Abstract
Purpose
The aim of this study is to provide some useful insights into the treatments, outcomes, and prognostic factors of patients with breast cancer spine metastases (BCSM).
Methods
We report a retrospective case series analyzing 87 patients with BCSM who underwent surgical interventions. Independent prognostic factors for SMFS and OS were extracted using univariate and multivariate analyses, the Kaplan–Meier method and the Cox proportional hazards model.
Results
The mean time between primary diagnoses and spinal metastases was 46.8 (median 41, range 0–147 months) months. The analysis showed that lymph node metastasis (p = 0.043, HR 10.498, 95%CI 1.074–102.588) and estrogen receptor (ER) status (p = 0.004, HR 0.368, 95%CI 0.189–0.721) can significantly affect SMFS. Furthermore, visceral metastasis (p = 0.042, HR 2.383, 95%CI 1.032–5.501), multiple metastases (p = 0.035, HR 2.538, 95%CI 1.066–6.048) and post-op chemotherapy (p = 0.003, HR 0.312, 95%CI 0.144–0.675) have significant effects on OS. Lastly, patients identified as Luminal A subtype have longer OS.
Conclusions
Lymph node metastases and ER status are independent risk factors in predicting BCSM. Moreover, visceral metastasis, multiple metastases of the spine and post-op chemotherapy are independent prognostic factors. Luminal subtypes have higher rate, but late onset of spine metastases and prolonged survival.
Keywords: Breast cancer spine metastasis, Prognostic factors, Survival, Kaplan–Meier method, Perou's classification
1. Introduction
Breast cancer (BC) is a leading cause of morbidity among females in China. It was estimated that 270,000 Chinese were diagnosed with the disease in 2015 and approximately 70,000 people died of the disease [1], [2]. With the advances in detection and treatment of primary tumors, life expectancies of breast cancer patients are prolonged whereby metastases have become the leading cause of death [3]. The multidisciplinary treating protocol, which is a personalized combination of surgery and adjuvant therapies can prolong life expectancies of breast cancer patients significantly [4]. However, longer survival leads to greater incidence of tumor metastasis, including bone metastasis.
It is reported that over 65% of patients with advanced breast cancer develop osteolytic bone metastases which mostly occurred in the spine [5], [6], and histologically, the Luminal subtypes of BC have the best prognostic outcomes but are also more likely to be associated with bone metastases [7], [8]. Spinal metastases can cause pathological fractures and spinal cords compression, which lead to severe pain and neurological dysfunction, such as paraplegia and bladder and/or bowel dysfunction. These changes significantly impact on patients’ quality of life [9], leading to unfavorable prognoses. Idea treatment strategies for spinal metastatic diseases, including BCSM, requires multidisciplinary collaborations, which calls for specialists in surgery, oncology, radio-/chemo-therapy, pain management, rehabilitation and nursing [10], [11]. With the advances of surgical techniques and inplant instruments, aggressive interventions were becoming more available and accessible. Tumor excision, pain relief, mechanical stabilization and even regain of neurological functions could all be obtained immediately after surgical treatment, which provided an opportunity for further systematic therapies.
Several studies on outcomes and prognostic factors of patients with spinal metastases from heterogeneous types of primary tumors concluded some recommendations for treatment opinions. However, only a limited number of previous studies have specially focused on BCSM [12]. Moreover, these studies investigated a relatively small number of patients and limited follow-up except for the study by Sciubba et al. [13]. Moreover, most of these studies are based on North America and Western Europe, related researches for Asian people are missing. Furthermore, although Sciubba's and Tan's studies involved some receptor expression data of the tumor, the importance of prognostic and indicative value of pathological classification on BCSM are still under consideration [13], [14]. In order to provide some useful insights into the treatments, outcomes, and prognostic factors for this challenging disease, we retrospectively analyzed a series of 87 patients with BCSM in our spine tumor center.
2. Materials and methods
2.1. Patients
With the inclusion criteria being pathologically diagnosed spinal metastatic breast cancer that underwent surgical interventions in our department, a total of 87 consecutive hospitalized patients from January 2005 to December 2015 were reviewed. This study was approved by the hospital ethics committee, and informed consents were obtained from the participants.
Patients’ medical records, images and pathology reports were retrospectively reviewed and analyzed. All patients were followed every 3 months for the first 6 months after surgery, every 6 months for the following 1.5 years, and once a year thereafter. Patients were followed for at least 1 year or until death. Information including general conditions, local tumor control, new lesions onset, neurological status, current systematic treatment strategies are reviewed and documented. Final statuses (died of disease/alive with disease/no evidence of disease) were acquired through telephone interviews.
2.2. Treatment
All patients in this series received surgical interventions after thorough assessments. Surgery indications are as follow: (1) Confirmed spinal metastases along with intolerable pain and neurological defects. (2) Tumor was not significantly reduced or even enlarged after adjuvant therapies. (3) An estimated life expectancy of greater than 3 months. Individualized surgical strategy was decided for each patient mainly based on their Tomita, revised Tokuhashi and Spinal Instability Neoplastic Score (SINS). Postoperatively, all tumor samples underwent pathological analysis for diagnosis and classification. Postoperative adjuvant treatments were delivered to patients according to their pathological subtypes and individual status.
2.3. Statistical analysis
Quantitative data are described by means (median, range), and qualitative data are described as counts and percentages. A series of clinical factors as follow were analyzed to identify independent variables that could predict prognosis. Patient factors include age, sex, age of breast cancer diagnosis (age BCdiag), time between the primary diagnose and spinal metastasis (spine metastasis free survival, SMFS), primary tumor grade, menstrual history (postmenopausal status at primary diagnosis), visceral and lymph node metastatic status, preoperative Eastern Cooperative Oncology Group (ECOG) score and preoperative Frankel score. Tumor factors include tumor location and its pathological subtype. Treatment factors include surgical intervention, intraoperative blood loss, bisphosphonate treatment and adjuvant therapies used. Factors such as the Tomita, revised Tokuhashi and SINS scores were ruled out for the purpose of this study because they are considered to have similar or combined significance with factors already mentioned.
SMFS was defined as the date from primary tumor diagnoses to spine metastasis. OS was measured as the date from spine metastasis to cancer related death, or December 2016. Survival curves were constructed according to the Kaplan–Meier method, and the log-rank test was used to compare the survival curves. Univariate analysis was carried out to assess clinical prognostic factors for SMFS and OS. Factors with p values of <0.1 were subjected to multivariate analysis using the Cox proportional hazards model to further explore observed differences in SMFS and OS respectively to identify independent prognostic factors. A p value (two-sided) of <0.05 was considered significant. All analyses were carried out using SPSS for Windows, version 22.0.0 (SPSS, IBM corp., New York, USA).
3. Result
A total of 85 women and 2 men with a mean age of 52 (median 52, range 27–77) years were retrospectively reviewed (detailed in Table 1). The mean age BCdiag was 48 (median 48, range 27–73) years. The mean SMFS, including 9 patients who were diagnosed of breast cancer with bone metastasis prior to primary site, was 46.8 (median 41, range 0–147 months) months. The locations of spinal metastatic lesions were noted, including 20 on the cervical spine, 46 on the thoracic spine, 37 on the lumber spine and 11 on the sacrum. Among them, 58 patients have multi-level, connected or separated metastases.
Table 1.
Demographics and clinical characteristics of 87 patients with breast cancer spine metastases.
| Age (year) | Mean 52 | Median 52 | Range 27–77 |
| Sex (F/M) | 85 | 2 | |
| Age BCdiag (year) | Mean 48 | Median 48 | Range 27–73 |
| Time of symptom (week) | Mean 4.8 | Median 3 | Range 1–36 |
| SMFS (month) | Mean 46.8 | Median 41 | Range 0–147 |
| Primary surgery | 77 | 88.51% | |
| Bone metastasis first | 9 | 10.34% | |
| Tumor location | C | 20 | 22.99% |
| T | 46 | 52.87% | |
| L | 37 | 42.53% | |
| S | 11 | 12.64% | |
| Multiple | 58 | 66.67% | |
| ECOG | Mean 2.85 | Median 3 | Range 2–4 |
| Tomita | Mean 4.05 | Median 4 | Range 3–7 |
| Tokuhashi | Mean 11.46 | Median 12 | Range 6–14 |
| SINS | Mean 10.10 | Median 10 | Range 5–15 |
| Tumor excision/PVP | 78/9 | ||
| Blood loss (ml) | Mean 1471.8 | Median 1350 | Range 100–4500 |
| Frankel | Pre | Post | |
| A | 5 | 0 | |
| B | 5 | 6 | |
| C | 31 | 3 | |
| D | 45 | 28 | |
| E | 1 | 48 | |
| ER(+/−) | 33 | 30 | |
| PR(+/−) | 22 | 41 | |
| Her2(+/−) | 28 | 26 | |
| Subtypes (Luminal (A/B)/Her2/TNBC) | 34(8/26) | 13 | 11 |
| CA-153(+/−) | 40 | 5 | |
| E-cadherin(+/−) | 35 | 7 |
A total of 8 patients received percutaneous vertebroplasty (PVP) therapies while the others underwent tumor excision. Vertebrectomies (in 75 patients) or laminectomies (in 3 patients) with reconstruction were adopted for thoracic/lumbar vertebrae using a posterior approach and for cervical vertebrae using an anterior or anterior-posterior approach. The average blood loss was 1471.8 (median 1350, range 100–4500) ml.
All patients who underwent surgeries had their preoperative symptoms alleviated (detailed in Table 1) and no local recurrences were observed within 6 months. Most patients experienced smooth recoveries except 2 patients suffered from acute post-op hematomas who underwent immediate removal surgeries, and another 2 patients developed pleural effusions, which were managed with chest tube placement. Their outcomes were satisfactory.
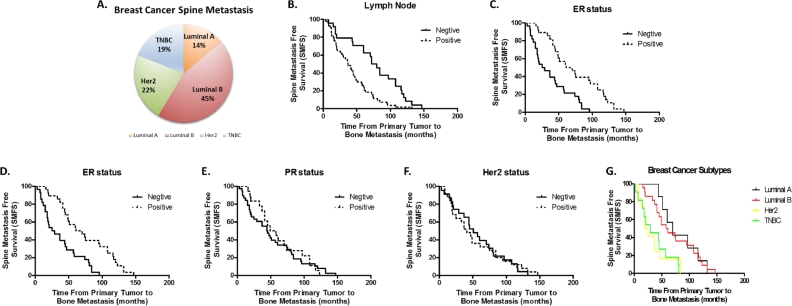
All or part of the ER, progesterone receptor (PR) and Her2 statuses were documented in pathological reports of 64 patients (Table 1). More specifically, 8 patients were subtyped as Luminal A (14%), 26 patients were subtyped as Luminal B (45%), 13 patients were subtyped as Her-2 (22%) and 11 patients were Triple negative (19%) (Fig. 1A).
Fig. 1.
Analysis of factors affecting BCSM. (A) Distribution of breast cancer subtypes with spine metastases. (B)–(C) Kaplan–Meier curves of Spine Metastasis Free Survival (SMFS) for (B) Lymph Node and (C) ER status. (D)–(G) Kaplan–Meier curves of SMFS for (D) ER, (E) PR, (F) Her2 status and (G) different subtypes of breast cancer.
3.1. Univariate and multivariate analysis of prognostic factors for SMFS
The 1-year, 3-year and 5-year SMFS rate were 85.9% ± 3.9%, 61.5% ± 5.5% and 35.9% ± 5.4% respectively, with a median SMFS of 44.0 ± 3.9 months (95%CI 36.3–51.7). Potential prognostic factors were listed in Table 2. Univariate analysis showed that patients with elder age (age BCdiag, p = 0.092), postmenopausal status at primary tumor diagnosis (p = 0.073), higher grade (p = 0.001) and lymph node positive (p < 0.001) of primary tumor had a significantly lower SMFS, while the ER positive (p < 0.001) ones had a prolonged SMFS.
Table 2.
Univariate and multivariate analysis of Spine Metastasis Free Survival (SMFS).
| Factors | N | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| Median survival (months) | p | B | HR(95% CI) | p | ||
| Age of primary tumor (≤47/>47, years) | 36/42 | 60/37 | 0.092* | NS | ||
| Postmenopausal (no/yes) | 45/31 | 60/37 | 0.073* | NS | ||
| Primary tumor grade (low/high) | 27/51 | 72/37 | 0.001* | NS | ||
| Lymph node (negative/positive) | 24/54 | 72/37 | <0.001* | 2.351 | 10.498(1.074,102.588) | 0.043* |
| Visceral metastasis (no/yes) | 65/13 | 43/58 | 0.962 | NI | ||
| Pre-op chemotherapy (no/yes) | 21/57 | 47/44 | 0.871 | NI | ||
| Pre-op radiotherapy (no/yes) | 47/31 | 49/42 | 0.458 | NI | ||
| Pre-op endocrine/targeted therapy (no/yes) | 57/21(18/3) | 42/60 | 0.124 | NI | ||
| ER status (negative/positive) | 28/28 | 25/61 | <0.001* | −0.998 | 0.368(0.189,0.721 | 0.004* |
| PR status (negative/positive) | 38/18 | 44/49 | 0.748 | NI | ||
| Her2 status (negative/positive) | 23/25 | 51/41 | 0.971 | NI | ||
Results from multivariate analysis confirmed that lymph node metastasis (p = 0.043, HR 10.498, 95%CI 1.074–102.588) and ER status (p = 0.004, HR 0.368, 95%CI 0.189–0.721) were risk factors for spinal metastasis. However, age BCdiag, menstrual history and primary tumor grade were not independent prognostic factors for SMFS according to the Cox proportional hazard analysis outcome. The Kaplan–Meier curves of SMFS using lymph node and ER status are shown in Fig. 1B and C.
Kaplan–Meier survival analysis was performed by categorizing patients according to their ER, PR and Her2 statuses. Although patients with Luminal subtypes had higher rates of spinal metastases, their SMFS were prolonged compared to those of the other two subtypes (Fig. 1D–G).
3.2. Univariate and multivariate analysis of prognostic factors for OS
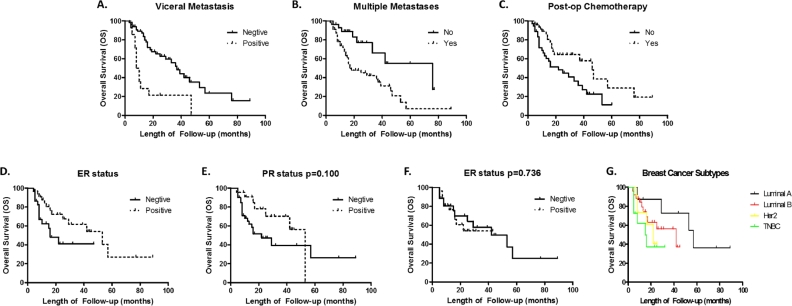
3 patients were lost to follow-up. In the remaining patients, 43 (51%) died of the disease. The 1-year, 3-year and 5-year OS rate were 77.6% ± 4.7%, 47.4% ± 6.7% and 19.9% ± 7.7% respectively, with a median OS of 36.0 ± 7.5 months (95%CI 21.3–50.7). Potential prognostic factors affecting OS are shown in Table 3. Statistical analysis using the Kaplan–Meier method showed that poorer OS was associated with patients whose age > 52 (p = 0.041); postmenopausal (p = 0.035); developed visceral metastasis (p < 0.001); multiple metastases in the spine (p = 0.006); ECOG score 3–5 (p < 0.001); pre-op Frankel score A–C (p = 0.002); and without post-op chemotherapy (p = 0.013), and bisphosphonate therapy (p = 0.001). These above factors, along with post-op endocrine/targeted therapy (p = 0.099), were used for further multivariable analysis, where visceral metastasis (p = 0.042, HR 2.383, 95%CI 1.032–5.501), multiple metastases (p = 0.035, HR 2.538, 95%CI 1.066–6.048) and post-op chemotherapy (p = 0.003, HR 0.312, 95%CI 0.144–0.675) remained highly significant. The Kaplan–Meier curves are shown in Fig. 2A–C.
Table 3.
Univariate and multivariate analysis of Overall Survival (OS).
| Factors | N | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| Median survival (months) | p | B | HR (95% CI) | p | ||
| Age (≤52/>52, years) | 42/42 | 42/18 | 0.041* | NS | ||
| Age of primary tumor (≤47/>47, years) | 38/46 | 33/36 | 0.447 | NI | ||
| Postmenopausal (no/yes) | 48/34 | 53/19 | 0.035* | NS | ||
| Primary tumor grade (low/high) | 28/51 | 29/33 | 0.989 | NI | ||
| Lymph node (negative/positive) | 24/55 | 37/33 | 0.701 | NI | ||
| Visceral metastasis (no/yes) | 65/14 | 37/8 | <0.001* | 0.868 | 2.383(1.032,5.501) | 0.042* |
| Pre-op chemotherapy (no/yes) | 27/57 | 39/33 | 0.757 | NI | ||
| Pre-op radiotherapy (no/yes) | 54/30 | 37/22 | 0.672 | NI | ||
| Pre-op endocrine/targeted therapy (no/yes) | 63/21(18/3) | 33/53 | 0.517 | NI | ||
| Cervical tumor (no/yes) | 66/18 | 36/33 | 0.945 | NI | ||
| Time of symptom (≤4/>4, weeks) | 57/27 | 36/29 | 0.714 | NI | ||
| Spine metastasis time (≤44/>44, months) | 37/39 | 37/18 | 0.116 | NI | ||
| Multiple metastases (no/yes) | 28/56 | 78/18 | 0.006* | 0.932 | 2.538(1.066,6.048) | 0.035* |
| ECOG Score (1,2/3–5) | 36/48 | 46/17 | <0.001* | NS | ||
| Pre-op Frankel score (A–C/D, E) | 39/45 | 16/39 | 0.002* | NS | ||
| Surgical excision or PVP (PVP/surgery) | 7/77 | 13/36 | 0.225 | NI | ||
| Blood loss (≤1500/>1500, ml) | 46/31 | 33/46 | 0.197 | NI | ||
| Post-op chemotherapy (no/yes) | 36/48 | 22/46 | 0.013* | −1.164 | 0.312(0.144,0.675) | 0.003* |
| Post-op radiotherapy (no/yes) | 63/21 | 29/46 | 0.200 | NI | ||
| Post-op endocrine/targeted therapy (no/yes) | 60/24(18/7) | 25/53 | 0.099* | NS | ||
| Bisphosphonate therapy (no/yes) | 25/59 | 16/46 | 0.001* | NS | ||
Fig. 2.
Analysis of factors affecting Overall Survival (OS). (A)–(C) Kaplan–Meier curves of OS for (A) Visceral Metastasis, (B) Multiple Metastasis and (C) Post-op Chemotherapy. (D)–(G), Kaplan–Meier curves of OS for ER (D), PR (E), Her2 (F) status and different subtypes of breast cancer (G).
In order to gain a deeper understanding of whether molecular markers, or in other words, breast cancer histopathology subtypes, affects OS rates, we did subgroup analysis as for SMFS. The results showed that patients of Luminal A subtypes were likely to have longer OS, and ER negative tumors metastasize to the spine faster than ER positive ones (p = 0.038). The presence or absence of the other two markers showed no significant differences (p = 0.100 and p = 0.736) (Fig. 2D–G).
4. Discussion
Bone metastases often present as challenges since the treatment strategies for primary breast tumors are well developed, while those for bone lesions are still unsatisfying [3]. Currently, surgical decompression followed with systematic treatments are widely accepted, but patients’ outcome variates and the median OS ranges from 18 to 36 months science 2002 [12]. In the present study, the median OS and the 1-year, 3-year and 5-year OS rate were all of dominance compared with published literatures. Our results were also in accordance with the latest published data concerning on outcomes of solid tumors spine metastases, of which the 1-year, 3-year and 5-year OS were 62, 42, and 25% with stable spinal bone metastases (SBM) and 57, 38, and 22% with unstable SBM [15].
Studies concerning on risk factors of BC bone metastases were limited, and less for BCSM. Chen et al reviewed a total of 2133 patients with BC, including 327 with bone metastases, of which mostly in the spine [6]. Their results indicated that axillary lymph node metastases and the concentrations of CA125, CA153, ALP and hemoglobin were the independent risk factors for bone metastases in patients with breast cancer. We also concluded from our series that lymph node metastasis is a critical factor affecting SMFS. This is logical since the sentinel node status is considered a critical indicator for tumor metastases [16]. Therefore, patients with lymphadenectasis should be monitored closely for spine metastases at indicated period.
A well-accepted classification scheme for breast cancer is based on the status of molecular markers ER, PR and Her2. Breast cancers can be classified as Luminal A (ER and/or PR positive, Her2 negative), Luminal B (ER and/or PR positive, Her2 positive, or ER and/or PR positive, Her2 negative, but Ki67 > 14%), Her2 (ER and PR negative, Her2 positive) and Triple negative (ER, PR and Her2 negative) [17]. Published literatures have shown that Luminal subtypes have better prognoses compared with other subtypes as they are responsive to adjuvant endocrine and targeted therapies, while in the meantime, higher incidence of bone metastases [8], [18], [19]. This is confirmed in our case series, where the Luminal subtypes had a higher rate of spine metastases and better outcomes. However, in our series, the ER positive and the Luminal subtypes showed a delayed onset of spine metastases, which is contrary with Chan-Seng's study [19]. Further studies based on larger numbers of patients are need to make the clarification of this confusion. While as far as it is concerned, reasons for out preliminary finding may due to longer survival broadening the time window for metastases. With additional time, the dormant breast cancer cells were reactivated in the bone microenvironment, leading to subsequent life-threatening resurgence [20].
Since the biological behavior of primary breast cancer is rather mild compared to other malignancies such as liver or lung cancer, the Tomita score system classifies it as ‘low-speed growth’ and represents 1 point [21]. Additionally, in the revised Tokuhashi score system [22], breast cancer represents 5 points, the highest among all primary tumor types. These suggest a relatively good prognosis for patients with BCSM and give solid indications for aggressive surgical interventions. However, Tan KA et al. argued that for hormone and Her2 receptor negative tumors, the Tokuhashi score should be decreased to 3 points due to their poor prognosis compared with receptor positive ones [14]. In our series, all 87 patients received appropriate surgical interventions and satisfactory outcomes. Although patients with negative receptors also had poor prognosis, we still advocate surgical interventions when patients’ general status were suitable for surgery. Poor prognosis of receptor negative tumors mostly due to lacks of effective systematic treatment agents, however, the advantages of surgical interventions are of prominent. Bone metastases and other organ metastases are unparalleled in the disease process. In the current study, visceral metastases were not associated with SMFS, however, visceral metastases significantly affected OS. This is consistent with the results from Tokuhashi score, Tomita score, and literatures on spine metastases from varied histology [23], [24], [25], because visceral metastases present the terminal stage of cancers.
Multiple metastases in the spine is another factor that directly affects OS, and also is an aspect in the scoring systems [22], [24]. Metastatic tumor masses that erode more than one vertebral segment usually reflect aggressive biological behavior, causing serious pain and instability. This can lead to great risk of pathological fractures followed by other devastating symptoms, thus shorter survival. Sciubba et al. reported that metastases in the cervical region are associated with a worse OS rate than those in other parts of the spine [26]. However, this study did not demonstrate such findings. Postoperative chemotherapy is the strongest indicator on OS rate while no other adjuvant therapies show significant association. Chemotherapy protocol such as combined cyclophosphamide, adriamycin and fluorouracil (CAF) has been proved to be effective in chemo-sensitive breast cancers [27]. More importantly, none of the preoperative interventions affects SMFS or OS. Conservative therapeutic protocols can hardly eradicate tumor cells since the bone microenvironment may provide firm shelter for tumor cells from harm [28]. Thus, innovations that specifically target bone metastases are urgently needed to deal with this intractable situation [29], [30]. This also emphasizes that bone surgery remains the keystone of the treatment process.
Bisphosphonates, such as zoledronic acid, are widely used in the treatment for osteoporosis and osteolytic bone tumors, preventing skeletal-related events (SREs) [31]. A previous study in our department had demonstrated that patients with metastatic non-small-cell lung cancer had better survival after bisphosphonate treatment [32]. However, the current study only reported significant impact on OS in the univariate, but not multivariate analysis. This was probably due to interactions amongst analyzed factors that led to statistically insignificant result. This question requires further investigation, at this stage, we still advocate for the routine use of bisphosphonate in the treatments of BCSM.
Several limitations of this study should be mentioned. Firstly, the results for SMFS may be more valid if the analysis can include random primary breast cancer patients with and without spine metastasis. However, because there are limited reports in this area, our results may still provide valuable information. Secondly, as a retrospective study, indications for therapies may act as confounding factors causing bias when interpreting our findings with respect to therapies.
5. Conclusion
We demonstrated that lymph node metastases and ER status are independent risk factors in predicting BCSM. And visceral metastasis, multiple metastases of the spine and post-op chemotherapy are independent prognostic factors for OS. Furthermore, Luminal subtypes had higher rate, but late onset of spine metastasis and prolonged survival. For patients with BCSM, a promising outcome could be achieved by surgical intervention followed by proper adjuvant therapies.
Conflict of interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2018.03.003.
Contributor Information
Chenglong Zhao, Email: ccyyzcl@smmu.edu.cn.
Zhenxi Li, Email: zhenxili@smmu.edu.cn.
Tielong Liu, Email: czyyltl@smmu.edu.cn.
Jianru Xiao, Email: jianruxiao83@smmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Baade P.D., Zhang S. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Cicek M., Oursler M.J. Breast cancer bone metastasis and current small therapeutics. Cancer Metastasis Rev. 2006;25(4):635–644. doi: 10.1007/s10555-006-9035-x. [DOI] [PubMed] [Google Scholar]
- 4.Turner N.C., Neven P., Loibl S., Andre F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet. 2017;389(10087):2403–2414. doi: 10.1016/S0140-6736(16)32419-9. [DOI] [PubMed] [Google Scholar]
- 5.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 6.Chen W.Z., Shen J.F., Zhou Y., Chen X.Y., Liu J.M., Liu Z.L. Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Sci. Rep. 2017;7(1):11325. doi: 10.1038/s41598-017-11700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 8.Smid M., Wang Y., Zhang Y., Sieuwerts A.M. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 9.Kelly M.L., Kshettry V.R., Rosenbaum B.P., Seicean A., Weil R.J. Effect of a randomized controlled trial on the surgical treatment of spinal metastasis, 2000 through 2010: a population-based cohort study. Cancer. 2014;120(6):901–908. doi: 10.1002/cncr.28497. [DOI] [PubMed] [Google Scholar]
- 10.Sciubba D.M., Gokaslan Z.L. Diagnosis and management of metastatic spine disease. Surg. Oncol. 2006;15(3):141–151. doi: 10.1016/j.suronc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Sciubba D.M., Petteys R.J., Dekutoski M.B., Fisher C.G. Diagnosis and management of metastatic spine disease. A review. J. Neurosurg. Spine. 2010;13(1):94–108. doi: 10.3171/2010.3.SPINE09202. [DOI] [PubMed] [Google Scholar]
- 12.Sciubba D.M., Goodwin C.R., Yurter A., Ju D. A systematic review of clinical outcomes and prognostic factors for patients undergoing surgery for spinal metastases secondary to breast cancer. Global Spine J. 2016;6(5):482–496. doi: 10.1055/s-0035-1564807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sciubba D.M., Gokaslan Z.L., Suk I., Suki D. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur. Spine J. 2007;16(10):1659–1667. doi: 10.1007/s00586-007-0380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan K.A., Tan J.H., Zaw A.S., Tan J.Y.H., Hey H.W.D., Kumar N. Evaluation of prognostic factors and proposed changes to the modified Tokuhashi score in patients with spinal metastases from breast cancer. Spine (Phila Pa 1976) 2018;43(7):512–519. doi: 10.1097/BRS.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 15.Wolf R.J., Foerster R., Bruckner T., Bostel T. Survival and prognostic factors in patients with stable and unstable spinal bone metastases from solid tumors: a retrospective analysis of 915 cases. BMC Cancer. 2016;16(528) doi: 10.1186/s12885-016-2571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsueh E.C., Hansen N., Giuliano A.E. Intraoperative lymphatic mapping and sentinel lymph node dissection in breast cancer. CA Cancer J. Clin. 2000;50(5):279–291. doi: 10.3322/canjclin.50.5.279. [DOI] [PubMed] [Google Scholar]
- 17.Perou C.M., Sørlie T., Eisen M.B., van de Rijn M. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 18.Sihto H., Lundin J., Lundin M., Lehtimäki T. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 2011;13(5):R87. doi: 10.1186/bcr2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan-Seng E., Charissoux M., Larbi A., Tétreau R. Spinal metastases in breast cancer: single center experience. World Neurosurg. 2014;82(6):1344–1350. doi: 10.1016/j.wneu.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Bartosh T.J., Ullah M., Zeitouni S., Beaver J., Prockop D.J. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs) Proc. Natl. Acad. Sci. USA. 2016;113(42):E6447–E6456. doi: 10.1073/pnas.1612290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita K., Kawahara N., Kobayashi T., Yoshida A., Murakami H., Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 22.Tokuhashi Y., Matsuzaki H., Oda H., Oshima M., Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30(19):2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 23.Meng T., Chen R., Zhong N., Fan T. Factors associated with improved survival following surgical treatment for metastatic prostate cancer in the spine: retrospective analysis of 29 patients in a single center. World J. Surg. Oncol. 2016;14(1):200. doi: 10.1186/s12957-016-0961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulino P.N.R., Janssen S.J., van Dijk E., Harris M.B. Development of a prognostic survival algorithm for patients with metastatic spine disease. J. Bone Joint Surg. Am. 2016;98(21):1767–1776. doi: 10.2106/JBJS.15.00975. [DOI] [PubMed] [Google Scholar]
- 25.Han S., Wang T., Jiang D., Yu Y. Surgery and survival outcomes of 30 patients with neurological deficit due to clear cell renal cell carcinoma spinal metastases. Eur. Spine J. 2015;24(8):1786–1791. doi: 10.1007/s00586-015-3912-3. [DOI] [PubMed] [Google Scholar]
- 26.Zadnik P.L., Hwang L., Ju D.G., Groves M.L. Prolonged survival following aggressive treatment for metastatic breast cancer in the spine. Clin. Exp. Metastasis. 2014;31(1):47–55. doi: 10.1007/s10585-013-9608-3. [DOI] [PubMed] [Google Scholar]
- 27.Gradishar W.J., Anderson B.O., Balassanian R., Blair S.L. Invasive breast cancer version 1. 2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Network. 2016;14(3):324–354. doi: 10.6004/jnccn.2016.0037. [DOI] [PubMed] [Google Scholar]
- 28.Neville-Webbe H.L., Cross N.A., Eaton C.L., Nyambo R. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res. Treat. 2004;86(3):269–279. doi: 10.1023/b:brea.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- 29.Coleman R.E. Adjuvant bone-targeted therapy to prevent metastasis: lessons from the AZURE study. Curr. Opin. Support Palliat. Care. 2012;6(3):322–329. doi: 10.1097/SPC.0b013e32835689cd. [DOI] [PubMed] [Google Scholar]
- 30.Coleman R., Gnant M., Morgan G., Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J. Natl. Cancer Inst. 2012;104(14):1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 31.Wilson C., Coleman R. Adjuvant bone-targeted therapies for postmenopausal breast cancer. JAMA Oncol. 2016;2(4):423–424. doi: 10.1001/jamaoncol.2015.5768. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y., Qu J., Wu J., Li S., Zhou Y., Xiao J. Metastatic spinal cord compression from non-small-cell lung cancer treated with surgery and adjuvant therapies: a retrospective analysis of outcomes and prognostic factors in 116 patients. J. Bone Joint Surg. Am. 2015;97(17):1418–1425. doi: 10.2106/JBJS.N.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.