Abstract
Melatonin, discovered in 1958 in the bovine pineal tissue, is an indoleamine that modulates circadian rhythms and has a wide variety of other functions. Haematological neoplasms are the leading cause of death in children and adolescents throughout the world. Research has demonstrated that melatonin is a low‐toxicity protective molecule against experimental haematological neoplasms, but the mechanisms remain poorly defined. Here, we provide an introduction to haematological neoplasms and melatonin, especially as they relate to the actions of melatonin on haematological carcinogenesis. Secondly, we summarize what is known about the mechanisms of action of melatonin in the haematological system, including its pro‐apoptotic, pro‐oxidative, anti‐proliferative and immunomodulatory actions. Thirdly, we discuss the advantages of melatonin in combination with other drugs against haematological malignancy, as well as its other benefits on the haematological system. Finally, we summarize the findings that are contrary to the suppressive effects of melatonin on cancers of haematological origin. We hope that this information will be helpful in the design of studies related to the therapeutic efficacy of melatonin in haematological neoplasms.
Linked Articles
This article is part of a themed section on Recent Developments in Research of Melatonin and its Potential Therapeutic Applications. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.16/issuetoc
Abbreviations
- ALL
acute lymphocytic leukaemia
- AML
acute myeloid leukaemia
- APL
acute promyelocytic leukaemia
- auto‐SCT
autologous stem cell transplantation
- CLL
chronic lymphocytic leukaemia
- DR
death receptor
- NHL
non‐Hodgkin lymphoma
- NK
natural killer
- Th1
T‐helper 1
Introduction
According to the Global Cancer Statistics from the American Cancer Society, about 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide. Among all tumours, haematological neoplasms are the leading cause of death in children and adolescents throughout the world (Torre et al., 2015). Haematological neoplasms, also known as haematological malignancies, are tumours that originate from the blood and blood‐forming system (marrow and lymphatic tissue), including leukaemias, lymphomas and multiple myelomas. Most haematological neoplasms are malignant and derive from the abnormal growth of myeloid/lymphoid cell lines. Chemotherapy is an effective method for treating haematonosis but such chemotherapy agents may also have serious adverse effects such as respiratory distress, pulmonary infiltrates, renal and cardiac failure (Frankel et al., 1992). Although 90% of patients with acute promyelocytic leukaemia (APL) may improve after treatment with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2644 (tretinoin), this compound is used only for APL (Mantha et al., 2017). Additionally, haematopoietic stem cell transplantation is an effective method for haematological neoplasms, especially leukaemia. However, the medical cost is high and immunological rejection is sometimes intense (Iwamoto et al., 2014). Therefore, much effort has been devoted to searching for a novel drug(s) in order to overcome the deficiencies of these strategies.
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224 is an indoleamine derived from the pineal gland, which modulates circadian rhythms and participates in a wide range of physiological processes (Cagnacci, 1996; Carrillo‐Vico et al., 2013; Silvestri and Rossi, 2013; Cipolla‐Neto et al., 2014; Iwamoto et al., 2014; Reiter et al., 2014a; Manchester et al., 2015; Reiter et al., 2016). Notably, it has low toxicity in humans and other animals. In mammals, melatonin is produced by the pineal gland in the vertebrate brain and in numerous other organs (Sugden et al., 2004; Reiter et al., 2013; Acuna‐Castroviejo et al., 2014). The activation of two well‐characterized G‐protein‐coupled seven‐transmembrane‐domain receptors, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=287 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=288, inhibits cAMP formation through Pertussis toxin‐sensitive inhibitory G proteins Moreover, activation of melatonin receptors might also promote the apoptosis of cancer cells and inhibit cancer development (Sanchez‐Hidalgo et al., 2012).
In 1917, McCord and Allen reported that feeding tadpoles macerated bovine pineal tissue lightened their skin by causing the accumulation of melatonin in epidermal melanophores. The pineal factor that causes the change in amphibian skin pigmentation was isolated from the bovine pineal gland in 1958 and identified as N‐acetyl‐5‐methoxytryptamine (Lerner et al., 1960). This compound was subsequently called melatonin and was found in all animals, plants, bacteria and fungi (Tan et al., 2010, 2014; Acuna‐Castroviejo et al., 2014; Arnao and Hernandez‐Ruiz, 2015; Erland et al., 2015; Yu et al., 2016). In mammalian pineal glands, melatonin synthesis is activated by daily onset of darkness, and, after its secretion, it affects sleep, mood and circadian rhythms (Santoro et al., 2012; Yun et al., 2014; Hill et al., 2015; Xiang et al., 2015; Borin et al., 2016).
Earlier research documented that melatonin is a pivotal oncostatic agent in various tumours including haematological neoplasms (Santoro et al., 2012), breast cancer (Hill et al., 2015; Xiang et al., 2015; Borin et al., 2016), lung cancer (Yun et al., 2014; Fan et al., 2015), hepatocellular carcinoma (Ordonez et al., 2015), gastrointestinal cancer (Wei et al., 2015), renal cancer (Park et al., 2014), pancreatic cancer (Ruiz‐Rabelo et al., 2011), uterine (Mao et al., 2016), cervical cancer (Pariente et al., 2016), neural cancer (Chen et al., 2016) and melanoma (Cabrera et al., 2010). Antineoplastic mechanisms of melatonin include proapoptotic, pro‐oxidative, anti‐proliferative and immunomodulatory actions. Also, via its actions as a potent antioxidant, melatonin has protective actions against myocardial ischaemia (Yang et al., 2013; Yu et al., 2014, 2015a, b, 2016), ischaemic stroke (Yang et al., 2015), septic encephalopathy (Zhao et al., 2015), subarachnoid haemorrhage (Dong et al., 2016) and flow shear stress (Yang et al., 2016). The melatonin‐mediated anti‐tumour effects have been reviewed for lung cancers (Ma et al., 2016) and gastrointestinal cancers (Xin et al., 2015), but information on its relationship to haematological neoplasms remains unsummarized.
Here, we have reviewed the publications related to the role of melatonin in haematological neoplasms, focusing on the therapeutic actions of melatonin against haematological cancers. Initially, we introduce the basic background information on haematological neoplasms and melatonin, as well as melatonin's suppressive actions on haematological tumours. Secondly, we summarize the antineoplastic mechanisms of melatonin in haematological cancers and drug synergy of melatonin, concomitant with its detoxification of anti‐haematological malignancy drugs. Thirdly, other benefits of melatonin on the haematological system are summarized along with contrary opinions, potential direction for future research, as based on the current information and experience. This review highlights recent advances and provides a thorough evaluation of melatonin's oncostatic potential. This information will hopefully benefit the design of studies for the clinical use of melatonin against haematological neoplasms.
Melatonin and haematological carcinogenesis
Melatonin is a pivotal component of the body's internal time‐keeping system that is associated with human health (Bonmati‐Carrion et al., 2014; Hernandez‐Resendiz and Zazueta, 2014; Reiter et al., 2014b; Vriend and Reiter, 2015). A conventional definition of carcinogenesis is that it is a pathological alteration of normal cells in response to stimulation by carcinogenic factors, such as chemicals, radiation and microorganisms (Vineis et al., 2010). As mentioned above, numerous studies have shown that melatonin inhibits carcinogenesis in both human and animals (Tanaka et al., 2009; Sanchez‐Barcelo et al., 2010). Epidemiological surveys show that melatonin disruption may increase the risk of haematological neoplasms, which is consistent with reduced levels of melatonin in patients with these tumours (Rana et al., 2014b). The so‐called melatonin hypothesis proposed that decreased nocturnal production of melatonin may explain the increased risk of cancer in some patients. Disruption of the circadian clockwork is one of the factors that predispose individuals to haematological neoplasms. Moreover, nocturnal work (night shifts) might disturb the normal secretion of melatonin and elevate the risk of myeloid tumours and lymphoma (Lahti et al., 2008; Yong et al., 2014; Costas et al., 2016). Further research suggests that night shift work might elevate cancer risk by suppressing melatonin secretion (Stevens and Davis, 1996; Reiter et al., 2006). In relation to haematological cancer, Garbazza et al. (2016) reported a case of a 40‐year‐old sighted male who developed a disordered day–night rhythm and was diagnosed with Hodgkin's lymphoma. Laboratory examination revealed that the circadian rhythm of melatonin was disrupted in this patient (Garbazza et al., 2016). Moreover, the circadian rhythm of melatonin was disordered in a lymphoma patient with the levels of melatonin being much lower than in healthy subjects. These findings, although they do not prove an association, are consistent with the notion that low melatonin levels may predispose to haematological malignancies.
Some retrospective clinical studies have reported a relationship of melatonin disruption with haematological neoplasms. In 37 chronic lymphocytic leukaemia (CLL) patients, serum melatonin levels were significantly lower than those in an equal number of age‐ and sex‐matched healthy volunteers (P < 0.05). In this case, the assay used was the human melatonin elisa. Melatonin levels of shift workers with CLL patients were significantly lower than those in non‐shift workers with CLL (P < 0.0001). Rana et al. (2014b) also reported that serum melatonin levels were markedly lower in CLL subjects, compared with healthy controls (P < 0.0001) and levels were found even more depressed in shift workers as compared to non‐shift workers in CLL group (P < 0.01). These findings are consistent with the possibility that shift work may relate to the aetiology of CLL by perturbing the circadian secretion of melatonin (Rana et al., 2014a). In Canada, Parent et al. (2012) performed a population‐based case–control study that enrolled 3137 males with incident cancer and 512 healthy controls. Compared to the men who never worked at night, the adjusted odds ratio of non‐Hodgkin's lymphoma (NHL) among men who ever worked at night are 2.31, suggesting that disrupted melatonin secretion is a potent etiological factor for haematological neoplasms (Table 1).
Table 1.
Studies suggest a relationship of melatonin and haematological carcinogenesis
| Patients | Managements/phenomenal description | Effects/discovery |
|---|---|---|
| Thirty‐seven CLL patients (Rana et al., 2014b) | Serum melatonin concentrations were determined by elisa. | Significantly lower serum melatonin levels were observed in CLL patients compared to healthy subjects. |
| A 40‐year‐old sighted male with Hodgkin's lymphoma (Garbazza et al., 2016) | Patient had misalignment of the internal clock with the external light–dark cycle. | The circadian rhythm of melatonin was disrupted, and the levels of melatonin were low compared to healthy subjects. |
| Thirty‐seven CLL patients (Rana et al., 2014a) | Aberrant expression of circadian clock and cell cycle genes (melatonin) were detected by elisa. | Serum melatonin levels were remarkably low in CLL subjects compared to healthy controls, and levels were still lower in shift‐workers compared to non‐shift‐workers in CLL group. |
| A total of 3137 males with incident cancer and 512 healthy controls (Parent et al., 2012) | Population‐based case–control study. | Adjusted odds ratio of low‐grade NHL among men with night work was 2.31 similar to controls. |
Function of melatonin in haematological neoplasms
Apoptosis
Apoptosis is a pathophysiological process of programmed cell death in multicellular organisms (Jiang et al., 2016; Jiang et al., 2017; Li et al., 2017). Resistance to cell death is one of the most important characteristics of a tumour. Therefore, apoptosis is a pivotal mechanism for restraining tumour progression and several studies have demonstrated that melatonin promotes apoptosis of myeloid leukaemia cells. Rubio et al. (2007) reported that melatonin enhances cytochrome c release from mitochondria, augments activity of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1619, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1625, and down‐regulates http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2844 in cancer cells. They also tested whether the apoptotic actions of melatonin in HL‐60 cells are mediated by the classic membrane MT receptors and discovered that a non‐specific MT receptor antagonist did not reverse the effects mentioned above, suggesting that the apoptotic response of myeloid leukaemia cells to melatonin is independent of these receptors. The combination of melatonin and puromycin restrains the expression of anti‐apoptotic proteins (Bcl‐2 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2845) and enhances activity of caspase‐3 and cleavage of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2771 compared to puromycin alone in human leukaemia HL‐60 cells. This suggests that melatonin may be effectively used in leukaemia patients as a potential chemotherapeutic agent (Koh et al., 2011). Melatonin induces a significant increase in caspase‐3 and ‐9 activities and evokes depolarization of the mitochondrial membrane and activation of permeability transition pore, thereby leading to elevated apoptosis of HL‐60 cells as determined by propidium iodide positive‐staining. These workers also discovered that melatonin‐induced apoptosis is time‐dependent and reaches to a maximum at 12 h and a minimum at 72 h (Bejarano et al., 2009). Jang et al. (2009) used both normal mice splenocytes and Jurkat T‐leukaemia cells subjected to 2 Gy X‐ray radiation. Pretreatment with 250 mg·kg−1 melatonin enhanced the radiation‐induced apoptosis of leukaemia cells while inhibiting radiation‐induced apoptosis in normal mouse splenocytes, as shown by the reduced http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=910/Bcl‐2 ratio and p53 RNA in normal splenocytes. This suggests that melatonin may promote radiation‐induced apoptosis via p53 expression. The apparent differential action of melatonin on radiation‐induced apoptosis in normal and cancer cells provides an opportunity to increase the therapeutic ratio between tumour control and protection of normal cells in radiotherapy (Dong et al., 2016).
Melatonin also promotes apoptosis of acute lymphocytic leukaemia (ALL). Melatonin (10−3 M concentration) may induce the apoptosis of MOLT‐4 cells by promoting the production of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1840, concomitant with reduced levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2613. Notably, they discovered that 10−5 M melatonin fails to induce the apoptosis of MOLT‐4 cells (Buyukavci et al., 2006). Melatonin promoted apoptosis of leukaemia Molt‐3 cells by increasing the activities of caspase‐3, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1622, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1623 and 9, which are associated with an elevation of Bax and the release of cytochrome c from mitochondria; this documents that melatonin induces apoptosis of ALL cells through a caspase‐dependent pathway (Perdomo et al., 2013). An above‐normal level of apoptosis is also observed in human leukaemia REH cells after pretreatment with melatonin and this rise correlates with increased expression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1875, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1883, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=334#1880 and their ligands. Thus, melatonin may find utility as a potential anti‐ALL agent (Casado‐Zapico et al., 2011).
Apart from leukaemia, melatonin also induces apoptosis of lymphoma cells. Sanchez‐Hidalgo et al. (2012) reported that apoptosis appears, with an increased caspase‐3 and PARP cleavage, within 0.5–1 h after melatonin treatment in three types of malignant Burkitt's lymphoma cells (Ramos and DoHH2 cells); this response correlated with a breakdown of the inner mitochondrial transmembrane potential. They also discovered that Ramos cells are the most sensitive to melatonin, but an explanation for this differential response was not uncovered (Sanchez‐Hidalgo et al., 2012). Paternoster et al. (2009) compared normal lymphocytes with lymphoma cells and discovered that melatonin is likely to induce apoptosis of lymphoma cells via ROS production. Moreover, melatonin causes apoptosis of RAMOS‐1 lymphoblastoid cells, a response that was associated with down‐regulation of Bcl‐2, mitochondrial membrane depolarization, cytochrome c release and activation of caspase‐3 (Trubiani et al., 2005). Collectively, the data summarized here demonstrate that melatonin should be considered a drug candidate for haematological neoplasms.
Anti‐proliferation
Proliferation is a physiological process characterized with increased cell division and cell numbers. Unlimited proliferation is a unique feature of tumours and often causes immense damage to normal growth of the surrounding cells. Previous evidence has suggested that melatonin inhibits the proliferation of different tumours including lymphocytic leukaemia. Melatonin displays anti‐proliferative properties in human Molt‐3 leukaemia cells arresting the cell cycle. The reported findings document a significant arrest at G1 phase at 12 h after treatment, followed by rise in the number of hypodiploid cells at 24 h (Perdomo et al., 2013). Melatonin also causes the arrest at G1 phase of the cell cycle in Ramos, DoHH2 and SU‐DHL‐4 cells, associated with a reduction in the proportion of cells in the S and G2/M phases (Reiter et al., 2014b). The anti‐proliferative effects of melatonin (at 10–3 M concentration) were readily apparent in CMK, Jurkat and MOLT‐4 cells. Melatonin also restrains proliferation of tumour cells by inducing the production of ROS, which are cytotoxic to leukaemia cells (Buyukavci et al., 2006).
Melatonin also exerts anti‐proliferative actions on myeloid leukaemia. Rubio et al. (2007) reported that melatonin suppressed the growth of the human myeloid leukaemia HL‐60 cells by blocking the progression from G1 to S phase, which was accompanied by a significant inhibition of cell growth and reduced cell number. Melatonin induces the phosphorylation of p53 at Ser15 and restrains cell proliferation in PML cells. The group also showed that melatonin‐induced anti‐proliferative actions are mediated by p38 MAPK signalling (Santoro et al., 2012). Clearly, melatonin is a powerful anti‐proliferative agent for haematological neoplasms.
Pro‐oxidation
Pro‐oxidation is associated with elevated levels of oxidative stress. As discussed above, melatonin is a powerful antioxidant in otherwise normal cells, but it becomes pro‐oxidant in tumour cells (Bizzarri et al., 2013; Zhang and Zhang, 2014). The experimental data confirm that the antineoplastic effects of melatonin are attributed to its ability to induce free radical generation and oxidative stress (Ghibelli et al., 1998; Bizzarri et al., 2013). Melatonin stimulates the production of ROS and elevates the oxidizing environment in human myeloid HL‐60 cells, with cytotoxic effects (Bejarano et al., 2011). For example, melatonin increases the activity of enzymes (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=271 or http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=269) and promotes the production of ROS in Burkitt lymphoma BL41 cells (Paternoster et al., 2009). Similarly, the indole combined with 4 Gy X‐ray irradiation causes a significant rise in ROS in Jurkat cells and enhances radiation‐induced cell death via a pro‐oxidant pathway (Jang et al., 2009). Thus, melatonin is a documented pro‐oxidant molecule in haematological neoplasms.
Immunomodulation
The attack on tumour cells by the immune system is a dynamic and constant process throughout tumour growth including progression and metastasis (Diken et al., 2017; Porter and Raviprakash, 2017). Melatonin is also a modulator of immune cell function and haematopoiesis (Miller et al., 2006; Carrillo‐Vico et al., 2013). Physiologically, melatonin is associated with T‐helper 1 (Th1) cytokines and induces activation of Th1. In both normal mice and those with acute mid‐stage erythroleukemia, melatonin administration results in a quantitative and functional enhancement of natural killer (NK) cells, which mediate endogenous defences against cancer cells. Melatonin regulates cell dynamics of host defence, including the proliferative and maturational stages of haematopoietic and immune cells (NK cells, T and B lymphocytes, granulocytes and monocytes), thereby improving their normal physiological function. Notably, in mice bearing mid‐stage leukaemia, daily administration of melatonin results in a survival index of 30–40% (more than 3 months) compared with 0% in untreated mice (Miller et al., 2006).
A high‐dose cytotoxic chemotherapy is a commonly used treatment for patients with ALL and is partly effective (Lissoni et al., 2000; Raj et al., 2013). However, this therapy usually contributes to considerable side effects such as myelosuppression, which leads to a weakened immune system that is difficult to correct later. Relative to this serious problem, Lissoni et al. (2000) carried out a phase II study of low‐dose http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4985 plus melatonin in 12 patients with an untreatable advanced haematological malignancy, including NHL (six cases), Hodgkin's disease (two cases), multiple myeloma (two cases), acute myeloid leukaemia (AML; one case) and chronic myelomonocytic leukaemia (one case). IL‐2 was injected subcutaneously at a dose of 3 million IU·day−1, and melatonin was given orally at 20 mg·day−1 in the evening for 4 weeks. The results showed that tumour progression was markedly suppressed in eight patients and a median duration of survival was extended by 21 months (14–30 months) for these patients. This suggests that the combination of low‐dose IL‐2 plus melatonin may prolong the survival time in untreatable advanced haematological malignancy, compared to high‐dose IL‐2 toxic immunotherapy alone (Lissoni et al., 2000). Extracts of Echinacea purpurea and melatonin elevated the number and activity of NK cells and enhanced the killing competence to tumour cells in leukaemia mice. Notably, the combination prolongs the lifespan of leukemic mice compared to melatonin alone (Currier and Miller, 2001). Taken together, the observations suggest melatonin‐induced immunomodulation may be useful as an effective management for haematological neoplasms (Figure 1).
Figure 1.
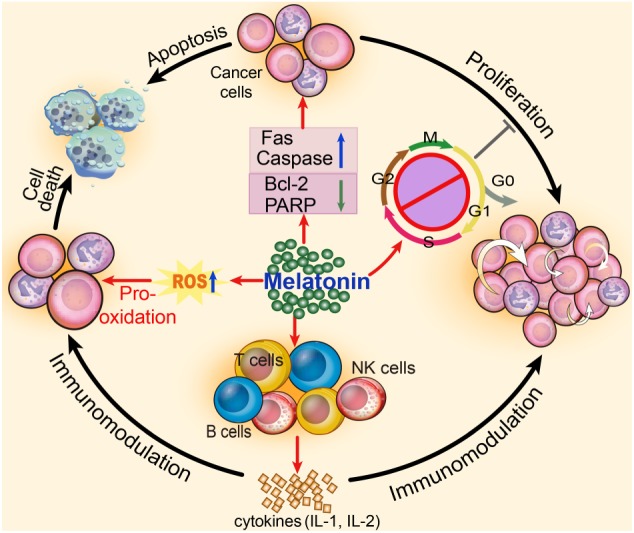
Roles of melatonin in haematological neoplasms. Melatonin is a pivotal endocrine compound, with actions against haematological carcinogenesis. Melatonin up‐regulates the levels of Fas and caspase family and down‐regulates the levels of PARP and Bcl‐2, as well as enhancing the production of ROS for promoting the apoptosis of haematological cancer cells. Melatonin also blocks the cell cycle of cancer cells to inhibit their proliferation. Additionally, activity of immunocytes and secretion of cytokines can be enhanced by melatonin, thereby inducing immunomodulation against haematological neoplasms.
Melatonin ameliorates toxicity of anti‐haematological malignancy drugs
Myelotoxicity, also known as myelosuppression, is the decrease of haematopoietic cells and haemocytes, which results from chemotherapy (e.g. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5687) during the treatment of these neoplasms and other drugs that restrain the immune system (e.g. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7120) (Kantarjian et al., 2006; Hirbe et al., 2007; Von Hoff et al., 2013). Doxorubicin and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4827 are two classical drugs used to treat haematological malignancy but they cause considerable haematotoxicity. Melatonin attenuates the reduction of marrow granulocyte macrophage‐colony forming unit, CD3+, CD4+ and CDS+ splenic T‐lymphocytes after doxorubicin treatment, concomitant with increased total GSH and reduced lipid peroxidation (Rapozzi et al., 1998). Melatonin reverses the fall in red blood cells, total leucocytes and platelets after cytarabine treatment. Additionally, melatonin significantly increases the amount of total protein, globulin and reduces the albumin/globulin ratio. These findings indicate that melatonin protects marrow and lymphoid tissue from injury by cytotoxic drugs as well as stimulating marrow regeneration (Nakayashiki et al., 2001). Moreover, melatonin protects the myeloid and erythroid series against intercellular oxidative stress induced by H2O2 during or 1 h after doxorubicin treatment (Greish et al., 2005).
Melatonin also ameliorates extra‐haematotoxicity of anti‐haematological malignancy drugs. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7278 is an effective chemotherapeutic drug especially in lymphoma whereas the testicular toxicity that induces sterility is a limiting factor. Melatonin in combination with this drug significantly lowers the levels of malondialdehyde and increases the levels of antioxidant enzymes, including GSH peroxidise, and nitrite values while exhibiting no side effects. These effects correlated with increased testicle size as indicated by their length, weight and sperm count (Alp et al., 2014). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4815 is widely used as a chemotherapeutic agent for leukaemia. The efficacy of this drug is often limited by intestinal mucositis in children and adults. Pretreatment with melatonin significantly attenuates methotrexate‐induced oxidative stress and restores the activities of the antioxidant enzymes (GSH reductase and superoxide dismutase), thereby ameliorating methotrexate‐induced small intestinal mucositis. This shows that melatonin protects against methotrexate‐induced mucositis in humans with leukaemia (Kolli et al., 2013). Together, melatonin not only acts as an antineoplastic drug but also a protector against the toxicity of anti‐haematological malignancy drugs. These results are consistent with other publications, which show that melatonin is an effective countermeasure to the molecular damage that is a consequence of many chemotherapies (Reiter et al., 2002).
Further perspectives
Drug synergy of melatonin
Melatonin is an endogenous molecule with low toxicity and favourable compatibility. When given in combination with other drugs, melatonin may improve their beneficial effects on haematological neoplasms (Oka et al., 1997; Orendas et al., 2014). Most of the published cases of combined therapy are focused on NHL. Patients with high‐grade NHL that receive autologous stem cell transplantation (auto‐SCT) usually relapse and have a poor prognosis. However, the combination of melatonin with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7154, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2019, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=35, retinoids or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3633 in patients with high‐grade relapsing NHL after auto‐SCT, allowed them to recover completely and to function normally at home (Todisco et al., 2001; Todisco, 2006; Todisco, 2009). The beneficial effects were also confirmed by Todisco's group, who reported that a patient with advanced low‐grade NHL recovered after a similar combination of drugs (Todisco, 2007). The Di Bella Method (melatonin, retinoids, vitamins C, D3 and E, somatostatin and prolactin inhibitors) prolonged the 1, 3 and 5 year survival rates and improved the quality of life in 55 subjects with lymphoma, concomitant with low toxicity during the therapeutic process (Di Bella et al., 2012).
In vitro, incubation with 200–1000 pg·mL−1 melatonin caused a significant and dose‐dependent partial sensitization in doxorubicin‐resistant P388 mouse leukaemia cells as shown by increased survival times of these cells. Interestingly, melatonin affects membrane http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=152#768 and elevates intracellular concentrations of doxorubicin in leukaemia cells (Granzotto et al., 2001) (Table 2).
Table 2.
Drug synergy of melatonin
| Patients/cells | Drug synergy | Results |
|---|---|---|
| Eight patients with relapsed low‐grade NHL (Todisco, 2006) | Melatonin, cyclophosphamide, somatostatin, bromocriptine, retinoids and ACTH | Patients recovered completely and did normal activities at home. |
| Twenty patients with relapsed low‐grade NHL (Todisco et al., 2001) | Melatonin, somatostatin, prolactin, retinoids and ACTH | Therapy was well tolerated and effective in 70% of these patients, concomitant with a mild toxicity. |
| Four patients with untreated progressive stage I CCL (Todisco, 2009) | Melatonin, cyclophosphamide, somatostatin, bromocriptine, retinoids and ACTH | No patients had recurrence and all did normal activities at home. |
| Twelve patients with low‐grade stage IV NHL (Todisco, 2007) | Melatonin, cyclophosphamide, somatostatin, bromocriptine, retinoids and ACTH | All patients had complete remission and did normal activities. |
| Resistant P388 mice leukaemia cells (Granzotto et al., 2001) | Melatonin and doxorubicin | Melatonin mediated membrane P‐glycoprotein and elevated intracellular concentrations of doxorubicin in leukaemia cells. |
Radiotherapy is a particular therapeutic method for haematological neoplasms (Johansen et al., 2017). A combination of melatonin with 4 Gy irradiation induced apoptosis of Jurkat leukaemia cells in C57BL/6 mice, concomitant with prolonged lifetime of the leukaemic animals. Thus, melatonin enhances radiation‐induced apoptosis and promotes survival of Jurkat leukaemia cells (Jang et al., 2009). It seems clear that the combination of melatonin with other oncostatic agents or radiation may be a promising method for improving the therapeutic efficacy and prolonging the lifespan of patients with haematological neoplasms (Figure 2).
Figure 2.
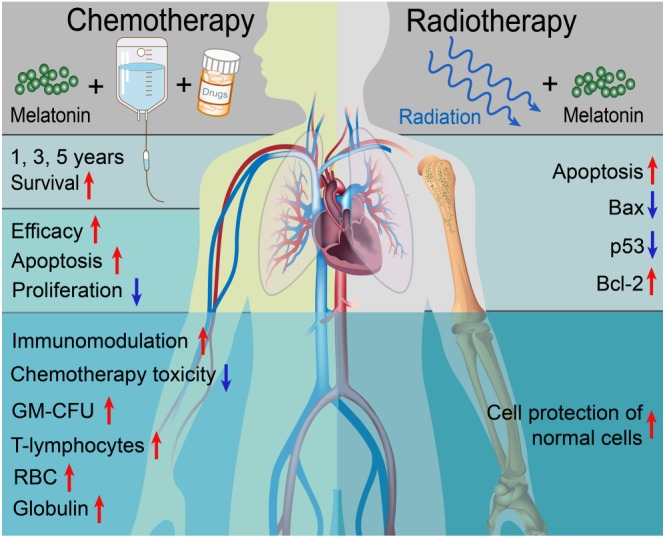
Actions of melatonin on chemo‐ and radiotherapy. Combination of melatonin with chemotherapeutic agents promotes survival, enhances the efficacy and immunomodulation and ameliorates toxicity due to chemotherapy, in patients with haematological neoplasms. Melatonin combined with radiotherapy promotes the apoptosis of haematological cancer cells while protecting the normal splenocytes, suggesting its selective cytotoxicity to haematological neoplasms.
Other beneficial actions
Although the study outcomes have been justifiably questioned, epidemiological studies have claimed an association between exposure to extremely low frequency electromagnetic fields and an increased risk of haematological neoplasms (Yellon, 1994). One proposed mechanism is that the power frequency fields may suppress the nocturnal production of melatonin, thereby contributing to a disturbed internal environment and increasing risk of haematological malignancy (Henshaw and Reiter, 2005; Henshaw et al., 2008). Flight personnel are often exposed to the non‐visible, low‐frequency electromagnetic fields and often have disrupted sleep patterns, which depend on normal melatonin rhythm. Buja et al. (2005)searched the online databases of male flight attendants and discovered that meta‐standardized incidence ratio of NHL was 2.49 (1.03–6.03) in these individuals and claimed that this was associated with low levels of circulating melatonin, suggesting that melatonin disruption is closely related to haematological malignancy. Nevertheless, the decrease of melatonin induced by occupational or environmental exposure to electric field is considerable (Kheifets et al., 1997; Ahlbom et al., 2001; Kheifets et al., 2009). Thus, maintenance of melatonin at normal levels might prevent haematological carcinogenesis.
Drug resistance remains a serious clinical problem in leukaemia therapy. Yamanishi et al. (2015) established two clofarabine‐resistant lymphoblastic leukaemia cell lines and discovered that clofarabine‐resistant cells exhibit a markedly reduced expression of mRNA for 2'‐deoxycytidine kinase after melatonin treatment. Meanwhile, histone acetylation of H3 and H4 was significantly lowered in resistant cells, as shown by the chromatin immunoprecipitation assay. Overall, melatonin treatment leads to significantly increased cytotoxicity with clofarabine in resistant cells via elevated acetylation, indicating that melatonin may be a useful candidate for overcoming resistance to drugs with anti‐haematological neoplasm actions (Yamanishi et al., 2015) as has been shown for other drug‐resistant tumours (Martin et al., 2010; Alonso‐Gonzalez et al., 2015). During the therapy of promyelocytic leukaemia, melatonin also triggers the over‐phosphorylation of p53 and prevents accumulation of damaged DNA in normal cells, thereby ameliorating the carcinogenic potential of normal cells (Santoro et al., 2012). Additionally, melatonin prevents the fall in bone marrow polychromatic erythroid, lymphocytes and neutrophils in lead‐treated rats. Notably, it also attenuates the dyserythropoiesis and megaloblastic lesion in the marrow, indicating that melatonin has the ability to protect haematopoietic cells from lead‐related toxicity (Othman et al., 2004). Again, these findings are consistent with the ability of melatonin to reduce lead‐mediated toxicity in other organs. Collectively, melatonin may well be a general protective molecule in the haematological system.
Contrary data regarding the efficacy of melatonin as an inhibitor of haematopoietic cancer
Throughout this report, we have summarized studies showing that melatonin reduces the growth of haematological neoplasms. Some publications, however, question the beneficial role of melatonin in haematological neoplasms and suggest otherwise. Several studies have reported increasing melatonin serum levels in patients with cancers of haematological origin. Plasma melatonin concentrations were determined in 46 patients with multiple myeloma and 31 age‐matched healthy subjects. The patients with multiple myeloma had significantly higher mean serum levels of melatonin than those in healthy subjects (22 ± 13.5 vs. 12 ± 4.8 pg·mL−1; P < 0.001) (Tarquini et al., 1995). Lissoni et al. (1987) enrolled 42 patients with solid tumours and 21 patients with lymphoma or leukaemia. They also noted that all patients had significantly higher serum levels of melatonin than those in control subjects. Additionally, there are reports claiming that melatonin accelerated the proliferation of lymphoma (Conti et al., 1992) and leukaemia (Sakano et al., 2004) and restrained apoptosis of lymphoma cells (Tanyi, 2006). One report argued that melatonin per se has no relationship with haematological neoplasms (Touitou and Selmaoui, 2012), and the review of the literature suggested that any relationship between magnetic field exposure and melatonin suppression was questionable (Touitou and Selmaoui, 2012). These contradictory findings are not easily reconciled. Tarquini et al. (1995) predicted that the elevated levels of melatonin in patients with haematological neoplasms are a consequence of compensatory rise of melatonin in an attempt to inhibit tumour growth. While the majority of findings confirm a suppressive effect of melatonin on cancers of haematopoietic origin, there are clearly some that do not support that conclusion.
Perspectives
Haematological neoplasms are still a major problem that concerns many medical professionals. So far, there is little evidence for a role of the MT receptors in haematological neoplasms. https://www.ncbi.nlm.nih.gov/pubmed/?term=S%C3%A1nchez-Hidalgo%20M%5BAuthor%5D&cauthor=true&cauthor_uid=22582944 and Rubio found that the effects of melatonin against haematological neoplasms were independent of MT1 and MT2 receptors, although they did not give a clear alternative explanation (Rubio et al., 2007; Sanchez‐Hidalgo et al., 2012). However, melatonin may increase the expression of the death receptors http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1875, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=334#1879 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=334#1880, thereby promoting the apoptosis of tumour cells (Rubio et al., 2007; Casado‐Zapico et al., 2011; Zheng et al., 2013). Based on the reports summarized here, we feel that melatonin is a potentially important agent for the treatment of these tumours. Chemotherapy is currently the main treatment for haematological neoplasms, but such compounds have marked side effects and so melatonin may be more effective as a treatment and, when combined with conventional chemotherapies, may significantly reduce their side effects (Reiter et al., 2002; Buyukavci et al., 2011). Melatonin receptor agonists have been developed and include http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1356 (Pandi‐Perumal et al., 2009) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=198 (Millan et al., 2003), but their indications are for sleep disorders only and whether they have any anti‐cancer actions has not been tested. Clinical trials using melatonin to treat haematological neoplasms have not been carried out and the evidence from animal experiments remains sketchy. New directions of melatonin research should involve the following: (i) evaluating the actions of melatonin alone on haematological neoplasms including defining melatonin's molecular effects on these cancers for providing a better treatment strategy; and (ii) using a combination of melatonin with chemotherapies to possibly increase their efficacy. Moreover, possibly of even greater importance would be the use of melatonin to reduce the toxicity of commonly used drugs to treat haematological cancers.
Concluding remarks
As summarized inthis review, melatonin appears to have beneficial actions against haematological neoplasms, overall. The normal circadian pattern of secretion of melatonin from the pineal gland may determine its protective actions against haematological cancers. The positive effects of melatonin are pro‐apoptotic, pro‐oxidative, anti‐proliferative and immunomodulatory. Thus, the timing of exogenous melatonin administration may be critical in determining its efficacy as an oncostatic agent. Importantly, melatonin also ameliorates the toxicity of many drugs used to treat haematological malignancies, including myelotoxicity and toxicity on non‐haematological tissues. Finally, clarification of the intracellular signalling network of melatonin's anti‐neoplastic actions will help to facilitate further basic research and clinical application of melatonin in the treatment of haematological neoplasms.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c,d,e).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81500263 and 81600306) and China Postdoctoral Science Foundation (2016T90973 and 2015M572681).
Li, T. , Yang, Z. , Jiang, S. , Di, W. , Ma, Z. , Hu, W. , Chen, F. , Reiter, R. J. , and Yang, Y. (2018) Melatonin: does it have utility in the treatment of haematological neoplasms?. British Journal of Pharmacology, 175: 3251–3262. 10.1111/bph.13966.
Contributor Information
Russel J Reiter, Email: reiter@uthscsa.edu.
Yang Yang, Email: yang200214yy@163.com.
References
- Acuna‐Castroviejo D, Escames G, Venegas C, Diaz‐Casado ME, Lima‐Cabello E, Lopez LC et al (2014). Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71: 2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlbom IC, Cardis E, Green A, Linet M, Savitz D, Swerdlow A et al (2001). Review of the epidemiologic literature on EMF and health. Environ Health Perspect 109 (Suppl 6): 911–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso‐Gonzalez C, Gonzalez A, Martinez‐Campa C, Gomez‐Arozamena J, Cos S (2015). Melatonin sensitizes human breast cancer cells to ionizing radiation by downregulating proteins involved in double‐strand DNA break repair. J Pineal Res 58: 189–197. [DOI] [PubMed] [Google Scholar]
- Alp BF, Kesik V, Malkoc E, Yigit N, Saldir M, Babacan O et al (2014). The effect of melatonin on procarbazine induced testicular toxicity on rats. Syst Biol Reprod Med 60: 323–328. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernandez‐Ruiz J (2015). Functions of melatonin in plants: a review. J Pineal Res 59: 133–150. [DOI] [PubMed] [Google Scholar]
- Bejarano I, Espino J, Marchena AM, Barriga C, Paredes SD, Rodriguez AB et al (2011). Melatonin enhances hydrogen peroxide‐induced apoptosis in human promyelocytic leukaemia HL‐60 cells. Mol Cell Biochem 353: 167–176. [DOI] [PubMed] [Google Scholar]
- Bejarano I, Redondo PC, Espino J, Rosado JA, Paredes SD, Barriga C et al (2009). Melatonin induces mitochondrial‐mediated apoptosis in human myeloid HL‐60 cells. J Pineal Res 46: 392–400. [DOI] [PubMed] [Google Scholar]
- Bizzarri M, Proietti S, Cucina A, Reiter RJ (2013). Molecular mechanisms of the pro‐apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets 17: 1483–1496. [DOI] [PubMed] [Google Scholar]
- Bonmati‐Carrion MA, Arguelles‐Prieto R, Martinez‐Madrid MJ, Reiter R, Hardeland R, Rol MA et al (2014). Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci 15: 23448–23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin TF, Arbab AS, Gelaleti GB, Ferreira LC, Moschetta MG, Jardim‐Perassi BV et al (2016). Melatonin decreases breast cancer metastasis by modulating Rho‐associated kinase protein‐1 expression. J Pineal Res 60: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja A, Lange JH, Perissinotto E, Rausa G, Grigoletto F, Canova C et al (2005). Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health 21: 273–282. [DOI] [PubMed] [Google Scholar]
- Buyukavci M, Ozdemir O, Buck S, Ravindranath Y, Savasan S (2011). Effect of melatonin on the cytotoxicity of chemotherapeutic drugs in human leukemia cells. In Vivo 25: 405–409. [PubMed] [Google Scholar]
- Buyukavci M, Ozdemir O, Buck S, Stout M, Ravindranath Y, Savasan S (2006). Melatonin cytotoxicity in human leukemia cells: relation with its pro‐oxidant effect. Fundam Clin Pharmacol 20: 73–79. [DOI] [PubMed] [Google Scholar]
- Cabrera J, Negrin G, Estevez F, Loro J, Reiter RJ, Quintana J (2010). Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK‐MEL‐1 cells. J Pineal Res 49: 45–54. [DOI] [PubMed] [Google Scholar]
- Cagnacci A (1996). Melatonin in relation to physiology in adult humans. J Pineal Res 21: 200–213. [DOI] [PubMed] [Google Scholar]
- Carrillo‐Vico A, Lardone PJ, Alvarez‐Sanchez N, Rodriguez‐Rodriguez A, Guerrero JM (2013). Melatonin: buffering the immune system. Int J Mol Sci 14: 8638–8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado‐Zapico S, Martin V, Garcia‐Santos G, Rodriguez‐Blanco J, Sanchez‐Sanchez AM, Luno E et al (2011). Regulation of the expression of death receptors and their ligands by melatonin in haematological cancer cell lines and in leukaemia cells from patients. J Pineal Res 50: 345–355. [DOI] [PubMed] [Google Scholar]
- Chen X, Hao A, Li X, Du Z, Li H, Wang H et al (2016). Melatonin inhibits tumorigenicity of glioblastoma stem‐like cells via the AKT‐EZH2‐STAT3 signaling axis. J Pineal Res 61: 208–217. [DOI] [PubMed] [Google Scholar]
- Cipolla‐Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014). Melatonin, energy metabolism, and obesity: a review. J Pineal Res 56: 371–381. [DOI] [PubMed] [Google Scholar]
- Conti A, Haran‐Ghera N, Maestroni GJ (1992). Role of pineal melatonin and melatonin‐induced‐immuno‐opioids in murine leukemogenesis. Med Oncol Tumor Pharmacother 9: 87–92. [DOI] [PubMed] [Google Scholar]
- Costas L, Benavente Y, Olmedo‐Requena R, Casabonne D, Robles C, Gonzalez‐Barca EM et al (2016). Night shift work and chronic lymphocytic leukemia in the MCC‐Spain case‐control study. Int J Cancer 139: 1994–2000. [DOI] [PubMed] [Google Scholar]
- Currier NL, Miller SC (2001). Echinacea purpurea and melatonin augment natural‐killer cells in leukemic mice and prolong life span. J Altern Complement Med 7: 241–251. [DOI] [PubMed] [Google Scholar]
- Di Bella G, Colori B, Mascia F (2012). The Di Bella Method (DBM) improved survival, objective response and performance status in a retrospective observational clinical study on 55 cases of lymphomas. Neuro Endocrinol Lett 33: 773–781. [PubMed] [Google Scholar]
- Diken M, Kranz LM, Kreiter S, Sahin U (2017). mRNA: a versatile molecule for cancer vaccines. Curr Issues Mol Biol 22: 113–128. [DOI] [PubMed] [Google Scholar]
- Dong Y, Fan C, Hu W, Jiang S, Ma Z, Yan X et al (2016). Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J Pineal Res 60: 253–262. [DOI] [PubMed] [Google Scholar]
- Erland LA, Murch SJ, Reiter RJ, Saxena PK (2015). A new balancing act: the many roles of melatonin and serotonin in plant growth and development. Plant Signal Behav 10: e1096469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Pan Y, Yang Y, Di S, Jiang S, Ma Z et al (2015). HDAC1 inhibition by melatonin leads to suppression of lung adenocarcinoma cells via induction of oxidative stress and activation of apoptotic pathways. J Pineal Res 59: 321–333. [DOI] [PubMed] [Google Scholar]
- Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP Jr (1992). The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med 117: 292–296. [DOI] [PubMed] [Google Scholar]
- Garbazza C, Bromundt V, Eckert A, Brunner DP, Meier F, Hackethal S et al (2016). Non‐24‐hour sleep‐wake disorder revisited – a case study. Front Neurol 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibelli L, Fanelli C, Rotilio G, Lafavia E, Coppola S, Colussi C et al (1998). Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J 12: 479–486. [DOI] [PubMed] [Google Scholar]
- Granzotto M, Rapozzi V, Decorti G, Giraldi T (2001). Effects of melatonin on doxorubicin cytotoxicity in sensitive and pleiotropically resistant tumor cells. J Pineal Res 31: 206–213. [DOI] [PubMed] [Google Scholar]
- Greish K, Sanada I, Saad Ael D, Hasanin E, Kawasuji M, Kawano F et al (2005). Protective effect of melatonin on human peripheral blood hematopoeitic stem cells against doxorubicin cytotoxicity. Anticancer Res 25: 4245–4248. [PubMed] [Google Scholar]
- Henshaw DL, Reiter RJ (2005). Do magnetic fields cause increased risk of childhood leukemia via melatonin disruption? Bioelectromagnetics Suppl 7: S86–S97. [DOI] [PubMed] [Google Scholar]
- Henshaw DL, Ward JP, Matthews JC (2008). Can disturbances in the atmospheric electric field created by powerline corona ions disrupt melatonin production in the pineal gland? J Pineal Res 45: 341–350. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Resendiz S, Zazueta C (2014). PHO‐ERK1/2 interaction with mitochondria regulates the permeability transition pore in cardioprotective signaling. Life Sci 108: 13–21. [DOI] [PubMed] [Google Scholar]
- Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L et al (2015). Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer 22: R183–R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbe AC, Uluckan O, Morgan EA, Eagleton MC, Prior JL, Piwnica‐Worms D et al (2007). Granulocyte colony‐stimulating factor enhances bone tumor growth in mice in an osteoclast‐dependent manner. Blood 109: 3424–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto C, Takenaka K, Urata S, Yamauchi T, Shima T, Kuriyama T et al (2014). The BALB/c‐specific polymorphic SIRPA enhances its affinity for human CD47, inhibiting phagocytosis against human cells to promote xenogeneic engraftment. Exp Hematol 42: 163–171 e161. [DOI] [PubMed] [Google Scholar]
- Jang SS, Kim WD, Park WY (2009). Melatonin exerts differential actions on X‐ray radiation‐induced apoptosis in normal mice splenocytes and Jurkat leukemia cells. J Pineal Res 47: 147–155. [DOI] [PubMed] [Google Scholar]
- Jiang S, Han J, Li T, Xin Z, Ma Z, Di W et al (2017). Curcumin as a potential protective compound against cardiac diseases. Pharmacol Res 119: 373–383. [DOI] [PubMed] [Google Scholar]
- Jiang S, Yang Y, Li T, Ma Z, Hu W, Deng C et al (2016). An overview of the mechanisms and novel roles of Nrf2 in cardiovascular diseases. Expert Opin Ther Targets 20: 1413–1424. [DOI] [PubMed] [Google Scholar]
- Johansen S, Norman MH, Dale E, Amdal CD, Furre T, Malinen E et al (2017). Patterns of local‐regional recurrence after conformal and intensity‐modulated radiotherapy for head and neck cancer. Radiat Oncol 12: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B et al (2006). Nilotinib in imatinib‐resistant CML and Philadelphia chromosome‐positive ALL. N Engl J Med 354: 2542–2551. [DOI] [PubMed] [Google Scholar]
- Kheifets L, Bowman JD, Checkoway H, Feychting M, Harrington JM, Kavet R et al (2009). Future needs of occupational epidemiology of extremely low frequency electric and magnetic fields: review and recommendations. Occup Environ Med 66: 72–80. [DOI] [PubMed] [Google Scholar]
- Kheifets LI, London SJ, Peters JM (1997). Leukemia risk and occupational electric field exposure in Los Angeles County, California. Am J Epidemiol 146: 87–90. [DOI] [PubMed] [Google Scholar]
- Koh W, Jeong SJ, Lee HJ, Ryu HG, Lee EO, Ahn KS et al (2011). Melatonin promotes puromycin‐induced apoptosis with activation of caspase‐3 and 5′‐adenosine monophosphate‐activated kinase‐alpha in human leukemia HL‐60 cells. J Pineal Res 50: 367–373. [DOI] [PubMed] [Google Scholar]
- Kolli VK, Abraham P, Isaac B, Kasthuri N (2013). Preclinical efficacy of melatonin to reduce methotrexate‐induced oxidative stress and small intestinal damage in rats. Dig Dis Sci 58: 959–969. [DOI] [PubMed] [Google Scholar]
- Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E (2008). Night‐time work predisposes to non‐Hodgkin lymphoma. Int J Cancer 123: 2148–2151. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Takahashi Y (1960). Isolation of melatonin and 5‐methoxyindole‐3‐acetic acid from bovine pineal glands. J Biol Chem 235: 1992–1997. [PubMed] [Google Scholar]
- Li T, Jiang S, Yang Z, Ma Z, Yi W, Wang D et al (2017). Targeting the energy guardian AMPK: another avenue for treating cardiomyopathy? Cell Mol Life Sci 74: 1413–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissoni P, Bastone A, Sala R, Mauri R, Rovelli F, Viviani S et al (1987). The clinical significance of melatonin serum determination in oncological patients and its correlations with GH and PRL blood levels. Eur J Cancer Clin Oncol 23: 949–957. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Bolis S, Brivio F, Fumagalli L (2000). A phase II study of neuroimmunotherapy with subcutaneous low‐dose IL‐2 plus the pineal hormone melatonin in untreatable advanced hematologic malignancies. Anticancer Res 20: 2103–2105. [PubMed] [Google Scholar]
- Ma Z, Yang Y, Fan C, Han J, Wang D, Di S et al (2016). Melatonin as a potential anticarcinogen for non‐small‐cell lung cancer. Oncotarget 7: 46768‐46784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester LC, Coto‐Montes A, Boga JA, Andersen LP, Zhou Z, Galano A et al (2015). Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 59: 403–419. [DOI] [PubMed] [Google Scholar]
- Mantha S, Goldman DA, Devlin SM, Lee JW, Zannino D, Collins M et al (2017). Determinants of fatal bleeding during induction therapy for acute promyelocytic leukemia in the ATRA era. Blood 129: 1763–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Dauchy RT, Blask DE, Dauchy EM, Slakey LM, Brimer S et al (2016). Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J Pineal Res 60: 167–177. [DOI] [PubMed] [Google Scholar]
- Martin V, Garcia‐Santos G, Rodriguez‐Blanco J, Casado‐Zapico S, Sanchez‐Sanchez A, Antolin I et al (2010). Melatonin sensitizes human malignant glioma cells against TRAIL‐induced cell death. Cancer Lett 287: 216–223. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman‐Tancredi A, Pasteau V et al (2003). The novel melatonin agonist agomelatine (S20098) is an antagonist at 5‐hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 306: 954–964. [DOI] [PubMed] [Google Scholar]
- Miller SC, Pandi‐Perumal SR, Esquifino AI, Cardinali DP, Maestroni GJ (2006). The role of melatonin in immuno‐enhancement: potential application in cancer. Int J Exp Pathol 87: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayashiki H, Ikeda K, Hashimoto Y, Tosa Y, Mayama S (2001). Methylation is not the main force repressing the retrotransposon MAGGY in Magnaporthe grisea. Nucleic Acids Res 29: 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S et al (1997). Caveolin interaction with protein kinase C. Isoenzyme‐dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem 272: 33416–33421. [DOI] [PubMed] [Google Scholar]
- Ordonez R, Fernandez A, Prieto‐Dominguez N, Martinez L, Garcia‐Ruiz C, Fernandez‐Checa JC et al (2015). Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. J Pineal Res 59: 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendas P, Kubatka P, Bojkova B, Kassayova M, Kajo K, Vybohova D et al (2014). Melatonin potentiates the anti‐tumour effect of pravastatin in rat mammary gland carcinoma model. Int J Exp Pathol 95: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman AI, al Sharawy S, el‐Missiry MA (2004). Role of melatonin in ameliorating lead induced haematotoxicity. Pharmacol Res 50: 301–307. [DOI] [PubMed] [Google Scholar]
- Pandi‐Perumal SR, Srinivasan V, Spence DW, Moscovitch A, Hardeland R, Brown GM et al (2009). Ramelteon: a review of its therapeutic potential in sleep disorders. Adv Ther 26: 613–626. [DOI] [PubMed] [Google Scholar]
- Parent ME, El‐Zein M, Rousseau MC, Pintos J, Siemiatycki J (2012). Night work and the risk of cancer among men. Am J Epidemiol 176: 751–759. [DOI] [PubMed] [Google Scholar]
- Pariente R, Pariente JA, Rodriguez AB, Espino J (2016). Melatonin sensitizes human cervical cancer HeLa cells to cisplatin‐induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res 60: 55–64. [DOI] [PubMed] [Google Scholar]
- Park EJ, Woo SM, Min KJ, Kwon TK (2014). Transcriptional and post‐translational regulation of Bim controls apoptosis in melatonin‐treated human renal cancer Caki cells. J Pineal Res 56: 97–106. [DOI] [PubMed] [Google Scholar]
- Paternoster L, Radogna F, Accorsi A, Cristina Albertini M, Gualandi G, Ghibelli L (2009). Melatonin as a modulator of apoptosis in B‐lymphoma cells. Ann N Y Acad Sci 1171: 345–349. [DOI] [PubMed] [Google Scholar]
- Perdomo J, Cabrera J, Estevez F, Loro J, Reiter RJ, Quintana J (2013). Melatonin induces apoptosis through a caspase‐dependent but reactive oxygen species‐independent mechanism in human leukemia Molt‐3 cells. J Pineal Res 55: 195–206. [DOI] [PubMed] [Google Scholar]
- Porter KR, Raviprakash K (2017). DNA vaccine delivery and improved immunogenicity. Curr Issues Mol Biol 22: 129–138. [DOI] [PubMed] [Google Scholar]
- Raj TA, Smith AM, Moore AS (2013). Vincristine sulfate liposomal injection for acute lymphoblastic leukemia. Int J Nanomedicine 8: 4361–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Munawar M, Shahid A, Malik M, Ullah H, Fatima W et al (2014a). Deregulated expression of circadian clock and clock‐controlled cell cycle genes in chronic lymphocytic leukemia. Mol Biol Rep 41: 95–103. [DOI] [PubMed] [Google Scholar]
- Rana S, Shahid A, Ullah H, Mahmood S (2014b). Lack of association of the NPAS2 gene Ala394Thr polymorphism (rs2305160:G>A) with risk of chronic lymphocytic leukemia. Asian Pac J Cancer Prev 15: 7169–7174. [DOI] [PubMed] [Google Scholar]
- Rapozzi V, Zorzet S, Comelli M, Mavelli I, Perissin L, Giraldi T (1998). Melatonin decreases bone marrow and lymphatic toxicity of adriamycin in mice bearing TLX5 lymphoma. Life Sci 63: 1701–1713. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Gultekin F, Manchester LC, Tan DX (2006). Light pollution, melatonin suppression and cancer growth. J Pineal Res 40: 357–358. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L (2016). Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61: 253‐278. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Galano A (2014a). Melatonin: exceeding expectations. Physiology (Bethesda) 29: 325–333. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Kim SJ, Cruz MH (2014b). Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow‐Robin perivascular spaces. Brain Struct Funct 219: 1873–1887. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Rosales‐Corral S, Manchester LC (2013). The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem 13: 373–384. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez‐Burillo S (2002). Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol 54: 1299–1321. [DOI] [PubMed] [Google Scholar]
- Rubio S, Estevez F, Cabrera J, Reiter RJ, Loro J, Quintana J (2007). Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid HL‐60 cells. J Pineal Res 42: 131–138. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Rabelo J, Vazquez R, Arjona A, Perea D, Montilla P, Tunez I et al (2011). Improvement of capecitabine antitumoral activity by melatonin in pancreatic cancer. Pancreas 40: 410–414. [DOI] [PubMed] [Google Scholar]
- Sakano K, Oikawa S, Hiraku Y, Kawanishi S (2004). Oxidative DNA damage induced by a melatonin metabolite, 6‐hydroxymelatonin, via a unique non‐o‐quinone type of redox cycle. Biochem Pharmacol 68: 1869–1878. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Barcelo EJ, Mediavilla MD, Tan DX, Reiter RJ (2010). Clinical uses of melatonin: evaluation of human trials. Curr Med Chem 17: 2070–2095. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Hidalgo M, Lee M, de la Lastra CA, Guerrero JM, Packham G (2012). Melatonin inhibits cell proliferation and induces caspase activation and apoptosis in human malignant lymphoid cell lines. J Pineal Res 53: 366–373. [DOI] [PubMed] [Google Scholar]
- Santoro R, Marani M, Blandino G, Muti P, Strano S (2012). Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene 31: 2931–2942. [DOI] [PubMed] [Google Scholar]
- Silvestri M, Rossi GA (2013). Melatonin: its possible role in the management of viral infections – a brief review. Ital J Pediatr 39: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG, Davis S (1996). The melatonin hypothesis: electric power and breast cancer. Environ Health Perspect 104 (Suppl 1): 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden D, Davidson K, Hough KA, Teh MT (2004). Melatonin, melatonin receptors and melanophores: a moving story. Pigment Cell Res 17: 454–460. [DOI] [PubMed] [Google Scholar]
- Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM et al (2010). The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev Camb Philos Soc 85: 607–623. [DOI] [PubMed] [Google Scholar]
- Tan DX, Zanghi BM, Manchester LC, Reiter RJ (2014). Melatonin identified in meats and other food stuffs: potentially nutritional impact. J Pineal Res 57: 213–218. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yasui Y, Tanaka M, Tanaka T, Oyama T, Rahman KM (2009). Melatonin suppresses AOM/DSS‐induced large bowel oncogenesis in rats. Chem Biol Interact 177: 128–136. [DOI] [PubMed] [Google Scholar]
- Tanyi RA (2006). Spirituality and family nursing: spiritual assessment and interventions for families. J Adv Nurs 53: 287–294. [DOI] [PubMed] [Google Scholar]
- Tarquini R, Perfetto F, Zoccolante A, Salti F, Piluso A, De Leonardis V et al (1995). Serum melatonin in multiple myeloma: natural brake or epiphenomenon? Anticancer Res 15: 2633–2637. [PubMed] [Google Scholar]
- Todisco M (2006). Relapse of high‐grade non‐Hodgkin's lymphoma after autologous stem cell transplantation: a case successfully treated with cyclophosphamide plus somatostatin, bromocriptine, melatonin, retinoids, and ACTH. Am J Ther 13: 556–557. [DOI] [PubMed] [Google Scholar]
- Todisco M (2007). Low‐grade non‐Hodgkin lymphoma at advanced stage: a case successfully treated with cyclophosphamide plus somatostatin, bromocriptine, retinoids, and melatonin. Am J Ther 14: 113–115. [DOI] [PubMed] [Google Scholar]
- Todisco M (2009). Chronic lymphocytic leukemia: long‐lasting remission with combination of cyclophosphamide, somatostatin, bromocriptine, retinoids, melatonin, and ACTH. Cancer Biother Radiopharm 24: 353–355. [DOI] [PubMed] [Google Scholar]
- Todisco M, Casaccia P, Rossi N (2001). Cyclophosphamide plus somatostatin, bromocriptin, retinoids, melatonin and ACTH in the treatment of low‐grade non‐Hodgkin's lymphomas at advanced stage: results of a phase II trial. Cancer Biother Radiopharm 16: 171–177. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A (2015). Global cancer statistics, 2012. CA Cancer J Clin 65: 87–108. [DOI] [PubMed] [Google Scholar]
- Touitou Y, Selmaoui B (2012). The effects of extremely low‐frequency magnetic fields on melatonin and cortisol, two marker rhythms of the circadian system. Dialogues Clin Neurosci 14: 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trubiani O, Recchioni R, Moroni F, Pizzicannella J, Caputi S, Di Primio R (2005). Melatonin provokes cell death in human B‐lymphoma cells by mitochondrial‐dependent apoptotic pathway activation. J Pineal Res 39: 425–431. [DOI] [PubMed] [Google Scholar]
- Vineis P, Schatzkin A, Potter JD (2010). Models of carcinogenesis: an overview. Carcinogenesis 31: 1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al (2013). Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend J, Reiter RJ (2015). Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res 58: 1–11. [DOI] [PubMed] [Google Scholar]
- Wei JY, Li WM, Zhou LL, Lu QN, He W (2015). Melatonin induces apoptosis of colorectal cancer cells through HDAC4 nuclear import mediated by CaMKII inactivation. J Pineal Res 58: 429–438. [DOI] [PubMed] [Google Scholar]
- Xiang S, Dauchy RT, Hauch A, Mao L, Yuan L, Wren MA et al (2015). Doxorubicin resistance in breast cancer is driven by light at night‐induced disruption of the circadian melatonin signal. J Pineal Res 59: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Jiang S, Jiang P, Yan X, Fan C, Di S et al (2015). Melatonin as a treatment for gastrointestinal cancer: a review. J Pineal Res 58: 375–387. [DOI] [PubMed] [Google Scholar]
- Yamanishi M, Narazaki H, Asano T (2015). Melatonin overcomes resistance to clofarabine in two leukemic cell lines by increased expression of deoxycytidine kinase. Exp Hematol 43: 207–214. [DOI] [PubMed] [Google Scholar]
- Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S et al (2013). JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res 55: 275–286. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fan C, Deng C, Zhao L, Hu W, Di S et al (2016). Melatonin reverses flow shear stress‐induced injury in bone marrow mesenchymal stem cells via activation of AMP‐activated protein kinase signaling. J Pineal Res 60: 228–241. [DOI] [PubMed] [Google Scholar]
- Yang Y, Jiang S, Dong Y, Fan C, Zhao L, Yang X et al (2015). Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1‐dependent mechanism during ischemic‐stroke in mice. J Pineal Res 58: 61–70. [DOI] [PubMed] [Google Scholar]
- Yellon SM (1994). Acute 60 Hz magnetic field exposure effects on the melatonin rhythm in the pineal gland and circulation of the adult Djungarian hamster. J Pineal Res 16: 136–144. [DOI] [PubMed] [Google Scholar]
- Yong M, Nasterlack M, Messerer P, Oberlinner C, Lang S (2014). A retrospective cohort study of shift work and risk of cancer‐specific mortality in German male chemical workers. Int Arch Occup Environ Health 87: 175–183. [DOI] [PubMed] [Google Scholar]
- Yu L, Li B, Zhang M, Jin Z, Duan W, Zhao G et al (2016). Melatonin reduces PERK‐eIF2alpha‐ATF4‐mediated endoplasmic reticulum stress during myocardial ischemia‐reperfusion injury: role of RISK and SAFE pathways interaction. Apoptosis 21: 809–824. [DOI] [PubMed] [Google Scholar]
- Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai M et al (2015a). Reduced silent information regulator 1 signaling exacerbates myocardial ischemia‐reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res 59: 376–390. [DOI] [PubMed] [Google Scholar]
- Yu L, Liang H, Lu Z, Zhao G, Zhai M, Yang Y et al (2015b). Membrane receptor‐dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia‐reperfusion injury: in vivo and in vitro studies. J Pineal Res 59: 420–433. [DOI] [PubMed] [Google Scholar]
- Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M et al (2014). Melatonin receptor‐mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res 57: 228–238. [DOI] [PubMed] [Google Scholar]
- Yun M, Kim EO, Lee D, Kim JH, Kim J, Lee H et al (2014). Melatonin sensitizes H1975 non‐small‐cell lung cancer cells harboring a T790M‐targeted epidermal growth factor receptor mutation to the tyrosine kinase inhibitor gefitinib. Cell Physiol Biochem 34: 865–872. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Zhang Y (2014). Melatonin: a well‐documented antioxidant with conditional pro‐oxidant actions. J Pineal Res 57: 131–146. [DOI] [PubMed] [Google Scholar]
- Zhao L, An R, Yang Y, Yang X, Liu H, Yue L et al (2015). Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J Pineal Res 59: 230–239. [DOI] [PubMed] [Google Scholar]
- Zheng T, Fu JJ, Hu L, Qiu F, Hu M, Zhu JJ et al (2013). Nanoarchitectured electrochemical cytosensors for selective detection of leukemia cells and quantitative evaluation of death receptor expression on cell surfaces. Anal Chem 85: 5609–5616. [DOI] [PubMed] [Google Scholar]


