cecon E, Markus RP, Ferreira ZS (2006). Effect of TNF‐alpha on the melatonin synthetic pathway in the rat pineal gland: basis for a 'feedback' of the immune response on circadian timing. J Pineal Res 41: 344–350. [DOI] [PubMed] [Google Scholar]
- Fernandes PACM, Bothorel B, Clesse D, Monteiro AWA, Calgari C, Raison S et al (2009). Local corticosterone infusion enhances nocturnal pineal melatonin production in vivo . J Neuroendocrinol 21: 90–97. [DOI] [PubMed] [Google Scholar]
- Ferreira ZS, Fernandes PACM, Duma D, Assreuy J, Avellar MCW, Markus RP (2005). Corticosterone modulates noradrenaline‐induced melatonin synthesis through inhibition of nuclear factor kappa B. J Pineal Res 38: 182–188. [DOI] [PubMed] [Google Scholar]
- Finocchiaro LM, Arzt ES, Fernandez‐Castelo S, Criscuolo M, Finkielman S, Nahmod VE (1988). Serotonin and melatonin synthesis in peripheral blood mononuclear cells: stimulation by interferon‐gamma as part of an immunomodulatory pathway. J Interferon Res 8: 705–716. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Akiyama N, Ikegami M, Takahashi H, Sasaki A, Oka H et al (2010). Expression of hydroxyindole‐O‐methyltransferase enzyme in the human central nervous system and in pineal parenchymal cell tumors. J Neuropathol Exp Neurol 69: 498–510. [DOI] [PubMed] [Google Scholar]
- Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L et al (2002). Prognostic significance of circulating IL‐10 and IL‐6 serum levels in colon cancer patients undergoing surgery. Clin Immunol 102: 169–178. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Grodzki C, Sugden D, Møller M, Odom S, Gaildrat P et al (2007). Neural adrenergic/cyclic AMP regulation of the immunoglobulin E receptor alpha‐subunit expression in the mammalian pinealocyte: a neuroendocrine/immune response link? J Biol Chem 282: 32758–32764. [DOI] [PubMed] [Google Scholar]
- Gbahou F, Cecon E, Viault G, Gerbier R, Jean‐Alphonse F, Karamitri A et al (2017). Design and validation of the first cell‐impermeant melatonin receptor agonist. Br J Pharmacol 174: 2409–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad E, Wong HR, Zingarelli B, Virag L, O'Connor M, Salzman AL et al (1998). Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: role of inhibition of NFkappaB activation. FASEB J 12: 685–693. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M (2010). Immunity, inflammation, and cancer. Cell 140): 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar C, Haussler D, Gupta D (1992). Effect of the pineal gland on circadian rhythmicity of colony forming units for granulocytes and macrophages (CFU‐GM) from rat bone marrow cell cultures. J Pineal Res 12: 79–83. [DOI] [PubMed] [Google Scholar]
- Han Y, Demorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A et al (2011). Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol 301: G623–G633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Rodriguez C (1995). Versatile melatonin – a pervasive molecule serves various functions in signaling and protection. Chronobiol Int 12: 157–165. [Google Scholar]
- van den Heiligenberg S, Depres‐Brummer P, Barbason H, Claustrat B, Reynes M, Levi F (1999). The tumor promoting effect of constant light exposure on diethylnitrosamine‐induced hepatocarcinogenesis in rats. Life Sci 64: 2523–2534. [DOI] [PubMed] [Google Scholar]
- Herman AP, Wojtulewicz K, Bochenek J, Krawczyńska A, Antushevich H, Pawlina B et al (2017). Endotoxin‐induced inflammation disturbs melatonin secretion in ewe. Asian‐Australas J Anim Sci 30: 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta CT, Gazarini ML, Beraldo FH, Varotti FP, Lopes C, Markus RP et al (2000). Calcium‐dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat Cell Biol 2: 466–468. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K (2009). An epigenetic switch involving NF‐kappaB, Lin28, Let‐7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang‐Shieh YF, Wu CH, Chien HF, Wei IH, Chang ML, Shieh JY et al (2005). Reactive changes of interstitial glia and pinealocytes in the rat pineal gland challenged with cell wall components from gram‐positive and ‐negative bacteria. J Pineal Res 38: 17–26. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Jorge S, Guerrero JM, Jimenez‐Caliani AJ, Naranjo MC, Lardone PJ, Carrillo‐Vico A et al (2007). Evidence for melatonin synthesis in the rat brain during development. J Pineal Res 42: 240–246. [DOI] [PubMed] [Google Scholar]
- Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G et al (2016). Update on melatonin receptors: IUPHAR Review 20. Br J Pharmacol 173: 2702–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M (2009). NF‐kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1: a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V (2006). Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124: 823–835. [DOI] [PubMed] [Google Scholar]
- Karper JC, Ewing MM, de Vries MR, de Jager SC, Peters EA, de Boer HC et al (2013). TLR accessory molecule RP105 (CD180) is involved in post‐interventional vascular remodeling and soluble RP105 modulates neointima formation. PLoS One 8: e67923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Singh J, Lim MK, Ng BL, Ling EA (1997). Macrophages/microglia as 'sensors' of injury in the pineal gland of rats following a non‐penetrative blast. Neurosci Res 27: 317–322. [DOI] [PubMed] [Google Scholar]
- Kinker GS, Oba‐Shinjo SM, Carvalho‐Sousa CE, Muxel SM, Marie SK, Markus RP et al (2016). Melatonergic system‐based two‐gene index is prognostic in human gliomas. J Pineal Res 60: 84–94. [DOI] [PubMed] [Google Scholar]
- Kollet O, Canaani J, Kalinkovich A, Lapidot T (2012). Regulatory cross talks of bone cells, hematopoietic stem cells and the nervous system maintain hematopoiesis. Inflamm Allergy Drug Targets 11: 170–180. [DOI] [PubMed] [Google Scholar]
- Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y et al (2006). Prospective cohort study of the risk of prostate cancer among rotating‐shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol 164: 549–555. [DOI] [PubMed] [Google Scholar]
- Laranjeira‐Silva MF, Zampieri RA, Muxel SM, Floeter‐Winter LM, Markus RP (2015). Melatonin attenuates Leishmania (L.) amazonensis infection by modulating arginine metabolism. J Pineal Res 59: 478–487. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Fong C (2010). The resolution of inflammation: anti‐inflammatory roles for NF‐kappaB. Int J Biochem Cell Biol 42: 519–523. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim SJ, Yoon HJ, Yu SY, Yang H, Jeong SI et al (2013). Genome‐wide profiling in melatonin‐exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J Pineal Res 54: 80–88. [DOI] [PubMed] [Google Scholar]
- Legros C, Chesneau D, Boutin JA, Barc C, Malpaux B (2014). Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J Neuroendocrinol 26: 151–163. [DOI] [PubMed] [Google Scholar]
- Lehallier B, Essioux L, Gayan J, Alexandridis R, Nikolcheva T, Wyss‐Coray T et al (2015). Alzheimer's disease neuroimaging initiative. combined plasma and cerebrospinal fluid signature for the prediction of midterm progression from mild cognitive impairment to Alzheimer disease. JAMA Neurol 14: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leston J, Harthe C, Mottolese C, Mertens P, Sindou M, Claustrat B (2015). Is pineal melatonin released in the third ventricle in humans? A study in movement disorders. Neurochirurgie 61: 85–89. [DOI] [PubMed] [Google Scholar]
- Levandovski R, Pfaffenseller B, Carissimi A, Gama CS, Hidalgo MP (2013). The effect of sunlight exposure on interleukin‐6 levels in depressive and non‐depressive subjects. BMC Psychiatry 13: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C, Mariano M, Markus RP (2001). Interaction between the adrenal and the pineal gland in chronic experimental inflammation induced by BCG in mice. Inflamm Res 50: 6–11. [DOI] [PubMed] [Google Scholar]
- Lotufo CM, Lopes C, Dubocovich ML, Farsky SH, Markus RP (2001). Melatonin and N‐acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur J Pharmacol 430: 351–357. [DOI] [PubMed] [Google Scholar]
- Lotufo CMC, Yamashita CE, Farsky SHP, Markus RP (2006). Melatonin effect on endothelial cells reduces vascular permeability increase induced by leukotriene B‐4. Eur J Pharmacol 534: 258–263. [DOI] [PubMed] [Google Scholar]
- Mack JM, Schamne MG, Sampaio TB, Pertile RA, Fernandes PA, Markus RP et al (2016). Melatoninergic system in Parkinson's disease: from neuroprotection to the management of motor and nonmotor symptoms. Oxid Med Cell Longev 2016: 3472032: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker B, Sherr DH, Vonderheide RH, von Bergwelt‐Baildon MS, Hirano N, Anderson KS et al (2003). The shared tumor‐associated antigen cytochrome P450 1B1 is recognized by specific cytotoxic T cells. Blood 102: 3287–3294. [DOI] [PubMed] [Google Scholar]
- Mahmoud F, Sarhill N, Mazurczak MA (2005). The therapeutic application of melatonin in supportive care and palliative medicine. Am J Hosp Palliat Care 22: 295–309. [DOI] [PubMed] [Google Scholar]
- Marçola M, Cruz‐Machado SD, Fernandes PACM, Monteiro AWA, Markus RP, Tamura EK (2013). Endothelial cell adhesiveness is a function of environmental lighting and melatonin level. J Pineal Res 54: 162–169. [DOI] [PubMed] [Google Scholar]
- Marçola M, Lopes‐Ramos CM, Pereira EP, Cecon E, Fernandes PA, Tamura EK et al (2016). Light/dark environmental cycle imposes a daily profile in the expression of microRNAs in rat CD133(+) cells. J Cell Physiol 231: 1953–1963. [DOI] [PubMed] [Google Scholar]
- Markus RP, Ferreira ZS, Fernandes PACM, Cecon E (2007). The immune‐pineal axis: a shuttle between endocrine and paracrine melatonin sources. Neuroimmunomodulation 14: 126–133. [DOI] [PubMed] [Google Scholar]
- Markus RP, Cecon E, Pires‐Lapa MA (2013). Immune‐pineal axis: nuclear factor kappa B (NF‐kappa B) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int J Mol Sci 14: 10979–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M, Coon SL, Klein DC (2013). RGS2 is a feedback inhibitor of melatonin production in the pineal gland. FEBS Lett 587: 1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima S, Reiter RJ (1975). Ultrastructural observations at pineal gland capillaries in four rodent species. Am J Anat 143: 265–281. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G, Carughi S, De Cata A, La Viola M, Vendemiale G (2005). Melatonin and cortisol serum levels in lung cancer patients at different stages of disease. Med Sci Monit 11: CR284–CR288. [PubMed] [Google Scholar]
- Mediavilla MD, Sanchez‐Barcelo EJ, Tan DX, Manchester L, Reiter RJ (2010). Basic mechanisms involved in the anti‐cancer effects of melatonin. Curr Med Chem 17: 4462–4481. [DOI] [PubMed] [Google Scholar]
- de Menezes JP, Saraiva EM, da Rocha‐Azevedo B (2016). The site of the bite: Leishmania interaction with macrophages, neutrophils and the extracellular matrix in the dermis. Parasit Vectors 9: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalaki V, Syrigos K, Charles P, Waxman J (2004). Serum levels of IL‐6 and TNF‐alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 90: 2312–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller M, Baeres FM (2002). The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res 309: 139–150. [DOI] [PubMed] [Google Scholar]
- Mortani Barbosa EJ, Ferreira ZS, Markus RP (2000). Purinergic and noradrenergic cotransmission in the rat pineal gland. Eur J Pharmacol 401: 59–62. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP (2008). Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GI, Taylor MC, McFadyen MC, McKay JA, Greenlee WF, Burke MD et al (1997). Tumor‐specific expression of cytochrome P450 CYP1B1. Cancer Res 57: 3026–3031. [PubMed] [Google Scholar]
- Muxel SM, Pires‐Lapa MA, Monteiro AWA, Cecon E, Tamura EK, Floeter‐Winter LM et al (2012). NF‐kappa B drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine‐N‐acetyltransferase (AA‐NAT) Gene. Plos One 7: e52010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muxel SM, Laranjeira‐Silva MF, Carvalho‐Sousa CE, Floeter‐Winter LM, Markus RP (2016). The RelA/cRel nuclear factor‐B (NF‐B) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J Pineal Res 60: 394–404. [DOI] [PubMed] [Google Scholar]
- Noseda AC, Rodrigues LS, Targa AD, Aurich MF, Vital MA, Da Cunha C et al (2014). Putative role of monoamines in the antidepressant‐like mechanism induced by striatal MT2 blockade. Behav Brain Res 275: 136–145. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S (2009). The NF‐kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1: a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangerl B, Pangerl A, Reiter RJ (1990). Circadian variations of adrenergic receptors in the mammalian pineal gland: a review. J Neural Transm Gen Sect 81: 17–29. [DOI] [PubMed] [Google Scholar]
- Papaioannou V, Mebazaa A, Plaud B, Legrand M (2014). 'Chronomics' in ICU: circadian aspects of immune response and therapeutic perspectives in the critically ill. Intensive Care Med Exp 2: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak J, Golab M, Markowska M, Majewski P, Skwarlo‐Sonta K (2009). Photoperiod‐related changes in hormonal and immune status of male Siberian hamsters, Phodopus sungorus. Comp Biochem Physiol A Mol Integr Physiol 152: 299–303. [DOI] [PubMed] [Google Scholar]
- Piesiewicz A, Kedzierska U, Adamska I, Usarek M, Zeman M, Skwarlo‐Sonta K et al (2012). Pineal arylalkylamine N‐acetyltransferase (Aanat) gene expression as a target of inflammatory mediators in the chicken. Gen Comp Endocrinol 179: 143–151. [DOI] [PubMed] [Google Scholar]
- Pinato L, Cruz‐Machado SD, Franco DG, Campos LMG, Cecon E, Fernandes PACM et al (2015). Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct Funct 220: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires‐Lapa MA, Tamura EK, Salustiano EMA, Markus RP (2013). Melatonin synthesis in human colostrum mononuclear cells enhances dectin‐1‐mediated phagocytosis by mononuclear cells. J Pineal Res 55: 240–246. [DOI] [PubMed] [Google Scholar]
- Pontes GN, Cardoso EC, Carneiro‐Sampaio MMS, Markus RP (2006). Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes) – melatonin in human colostrum and colostrum phagocytes. J Pineal Res 41: 136–141. [DOI] [PubMed] [Google Scholar]
- Pontes GN, Cardoso EC, Carneiro‐Sampaio MMS, Markus RP (2007). Pineal melatonin and the innate immune response: the TNF‐alpha increase after cesarean section suppresses nocturnal melatonin production. J Pineal Res 43: 365–371. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez‐Burillo S (2002). Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol 54: 1299–1321. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Rosales‐Corral SA, Tan DX, Acuna‐Castroviejo D, Qin L, Yang SF et al (2017). Melatonin, a full service anti‐cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci 18: pii: E843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Pevet P, Simonneaux V (2000). HIOMT drives the photoperiodic changes in the amplitude of the melatonin peak of the Siberian hamster. Am J Physiol Regul Integr Comp Physiol 278: R1339–R1345. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Hankinson SE (2005). Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst 97: 1084–1087. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Hankinson SE (2009). Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses' Health Study cohort. Cancer Epidemiol Biomarkers Prev 18: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I et al (2001). Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst 93: 1563–1568. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I et al (2003). Night‐shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst 95: 825–828. [DOI] [PubMed] [Google Scholar]
- Schildger BJ, Hafeli W, Matern B, Muller K, Wicker R (1991). The use of diagnostic imaging methods in reptiles. Berl Munch Tierarztl Wochenschr 104: 20–26. [PubMed] [Google Scholar]
- Shende VR, Kim SM, Neuendorff N, Earnest DJ (2014). MicroRNAs function as cis‐ and trans‐acting modulators of peripheral circadian clocks. FEBS Lett 588: 3015–3022. [DOI] [PubMed] [Google Scholar]
- Shukla M, Boontem P, Reiter RJ, Satayavivad J, Govitrapong P (2017). Mechanisms of melatonin in alleviating Alzheimer's disease. Curr Neuropharmacol 15: 1010–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira Cruz‐Machado S, Carvalho‐Sousa CE, Tamura EK, Pinato L, Cecon E, Fernandes PACM et al (2010). TLR4 and CD14 receptors expressed in rat pineal gland trigger NFKB pathway. J Pineal Res 49: 183–192. [DOI] [PubMed] [Google Scholar]
- da Silveira Cruz‐Machado S, Pinato L, Tamura EK, Carvalho‐Sousa CE, Markus RP (2012). Glia‐pinealocyte network: the paracrine modulation of melatonin synthesis by tumor necrosis factor (TNF). PLoS One 7: e40142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira Cruz‐Machado S, Tamura EK, Carvalho‐Sousa CE, Rocha VA, Pinato L, Fernandes PAC et al (2017). Daily corticosterone rhythm modulates pineal function through NFkappaB‐related gene transcriptional program. Sci Rep 7: 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Ribelayga C (2003). Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev 55: 325–395. [DOI] [PubMed] [Google Scholar]
- Skene DJ, Swaab DF (2003). Melatonin rhythmicity: effect of age and Alzheimer's disease. Exp Gerontol 38: 199–206. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Malpaux B (1999). High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology 140: 4399–4405. [DOI] [PubMed] [Google Scholar]
- Skwarlo‐Sonta K, Majewski P, Markowska M, Oblap R, Olszanska B (2003). Bidirectional communication between the pineal gland and the immune system. Can J Physiol Pharmacol 81: 342–349. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A et al (2002). Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J 16: 896–898. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Maloney B, Long JM, Ray B, Lahiri DK (2011). Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology 76: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006). NF‐kappaB‐dependent induction of microRNA miR‐146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103: 12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B (1981). Melatonin inhibition and pinealectomy enhancement of 7,12‐dimethylbenz(a)anthracene‐induced mammary tumors in the rat. Cancer Res 41: 4432–4436. [PubMed] [Google Scholar]
- Tamarkin L, Danforth D, Lichter A, DeMoss E, Cohen M, Chabner B et al (1982). Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science 216: 1003–1005. [DOI] [PubMed] [Google Scholar]
- Tamura EK, Fernandes PA, Marcola M, Cruz‐Machado SD, Markus RP (2010). Long‐lasting priming of endothelial cells by Pplasma melatonin levels. Plos One 5: e13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas F, Duranyildiz D, Argon A, Oguz H, Camlica H, Yasasever V et al (2005). Serum levels of leptin and proinflammatory cytokines in advanced‐stage non‐small cell lung cancer. Med Oncol 22: 353–358. [DOI] [PubMed] [Google Scholar]
- Tatsch‐Dias MDO, Levandovski RM, de Souza ICC, Rocha MG, Fernandes PA, Torres ILS et al (2013). The concept of the immune‐pineal axis tested in patients undergoing an abdominal hysterectomy. Neuroimmunomodulation 20: 205–212. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Najjar I, Bellissant E, Anderson GM, Barburoth M, Cohen D et al (2013). Advances in the research of melatonin in autism spectrum disorders: literature review and new perspectives. Int J Mol Sci 14: 20508–20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani ME, Reiter RJ, Vaughan MK, Gonzalez‐Brito A, Herbert DC (1988). The depression in rat pineal melatonin production after saline injection at night may be elicited by corticosterone. Brain Res 450: 18–24. [DOI] [PubMed] [Google Scholar]
- Tsai SY, O'Brien TE, McNulty JA (2001a). Microglia play a role in mediating the effects of cytokines on the structure and function of the rat pineal gland. Cell Tissue Res 303: 423–431. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Schluns KS, Le PT, McNulty JA (2001b). TGF‐beta1 and IL‐6 expression in rat pineal gland is regulated by norepinephrine and interleukin‐1beta. Histol Histopathol 16: 1135–1141. [DOI] [PubMed] [Google Scholar]
- Viswanathan AN, Hankinson SE, Schernhammer ES (2007). Night shift work and the risk of endometrial cancer. Cancer Res 67: 10618–10622. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang ZH, Wu YY, Tang H, Tan L, Wang X et al (2013). Melatonin attenuates scopolamine‐induced memory/synaptic disorder by rescuing EPACs/miR‐124/Egr1 pathway. Mol Neurobiol 47: 373–381. [DOI] [PubMed] [Google Scholar]
- Wang YM, Jin BZ, Ai F, Duan CH, Lu YZ, Dong TF et al (2012). The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta‐analysis of randomized controlled trials. Cancer Chemother Pharmacol 69: 1213–1220. [DOI] [PubMed] [Google Scholar]
- Whiteside TL (2008). The tumor microenvironment and its role in promoting tumor growth. Oncogene 27: 5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi WJ, Kim TS (2017). Melatonin protects mice against stress‐induced inflammation through enhancement of M2 macrophage polarization. Int Immunopharmacol 48: 146–158. [DOI] [PubMed] [Google Scholar]
- Yuwiler A, Wetterberg L (1989). Peptide T does not affect induction of pineal N‐acetyltransferase by vasoactive intestinal peptide. Regul Pept 25: 69–73. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lenardo MJ, Baltimore D (2017). 30 years of NF‐kappaB: a blossoming of relevance to human pathobiology. Cell 168: 37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Raichle ME, Wen J, Benzinger TL, Fagan AM, Hassenstab J et al (2017). In vivo detection of microstructural correlates of brain pathology in preclinical and early Alzheimer disease with magnetic resonance imaging. Neuroimage 148: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZY, Touitou Y (1993). Kinetic changes of melatonin release in rat pineal perifusions at different circadian stages. Effects of corticosteroids. Acta Endocrinol 129: 81–88. [DOI] [PubMed] [Google Scholar]
- Zhelev Z, Ivanova D, Bakalova R, Aoki I, Higashi T (2017). Synergistic cytotoxicity of melatonin and new‐generation anticancer drugs against leukemia lymphocytes but not normal lymphocytes. Anticancer Res 37: 149–159. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF (2003). Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res 35: 125–130. [DOI] [PubMed] [Google Scholar]


