Abstract
We aimed to investigate the risks of Alzheimer’s (AD) and Parkinson’s disease (PD) in the 10 years following diagnosis of open-angle glaucoma (OAG) using a nationwide cohort. This propensity score-matched retrospective cohort study included 1,025,340 subjects from the Korean National Health Insurance Service National Sample Cohort database. The OAG group (n = 1,469) included patients who were initially diagnosed with OAG between 2004 and 2007, and the subjects in the comparison group were matched in a 1:5 ratio using propensity scores. Cox regression analyses were performed to investigate the risks of developing AD or PD. The diagnosis of OAG was significantly associated with an increased incidence of AD (hazard ratio [HR] = 1.403, 95% confidence interval [CI] 1.180–1.669, p < 0.001), but not PD (HR = 0.995, 95% CI 0.620–1.595, p = 0.983) after adjusting for possible confounding factors. In subgroup analyses, participants with OAG aged ≥65 years were more likely to develop AD compared with those aged <65 years, and female OAG patients had a greater risk of developing AD than males. Patients diagnosed with OAG have a higher risk of developing AD, but not PD, and the risk differed according to age and sex.
Introduction
Open-angle glaucoma (OAG) is a leading cause of irreversible blindness and is characterized by chronic progressive glaucomatous optic neuropathy with corresponding visual field defects1,2. Retinal ganglion cells (RGCs), the main target of glaucomatous damage, are central nervous system neurons located in the inner layer of the retina that convey information from the retina to the brain. On developing glaucoma, injuries occur in optic nerve axons, leading to progression of RGC neurodegeneration3,4. It is widely accepted that intraocular pressure (IOP) plays a crucial role in the development and progression of OAG, and several non-IOP factors have been reported to be associated with OAG pathogenesis5–8. However, there are still many mysteries to unravel to determine the mechanisms involved in RGC damage in glaucoma patients.
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the two most common neurodegenerative disorders in the elderly9. In AD, neurons and synapses are lost in the cerebral cortex, in the presence of extracellular amyloid-β plaques (Aβ) and neurofibrillary tangles that consist of phosphorylated Tau protein, leading to progressive memory loss, behavioral disturbances, personality changes, and cognitive impairment10,11. PD is a chronic, progressive movement disorder in which patients show slow movement, difficulty walking, rigidity, and tremor, caused by the loss of dopaminergic neurons in the substantia nigra12,13. It is related to alpha-synuclein, the major biochemical component of Lewy bodies14. Neurodegenerative disease, i.e., the progressive loss of neuron structure or function, including AD and PD, is currently recognized as a major public health issue with the aging global population15,16. There is increasing evidence of neurodegenerative lesions found in AD are also observed in the optic nerve, lateral geniculate nucleus, and visual cortex in glaucoma patients, suggesting that OAG is a neurodegenerative disease of the eye17–24. Previous experimental and clinical studies have shown close associations between OAG and PD25–27. These two neurodegenerative diseases, AD and PD, and glaucoma share several biological features, such as being chronic, slowly progressive disorders with an age-related incidence and similar mechanisms of cell injury. Although there are strong associations between OAG and AD or PD, only a few epidemiological studies have been conducted with inconsistent results, leaving the relationship between the conditions inconclusive28–33.
In light of this, we investigated the risks of developing AD and PD after an initial diagnosis of OAG, and compared the risk of each neurodegenerative disease during the 10 years following diagnosis of OAG using a representative South Korean sample of approximately 1 million adults from the National Health Insurance Service-National Sample Cohort 2002–2013 (NHIS-NSC 2002–2013).
Results
Table 1 shows the baseline characteristics of the study population. The study ultimately enrolled 1,469 patients with OAG and 7,345 individuals for the comparison group. There was a significant difference in AD prevalence between the OAG group and the comparison group (p < 0.001). Hypertension (p < 0.001), diabetes mellitus (p < 0.001), hyperlipidemia (p < 0.001), and ischemic stroke (p = 0.003) were more prevalent in the OAG group than in the comparison group. There were no significant differences in the other parameters used for matching, which included sex, age group, income, residential area, Charlson comorbidity index (CCI), and the year of enrollment. When subjects were grouped according to the diagnosis of each neurodegenerative disease, subsequent AD developed in 742 subjects and subsequent PD developed in 118 subjects during the median follow-up period of 6.99 years. Subjects with AD and PD had higher proportions of female, old age, high income, and high CCI than patients without these diseases (Table 2).
Table 1.
Demographic characteristics of study population.
| Patient without OAG | Patient with OAG | P-value | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | ||
| Total | 7,345 | 100.0% | 1,469 | 100.0% | 8,814 | |
| Presence of AD or PD | ||||||
| No | 6,682 | 91.0% | 1,272 | 86.6% | <0.001 | 7,954 |
| AD | 567 | 7.7% | 175 | 11.9% | 742 | |
| PD | 96 | 1.3% | 22 | 1.5% | 118 | |
| Hypertension | ||||||
| No | 2,615 | 35.6% | 446 | 30.4% | <0.001 | 3,061 |
| Yes | 4,730 | 64.4% | 1,023 | 69.6% | 5,753 | |
| Diabetes | ||||||
| No | 3,112 | 42.4% | 539 | 36.7% | <0.001 | 3,651 |
| Yes | 4,233 | 57.6% | 930 | 63.3% | 5,163 | |
| Hyperlipidemia | ||||||
| No | 2,582 | 35.2% | 429 | 29.2% | <0.001 | 3,011 |
| Yes | 4,763 | 64.8% | 1,040 | 70.8% | 5,803 | |
| Ischemic stroke | ||||||
| No | 5,836 | 79.5% | 1,117 | 76.0% | 0.003 | 6,953 |
| Yes | 1,509 | 20.5% | 352 | 24.0% | 1,861 | |
| Variables for matching | ||||||
| Sex | ||||||
| Male | 3,886 | 52.9% | 781 | 53.2% | 0.856 | 4,667 |
| Female | 3,459 | 47.1% | 688 | 46.8% | 4,147 | |
| Age group | ||||||
| ≤49 | 1,852 | 25.2% | 372 | 25.3% | 0.958 | 2,224 |
| 50–59 | 1,447 | 19.7% | 287 | 19.6% | 1,734 | |
| 60–69 | 2,239 | 30.5% | 436 | 29.7% | 2,675 | |
| 70–79 | 1,477 | 20.1% | 306 | 20.8% | 1,783 | |
| ≥80 | 330 | 4.5% | 68 | 4.6% | 398 | |
| Income | ||||||
| High | 3,861 | 52.6% | 759 | 51.7% | 0.684 | 4,620 |
| Milddle | 2,142 | 29.2% | 428 | 29.1% | 2,570 | |
| Low | 1,342 | 18.3% | 282 | 19.2% | 1,624 | |
| Residential area | ||||||
| Metropolitan | 514 | 7.0% | 106 | 7.2% | 0.944 | 620 |
| City | 3,299 | 44.9% | 655 | 44.6% | 3,954 | |
| Rural | 3,532 | 48.1% | 708 | 48.2% | 4,240 | |
| CCI | ||||||
| ≤3 | 5,891 | 80.2% | 1,176 | 80.1% | 0.895 | 7,067 |
| >3 | 1,454 | 19.8% | 293 | 19.9% | 1,747 | |
| Year | ||||||
| 2004 | 1,435 | 19.5% | 287 | 19.5% | 1.000 | 1,722 |
| 2005 | 1,495 | 20.4% | 299 | 20.4% | 1,794 | |
| 2006 | 1,395 | 19.0% | 279 | 19.0% | 1,674 | |
| 2007 | 1,355 | 18.4% | 271 | 18.4% | 1,626 | |
| 2008 | 1,665 | 22.7% | 333 | 22.7% | 1,998 | |
OAG = open angle glaucoma; AD = Alzheimer’s disease; PD = Parkinson’s disease; CCI = Charlson comorbidity index.
Table 2.
Characteristics according to the presence of Alzheimer’s disease or Parkinson’s disease.
| Patients without AD or PD | AD | PD | Total | ||||
|---|---|---|---|---|---|---|---|
| Total | 7954 | (90.2%) | 742 | (8.4%) | 118 | (1.3%) | 8,814 |
| OAG | |||||||
| No | 6,682 | (84.0%) | 567 | (76.4%) | 96 | (81.4%) | 7,345 |
| Yes | 1,272 | (16.0%) | 175 | (23.6%) | 22 | (18.6%) | 1,469 |
| Hypertension | |||||||
| No | 2,988 | (37.6%) | 62 | (8.4%) | 11 | (9.3%) | 3,061 |
| Yes | 4,966 | (62.4%) | 680 | (91.6%) | 107 | (90.7%) | 5,753 |
| Diabetes | |||||||
| No | 3,506 | (44.1%) | 119 | (16.0%) | 26 | (22.0%) | 3,651 |
| Yes | 4,448 | (55.9%) | 623 | (84.0%) | 92 | (78.0%) | 5,163 |
| Hyperlipidemia | |||||||
| No | 2,873 | (36.1%) | 114 | (15.4%) | 24 | (20.3%) | 3,011 |
| Yes | 5,081 | (63.9%) | 628 | (84.6%) | 94 | (79.7%) | 5,803 |
| Ischemic stroke | |||||||
| No | 6,620 | (83.2%) | 279 | (37.6%) | 54 | (45.8%) | 6,953 |
| Yes | 1,334 | (16.8%) | 463 | (62.4%) | 64 | (54.2%) | 1,861 |
| Variables for matching | |||||||
| Sex | |||||||
| Male | 4,298 | (54.0%) | 318 | (42.9%) | 51 | (43.2%) | 4,667 |
| Female | 3,656 | (46.0%) | 424 | (57.1%) | 67 | (56.8%) | 4,147 |
| Age group | |||||||
| ≤49 | 2,221 | (27.9%) | 1 | (0.1%) | 2 | (1.7%) | 2,224 |
| 50–59 | 1,690 | (21.3%) | 36 | (4.9%) | 8 | (6.8%) | 1,734 |
| 60–69 | 2,423 | (30.5%) | 201 | (27.1%) | 51 | (43.2%) | 2,675 |
| 70–79 | 1,337 | (16.8%) | 399 | (53.8%) | 47 | (39.8%) | 1,783 |
| ≥80 | 283 | (3.6%) | 105 | (14.2%) | 10 | (8.5%) | 398 |
| Income | |||||||
| High | 4,095 | (51.5%) | 452 | (60.9%) | 73 | (61.9%) | 4,620 |
| Milddle | 2,395 | (30.1%) | 150 | (20.2%) | 25 | (21.2%) | 2,570 |
| Low | 1,464 | (18.4%) | 140 | (18.9%) | 20 | (16.9%) | 1,624 |
| Residential area | |||||||
| Metropolitan | 558 | (7.0%) | 58 | (7.8%) | 4 | (3.4%) | 620 |
| City | 3,597 | (45.2%) | 304 | (41.0%) | 53 | (44.9%) | 3,954 |
| Rural | 3,799 | (47.8%) | 380 | (51.2%) | 61 | (51.7%) | 4,240 |
| CCI | |||||||
| ≤3 | 6,655 | (83.7%) | 350 | (47.2%) | 62 | (52.5%) | 7,067 |
| >3 | 1,299 | (16.3%) | 392 | (52.8%) | 56 | (47.5%) | 1,747 |
| Year | |||||||
| 2004 | 1,524 | (19.2%) | 173 | (23.3%) | 25 | (21.2%) | 1,722 |
| 2005 | 1,594 | (20.0%) | 177 | (23.9%) | 23 | (19.5%) | 1,794 |
| 2006 | 1,524 | (19.2%) | 128 | (17.3%) | 22 | (18.6%) | 1,674 |
| 2007 | 1,483 | (18.6%) | 121 | (16.3%) | 22 | (18.6%) | 1,626 |
| 2008 | 1,829 | (23.0%) | 143 | (19.3%) | 26 | (22.0%) | 1,998 |
OAG = open angle glaucoma; AD = Alzheimer’s disease; PD = Parkinson’s disease; CCI = Charlson comorbidity index.
The risk of developing subsequent AD or PD during the 10-year follow-up period was evaluated using multivariate Cox hazard regression analyses with three different models, crude model, model 1 hypertension, diabetes mellitus, hyperlipidemia and ischemic stroke) and model 2 (adjusted for age, sex, residential area, income, CCI, hypertension, diabetes mellitus, hyperlipidemia and ischemic stroke). All three models revealed that the risk of developing subsequent AD during the 10-year follow-up period was significantly higher in the OAG group than in the comparison group (Crude: hazard ratio [HR] = 1.623, 95% confidence interval [CI] 1.369–1.924, p < 0.001; model 1: HR = 1.421, 95% CI 1.199–1.684, p < 0.001; model 2: HR = 1.403, 95% CI 1.180–1.669, p < 0.001) (Table 3). In model 1, hypertension, diabetes mellitus, ischemic stroke were also associated with an increased risk of AD development (Supplementary Table 1), while in model 2, old age, female gender, ischemic stroke and a high CCI score were significantly associated with an increased risk of developing AD (Supplementary Table 2). In comparison, the risk of developing PD in the OAG group was not significant compared with the comparison group in all three models (Crude: HR = 1.115, 95% CI 0.696–1.788, p = 0.650; model 1: HR = 0.984, 95% CI 0.612–1.582, p = 0.946; model 2: HR = 0.995, 95% CI 0.620–1.595, p = 0.983), and there were disparities in the development of PD according to hypertension and ischemic stroke in model 1, and ischemic stroke and subject age in model 2. (Supplementary Tables 1 and 2).
Table 3.
Hazard ratios for Alzheimer’s disease or Parkinson’s disease in multivariable Cox regression analysis.
| Outcome | Crude | Model 11 | Model 22 | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||
| AD | No OAG | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| OAG | 1.623 (1.369–1.924) | <0.001 | 1.421 (1.199–1.684) | <0.001 | 1.403 (1.180–1.669) | <0.001 | |
| PD | No OAG | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| OAG | 1.115 (0.696–1.788) | 0.650 | 0.984 (0.612–1.582) | 0.946 | 0.995 (0.620–1.595) | 0.983 | |
1Adjusted for hypertension, diabetes mellitus, hyperlipidemia and ischemic stroke.
2Adjusted for age, sex, residential area, income, Charlson comorbidity index, hypertension, diabetes mellitus, hyperlipidemia and ischemic stroke.
OAG = open angle glaucoma; AD = Alzheimer’s disease; PD = Parkinson’s disease.
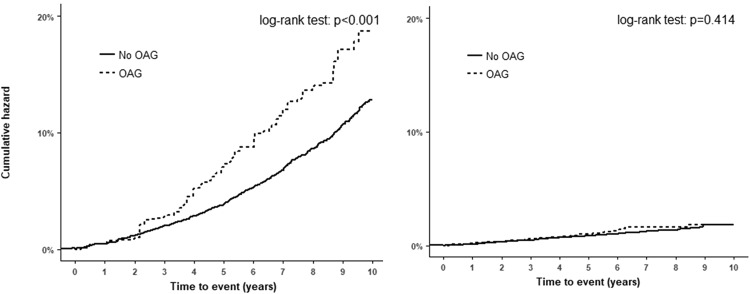
The Kaplan–Meier survival curves showed that the risk of developing AD among OAG patients was higher than in the comparison group (log-rank test, p < 0.001, (Fig. 1a), whereas no significant difference in the cumulative PD incidence was observed between the OAG and comparison groups (log-rank test, p = 0.414, Fig. 1b).
Figure 1.
Cumulative hazards of (a) Alzheimer’s disease and (b) Parkinson’s disease.
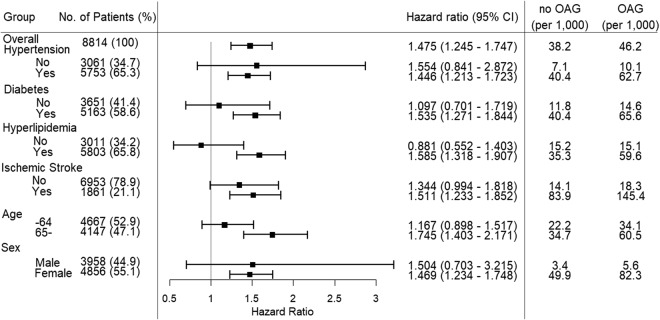
The incidence of AD per 1,000 person-years for the OAG and comparison groups was 38.2% and 46.2%, respectively (Fig. 2). In subgroup analyses based on multivariate Cox regression, the adjusted HR of AD in the OAG patients with diabetes mellitus was greater (HR = 1.535) than that in the subjects without diabetes mellitus (HR = 1.097). The risk of developing AD in the OAG group was higher in those with hyperlipidemia or ischemic stroke (HR = 1.585 and HR = 1.511, respectively) compared with those without hyperlipidemia or ischemic stroke (HR = 0.881 and HR = 1.344, respectively). The risk of developing AD in patients with OAG was higher in the older subgroup (age ≥ 65 years; HR = 1.745) than that in the younger subgroup (age < 65 years; HR = 1.167). Subgroups for hypertension and sex had a similar HR to that of the entire cohort regardless of the presence of the disease or gender.
Figure 2.
Subgroup analysis of Alzheimer’s disease based on multivariate Cox regression.
Discussion
Many studies have suggested that glaucoma is an age-related neurodegenerative disease, sharing many features of other well-known neurodegenerative diseases. The relationships between neurodegenerative diseases and glaucoma have been widely investigated, showing positive relationships between the disorders. However, there are still conflicting results regarding whether glaucoma is a risk factor for the development of neurodegenerative disorders. In this study, using longitudinal data from a nationwide cohort, we demonstrated an increased risk of neurodegenerative disease development in OAG patients during a 10-year follow-up period compared with a control group. Subgroup analyses revealed that primary OAG patients had a significantly higher risk of developing AD, whereas a non-significant association was found for PD. The relationship between AD and OAG was stronger in older patients. There was no significant (p = 0.222) difference in patients younger than 60 years; in patients over 70 years old, the relationship was stronger than in younger patients and the statistical significance was increased (HR = 2.614, p < 0.001). To our knowledge, this is the first nationwide population-based cohort study from South Korea on the association between OAG and the risk of AD and PD.
Experimental and clinical studies have demonstrated a strong link between AD and primary OAG. While the presence of extracellular Aβ plaques and neurofibrillary tangles consisting of phosphorylated Tau protein is a characteristic feature of AD, many experimental studies have revealed increased levels of Aβ and Tau protein in vivo and in vitro. A recent study showed an increased Aβ level in the optic nerve and RGC layer in glaucomatous animal models21,22, and tauopathy was also detected in the glaucomatous retina23. A recent study observed these AD-like pathologies in the lateral geniculate nucleus and primary visual cortex in a chronic hypertensive glaucoma monkey model24. However, the results of epidemiological studies are still conflicting. Retrospective registry-based studies conducted in Denmark and the United States found no increased risk of developing AD in OAG patients30,31,34. Conversely, other studies found a positive correlation between AD and OAG and found that patients with AD had a higher prevalence of OAG than controls35,36. In recent studies from Taiwan, patients with OAG had a 1.5-fold increased chance of AD37, and the prevalence of AD in OAG patients was higher than in the control group, implying that OAG is a significant predictor of AD in elderly patients29. Our findings from the Korean population are consistent with these studies supporting the significant relationship between primary OAG and subsequent AD development.
PD is a long-term neurodegenerative disease of the central nervous system that mainly affects the motor system13 and is the second most common neurodegenerative disease after AD, affecting approximately seven million people globally9. Previously, abnormalities in visual function have been reported in PD patients25 and numerous studies have reported changes in the retinal nerve fiber layer thickness26. One study found that patients with PD had a higher prevalence of OAG27. Subsequently, two studies examined whether primary OAG is an early manifestation of PD: one found a small correlation but a significant increase in the risk of PD (adjusted HR = 1.23)38, and the other found that the prevalence of PD did not increase in OAG patients29. In this study, we also examined whether OAG is an early manifestation of PD in Koreans, but found no evidence for this. AD and PD share neuroinflammation as a common pathogenic mechanism and the retina is an extension of the brain. Therefore, the neuroinflammatory response in the brain may also occur in the retina and may lead to the development of glaucoma39. However, these diseases are caused by the deposition of different protein aggregates in specific anatomical areas and neuronal cell death in one or more populations of neurons. In AD, beta-amyloid and neurofibrillary tangles consisting of phosphorylated Tau protein accumulate in the hippocampal and cortical neurons, while in PD, Lewy bodies consisting of alpha-synuclein accumulate in the nigrostriatal neurons14,39. These pathogenic differences may have caused the discordant results in the AD and PD groups in our study. Additional studies are needed to address this issue.
The results of our subgroup analyses suggested particular associations between AD and OAG, which, to our knowledge, have not been reported. In the OAG group, the risk of developing AD during the 10-year period was higher in OAG patients older than 65 years of age compared with those younger than 65 years of age. This relationship implies that the two disorders are mainly related to the neurodegeneration associated with aging. There were also different associations for males and females, with female OAG patients having a greater risk of developing AD than male patients. This was consistent with a report that not only was OAG more prevalent in women but also that the incidence of AD was higher in elderly women than in men40,41. Moreover, OAG patients with diabetes mellitus or hyperlipidemia had higher risks of developing AD. Although still controversial, numerous studies have identified diabetes mellitus and hyperlipidemia as risk factors for both OAG and AD7,42–45. Conversely, metformin and statins, which are the most prescribed medications for diabetes mellitus and hyperlipidemia, respectively, have shown protective effects on the neurodegenerative changes in both glaucoma and AD46–49, making it more difficult to conclude the exact relationships between the two comorbidities and OAG or AD. Hypertension and ischemic stroke are also related to either OAG or AD44,50–52, although we found similar HR values for the subgroups with or without hypertension or ischemic stroke.
There are several limitations to consider when interpreting our results. First, due to the inherent nature of the data used in our analyses, the diagnoses of OAG, AD, PD, and other comorbidities were based entirely on the KCD code system, and may be less accurate than diagnoses obtained through a standardized diagnostic process. To ensure the diagnosis of OAG, we included those who satisfied three inclusion criteria as OAG patients: the diagnostic code for OAG; the code for visual field tests; and prescriptions of antiglaucoma drugs. We believe that the OAG subjects included in our study had high specificity. Second, it is possible that the OAG patients may have been underestimated because the data are based on hospital visits. The data do not include initial patients who did not experience symptoms and patients who do not visit the hospital for economic reasons. However, the degree of underestimation is limited, due to the low cost and high accessibility of the Korean medical system, a government-run healthcare system including nearly the entire population of South Korea. Therefore, it is unlikely that inaccuracy and underestimation of the diagnosis affected the statistical results. Third, our data include only data for the Korean population, and there may be differences in other ethnic groups. Studies from Asian ethnicity have reported a positive association between OAG and AD28,29,37. By contrast, studies conducted in Denmark, the United Kingdom, and the United States showed that this was not relevant30,32,34. Further studies are required to reveal specific differences in different ethnic groups.
In conclusion, this 10-year nationwide cohort study demonstrated that OAG was associated with an increased risk of developing AD compared with the risk in the general population. However, there was no positive association between OAG and PD. In addition, OAG patients older than 65 years of age or female OAG patients were more likely to develop AD than those under 65 years of age or male OAG patients. The results of this study have clinical implication on current understandings on OAG and further add to the evidences that OAG is a type of neurodegenerative disease. OAG patients should be followed closely for the subsequent development of symptoms related to AD, with neurological examinations for an early diagnosis of AD.
Methods and Materials
Database and study sample
We used data from the NHIS-NSC 2002–2013. The Korean National Health Insurance Service (KNHIS) is a mandatory single medical insurer system that provides universal healthcare coverage in the Republic of Korea, and all citizens are obligated to enroll in the system. The KNHIS developed the National Health Information Database (NHID), which contains personal demographic records to collect insurance premium and subscription data for reimbursement. The NHIS-NSC sampled 1,025,340 individuals from among 46,605,433 individuals in the NHID in 2002 and followed them until 201353. The study design was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital, Gyeonggi-do, Korea. This study adhered to the tenets of the Declaration of Helsinki, and the need for written informed consent was waived.
Selection of glaucoma cases and controls
The index OAG cases were defined as patients who met all three of the following criteria: (1) diagnosed with primary OAG (Korean Classification of Diseases [KCD] code H401) as the main diagnosis code; (2) having a visual field test code (E6691); and (3) prescribed glaucoma medication. To exclude chronic OAG patients, we included patients who were diagnosed with OAG from 2004 to 2008. As a result, there were 1,587 index OAG cases. We excluded 118 patients who were diagnosed with AD (KCD codes F009, G300, G301, G308, and G309) or PD (code G20) before the OAG diagnosis, and ultimately enrolled 1,469 subjects.
Control subjects were selected from individuals who did not meet the OAG case criteria each year in a 1:5 ratio to glaucoma patients using propensity score with nearest neighbor matching54. Ultimately, 7,345 control subjects were included. Propensity scores were calculated using sociodemographic parameters, including age group (≤49, 50–59, 60–69, 70–79, ≥80), sex, income (≤40th, 41st–70th, ≥71st percentiles), residential area (urban, metropolitan cities, and cities, rural, all other areas), and Charlson comorbidity index (CCI; score <3, ≥3). We used the CCI to determine whether the subject visited medical facilities frequently55.
Outcomes and comorbidities
In the matched populations, we compared the incidence of AD and PD. Comorbid conditions included hypertension (codes I10–I15), diabetes (codes E10–E14), hyperlipidemia (codes E78.0–E78.5), and ischemic stroke (code I61) that were diagnosed between 2002 and 2008.
Statistical analysis
The baseline characteristics are shown as the mean and standard deviation or number and percentage. The chi-square test was used to compare the groups. Multivariate Cox proportional hazard regression analysis was performed to calculate the HR with the 95% confidence interval (CI) for estimating the incidence of AD or PD in the OAG patients. Three models were used to demonstrate HRs for developing subsequent AD or PD, which included crude model, minimal adjusted model 1 (adjusted for hypertension, diabetes mellitus, hyperlipidemia and ischemic stroke) and fully adjusted model 2 (adjusted for age, sex, residential area, income, CCI, hypertension, diabetes mellitus, hyperlipidemia and ischemic stroke). Subgroup analyses were conducted for age group (≥65 and <65), sex, and comorbidities. Survival curves for developing AD or PD were generated. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) and R 3.4.1 (R Project for Statistical Computing, Vienna, Austria).
Electronic supplementary material
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (Ministry of Education) (No. 2017R1D1A1B03029944). The sponsor or funding organization had no role in the design or conduct of this research.
Author Contributions
L.S.H. designed the study and M.Y., K.H.J. and L.S.H. wrote the main manuscript text. M.Y., P.Y.H., P.T.K., P.E., K.C.Y. and L.S.H. prepared figures and tables. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Jong Youn Moon and Hyung Jun Kim contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29557-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Resnikoff S, et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog. Retin. Eye Res. 2012;31:702–719. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- 5.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol. 1998;82:862–870. doi: 10.1136/bjo.82.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 2001;131:699–708. doi: 10.1016/S0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, et al. Vascular and metabolic comorbidities in open-angle glaucoma with low- and high-teen intraocular pressure: a cross-sectional study from South Korea. Acta Ophthalmol. 2017;95:e564–e574. doi: 10.1111/aos.13487. [DOI] [PubMed] [Google Scholar]
- 8.Nucci C, et al. Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog. Brain Res. 2015;221:49–65. doi: 10.1016/bs.pbr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 9.de Lau LML, Breteler MMB. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 10.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, et al. Biomarkers in Parkinson’s disease (recent update) Neurochem. Int. 2013;63:201–229. doi: 10.1016/j.neuint.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson’s disease. Prog. Neurobiol. 2002;68:325–340. doi: 10.1016/S0301-0082(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 13.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 14. Dickson, D. W. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb. Perspect. Med. 2 (2012). [DOI] [PMC free article] [PubMed]
- 15.Chan KY, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. 2013;381:2016–2023. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 16.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet390, 1211–1259 (2017). [DOI] [PMC free article] [PubMed]
- 17.Ghiso JA, Doudevski I, Ritch R, Rostagno AA. Alzheimer’s disease and glaucoma: mechanistic similarities and differences. J. Glaucoma. 2013;22(Suppl 5):S36–38. doi: 10.1097/IJG.0b013e3182934af6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wostyn P, Audenaert K, De Deyn PP. Alzheimer’s disease: cerebral glaucoma? Med. Hypotheses. 2010;74:973–977. doi: 10.1016/j.mehy.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013;13:12–15. doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Brown GC, Vilalta A. How microglia kill neurons. Brain Res. 2015;1628:288–297. doi: 10.1016/j.brainres.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, et al. Muscarinic activation attenuates abnormal processing of beta-amyloid precursor protein induced by cobalt chloride-mimetic hypoxia in retinal ganglion cells. Biochem. Biophys. Res. Commun. 2009;384:110–113. doi: 10.1016/j.bbrc.2009.04.080. [DOI] [PubMed] [Google Scholar]
- 22.McKinnon SJ. Glaucoma: ocular Alzheimer’s disease? Front. Biosci. 2003;8:s1140–1156. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- 23.Chiasseu M, et al. Tau Accumulation, Altered Phosphorylation, and Missorting Promote Neurodegeneration in Glaucoma. J. Neurosci. 2016;36:5785–5798. doi: 10.1523/JNEUROSCI.3986-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Z, et al. Elevated Intraocular Pressure Induces Amyloid-beta Deposition and Tauopathy in the Lateral Geniculate Nucleus in a Monkey Model of Glaucoma. Invest. Ophthalmol. Vis. Sci. 2017;58:5434–5443. doi: 10.1167/iovs.17-22312. [DOI] [PubMed] [Google Scholar]
- 25.Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci. 1990;13:296–302. doi: 10.1016/0166-2236(90)90113-O. [DOI] [PubMed] [Google Scholar]
- 26.Yu JG, et al. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One. 2014;9:e85718. doi: 10.1371/journal.pone.0085718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayer AU, Keller ON, Ferrari F, Maag KP. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am. J. Ophthalmol. 2002;133:135–137. doi: 10.1016/S0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 28.Su CW, Lin CC, Kao CH, Chen HY. Association Between Glaucoma and the Risk of Dementia. Medicine (Baltimore) 2016;95:e2833. doi: 10.1097/MD.0000000000002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin IC, et al. Glaucoma, Alzheimer’s disease, and Parkinson’s disease: an 8-year population-based follow-up study. PLoS One. 2014;9:e108938. doi: 10.1371/journal.pone.0108938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou Y, Grossman DS, Lee PP, Sloan FA. Glaucoma, Alzheimer disease and other dementia: a longitudinal analysis. Ophthalmic Epidemiol. 2012;19:285–292. doi: 10.3109/09286586.2011.649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessing LV, Lopez AG, Andersen PK, Kessing SV. No increased risk of developing Alzheimer disease in patients with glaucoma. J. Glaucoma. 2007;16:47–51. doi: 10.1097/IJG.0b013e31802b3527. [DOI] [PubMed] [Google Scholar]
- 32.Keenan TD, Goldacre R, Goldacre MJ. Associations between primary open angle glaucoma, Alzheimer’s disease and vascular dementia: record linkage study. Br. J. Ophthalmol. 2015;99:524–527. doi: 10.1136/bjophthalmol-2014-305863. [DOI] [PubMed] [Google Scholar]
- 33.Ekstrom C, Kilander L. Open-angle glaucoma and Alzheimer’s disease: a population-based 30-year follow-up study. Acta Ophthalmol. 2017;95:e157–e158. doi: 10.1111/aos.13243. [DOI] [PubMed] [Google Scholar]
- 34.Bach-Holm D, et al. Normal tension glaucoma and Alzheimer disease: comorbidity? Acta Ophthalmol. 2012;90:683–685. doi: 10.1111/j.1755-3768.2011.02125.x. [DOI] [PubMed] [Google Scholar]
- 35.Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur. Neurol. 2002;47:165–168. doi: 10.1159/000047976. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier AA, et al. Prevalence of glaucoma in hospitalized older adults with Alzheimer’s disease. Can. J. Neurol. Sci. 2014;41:206–209. doi: 10.1017/S0317167100016590. [DOI] [PubMed] [Google Scholar]
- 37.Lai SW, Lin CL, Liao KF. Glaucoma may be a non-memory manifestation of Alzheimer’s disease in older people. Int. Psychogeriatr. 2017;29:1–7. doi: 10.1017/S1041610217002617. [DOI] [PubMed] [Google Scholar]
- 38.Lai SW, Lin CL, Liao KF. Glaucoma correlates with increased risk of Parkinson’s disease in the elderly: a national-based cohort study in Taiwan. Curr. Med. Res. Opin. 2017;33:1511–1516. doi: 10.1080/03007995.2017.1322570. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez AI, et al. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017;9:214. doi: 10.3389/fnagi.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Curr. Opin. Ophthalmol. 2010;21:91–99. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vina J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J. Alzheimers Dis. 2010;20(2):S527–33. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 42.Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122:72–78. doi: 10.1016/j.ophtha.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 43.Lin HC, Chien CW, Hu CC, Ho JD. Comparison of comorbid conditions between open-angle glaucoma patients and a control cohort: a case-control study. Ophthalmology. 2010;117:2088–2095. doi: 10.1016/j.ophtha.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Polidori MC, Pientka L, Mecocci P. A review of the major vascular risk factors related to Alzheimer’s disease. J. Alzheimers Dis. 2012;32:521–530. doi: 10.3233/JAD-2012-120871. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, et al. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017;124:41–47. doi: 10.1016/j.diabres.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Lin HC, et al. Association of Geroprotective Effects of Metformin and Risk of Open-Angle Glaucoma in Persons With Diabetes Mellitus. JAMA Ophthalmol. 2015;133:915–923. doi: 10.1001/jamaophthalmol.2015.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koenig AM, et al. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis. Assoc. Disord. 2017;31:107–113. doi: 10.1097/WAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talwar N, Musch DC, Stein JD. Association of Daily Dosage and Type of Statin Agent With Risk of Open-Angle Glaucoma. JAMA Ophthalmol. 2017;135:263–267. doi: 10.1001/jamaophthalmol.2016.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geifman N, Brinton RD, Kennedy RE, Schneider LS, Butte AJ. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimers Res. Ther. 2017;9:10. doi: 10.1186/s13195-017-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoemann J, et al. Cerebral microinfarcts in primary open-angle glaucoma correlated with DTI-derived integrity of optic radiation. Invest. Ophthalmol. Vis. Sci. 2014;55:7241–7247. doi: 10.1167/iovs.14-14919. [DOI] [PubMed] [Google Scholar]
- 51.Rim TH, Lee SY, Kim SH, Kim SS, Kim CY. Increased incidence of open-angle glaucoma among hypertensive patients: an 11-year nationwide retrospective cohort study. J. Hypertens. 2017;35:729–736. doi: 10.1097/HJH.0000000000001225. [DOI] [PubMed] [Google Scholar]
- 52.Bae HW, et al. Systemic hypertension as a risk factor for open-angle glaucoma: a meta-analysis of population-based studies. PLoS One. 2014;9:e108226. doi: 10.1371/journal.pone.0108226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 54.Rassen JA, et al. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol. Drug Saf. 2012;21(Suppl 2):69–80. doi: 10.1002/pds.3263. [DOI] [PubMed] [Google Scholar]
- 55.Sundararajan V, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.