Abstract
GALNT14 is a member of N-acetylgalactosaminyltransferase enzyme family and mediates breast cancer cell development. Here, we find that GALNT14 regulates multidrug resistance (MDR) in breast cancer. The expression of GALNT14 is associated with MDR in breast cancer. Higher level of GALNT14 facilitates MCF-7 cells to resist Adriamycin, whereas knockdown of GALNT14 sensitizes cells to Adriamycin. Moreover, the expression of GALNT14 associates with the expression of P-gp, the efflux pump localized on the cell membrane, which could be the underlying mechanism of how GALNT14 induces MDR. In-depth analysis shows that GALNT14 regulates the stability of P-gp. Finally, GALNT14 associates with higher level of P-gp in chemotherapy-resistant human breast cancer tissues. Taken together, our studies reveal a molecular mechanism in breast cancer MDR.
Introduction
Breast cancer is one of the most common malignant tumors and the main cause of cancer-related mortality of women worldwide [1]. Chemotherapy plays an important role in the treatment of breast cancer. Drug resistance to chemotherapy is a major huddle for breast cancer treatment [2], [3], [4], [5].
Multidrug resistance (MDR) is associated with different mechanisms including enhanced drug efflux out of cancer cells through ATP-binding cassette family (ABC) transporters, reduced drug influx, activation of DNA repair, modulation of apoptotic pathways, and alteration of target molecules [6], [7]. Among these complicated molecular mechanisms, overexpression of glycosylated P-glycoprotein (P-gp) in the drug-treated cancer cells is one of the major causes for the failure of cancer chemotherapy. P-gp is a member of the ABC protein superfamily [8], [9] and is encoded by multidrug resistance 1 gene (mdr1). Overexpression of glycosylated P-gp in cancer cells results in an active efflux of anticancer agents from cells, especially those P-gp-binding drugs, such as Adriamycin [10], [11], [12].
Glycosylation is one of the most abundant posttranslational modifications found on more than half of all secreted and cellular proteins. Aberrant glycosylation is closely related to tumor growth, metastasis, as well as resistance to chemotherapy [13]. N-glycosylation and O-glycosylation are the two major types of protein glycosylation in mammalian cells. The most common O-glycosylation is the mucin type, initiated by the transfer of N-acetylgalactosamine to the hydroxyl group of serine or threonine residue [14]. The biochemical reaction is catalyzed by a large family of N-acetylgalactosaminyltransferases (GALNTs), consisting of at least 20 members in humans, namely, GALNT1 to 20 [14]. Earlier studies have shown that mislocalization of GALNTs or dysregulation of GALNTs’ expression results in aberrant glycosylation in cancer cells, denoting the critical roles of GALNTs in regulating cancer behaviors. GALNTs have different substrates and patterns of expression. Thus, GALNTs play distinct roles in carcinogenesis and tumor metastasis [15]. For example, GALNT14 mediates the O-glycosylation of death receptor in pancreatic carcinoma, non–small-cell lung carcinoma, and melanoma cells [16]. GALNT2, sharing a high amino acid sequence homology with GALNT14, regulates the malignant characteristics of hepatocellular carcinoma by modulating O-glycosylation of EGFR [17]. GALNT6 glycosylates Mucin1, regulates proliferation of breast cancer cells [18], and is essential for O-glycosylation and stabilization of Mucin4 in pancreatic cancer cells [19]. Yet very few studies have explored the role of GALNTs in MDR of chemotherapy. One recent study showed that high GALNT6 expression correlates with increased recurrence, lymph node metastasis, and chemoresistance in ovarian endometrioid and clear cell carcinomas [20].
Despite our previous study showing that overexpression of GALNT14 plays a critical role in cell migration, invasion, and proliferation of breast cancer by stimulating the epithelial mesenchymal transition of breast cancer cells [21], little is known about the role of GALNT14 in the chemoresistance of breast cancer. Therefore, the aim of this study is to explore the effect of GALNT14 on chemoresistance and the underlying molecular mechanism. Here, we provide the first evidence that the higher expression level of GALNT14 is associated with Adriamycin resistance in breast cancer cells. Moreover, we find that the expression of GALNT14 associates with the expression of P-gp during chemoresistance. Thus, our studies not only provide a molecular mechanism of chemoresistance but also reveal GALNT14 as a target for reversing the MDR in breast cancer treatment.
Result
GALNT14-dependant Regulation of Chemoresistance in Breast Cancer Cells
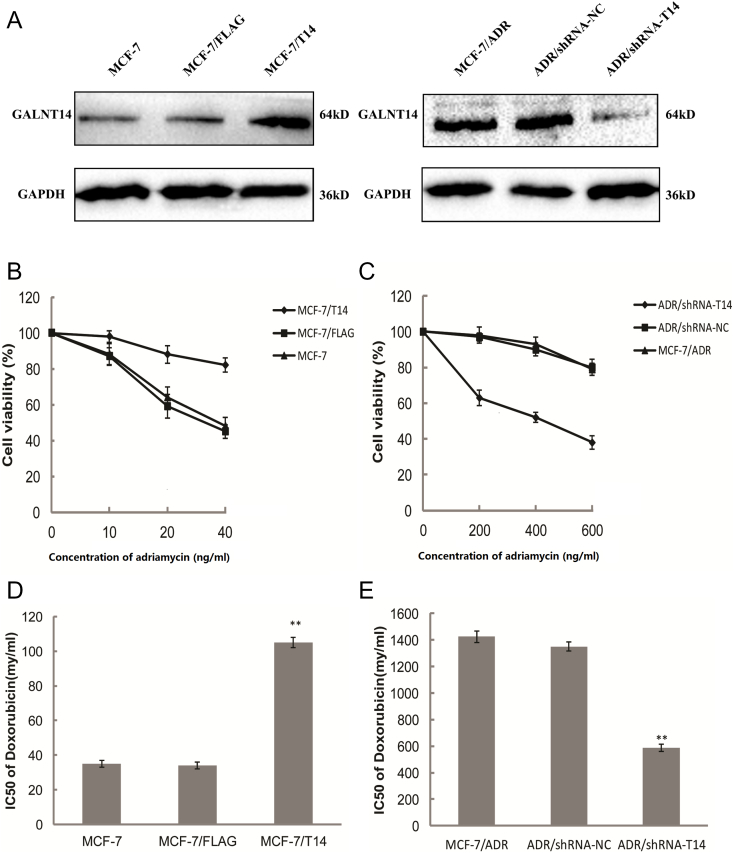
To examine the role of GALNT14 in chemoresistance, we chose MCF-7 breast cancer cells and MCF7 resistance to Adriamycin cells (MCF-7/ADR). We first examined the expression level of GALNT14 in both parental MCF7 cells and MCF-7/ADR cells. Notably, compared to that in the MCF7 cells, the mRNA level of GALNT14 was significantly elevated in MCF-7/ADR cells (Figure 1A). Consistently, using Western blot assays with anti-GALNT14 antibodies, we found that the protein level of GALNT14 was remarkably increased as well (Figure 1B), suggesting that higher level of GALNT14 is associated with MCF7 resistance to Adriamycin.
Figure 1.
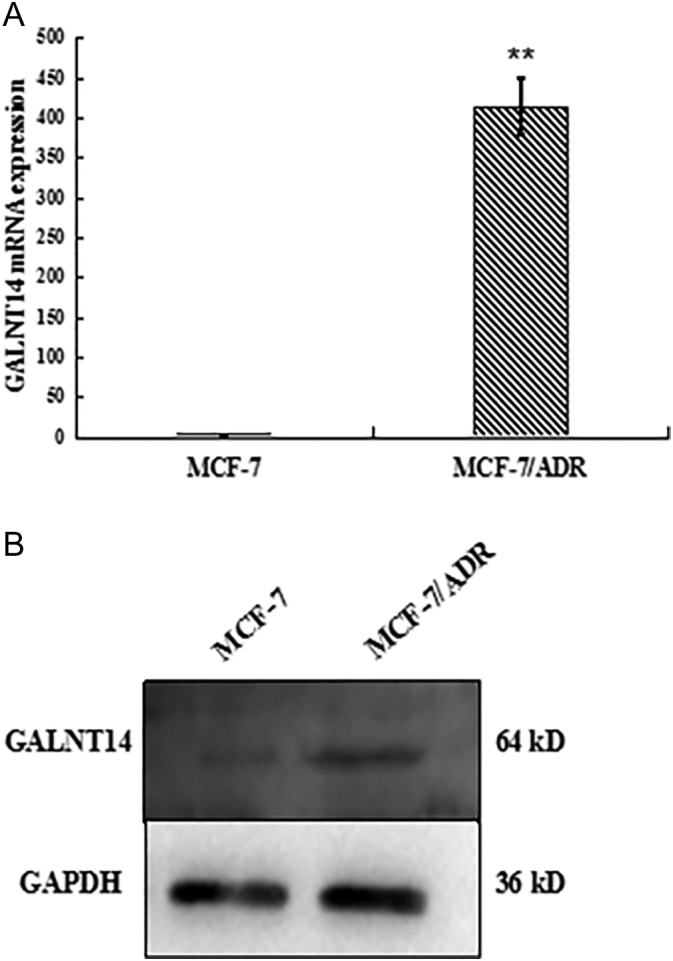
The levels of GALNT14 mRNA and protein are examined in MCF-7 and MCF-7/ADR cells. (A) GALNT14 mRNA expressions were assessed by quantitative PCR. (B) GALNT14 protein expressions were assessed by Western blot. GAPDH mRNA and protein served as loading controls. Bar graphs represent mean ± SEM of three independent experiments. **P< .01.
Next, to examine if GALNT14 regulates cell resistance to Adriamycin, we expressed the ectopic GALNT14 in the MCF-7 cells (MCF-7/T14) (Figure 2A). Compared to the control cells including the parental MCF-7 cells and MCF-7 cells only expressing the FLAG tag (MCF-7/FLAG), MCF-7/T14 cells were much less sensitive to Adriamycin in MTT assays (Figure 2B). Moreover, when we knocked down the expression of GALNT14 in the MCF-7/ADR cells (ADR/shRNA-T14), the cells were much more sensitive to the Adriamycin treatment compared to the parental MCF-7/ADR cells as well as the cells treated with control shRNA (ADR/shRNA-NC) (Figure 2, A and C). We also measured the IC50 of Adriamycin in these cells. In agreement with the results in the MTT assays, upregulation of GALNT14 in the MCF7 cells induced drug resistance, whereas downregulation of GALNT14 in the MCF-7/ADR cells sensitized the cells to the Adriamycin treatment (Figure 2, D and E). Taken together, the expression level of GALNT14 regulates Adriamycin resistance in the MCF-7 cells.
Figure 2.
GALNT14 regulates chemoresistance in breast cancer cells.
(A) Western blot analyses of the protein level of GALNT14 in GALNT14 overexpressed (FLAG-GALNT14) MCF-7 cells or GALNT14 knockdown (shRNA-GALNT14) MCF-7/ADR. (B, C) MCF-7 and MCF-7/ADR cells expressing ectopic GALNT14 or treated with shRNA-GALNT14 were further treated with Adriamycin for 48 hours. The cell viability was examined by MTT assays. (B) MCF-7 cells expressing GALNT14 (MCF7/T14) are resistant to Adriamycin. The parental MCF-7 or MCF-7 expressing FLAG tag empty vector was used as the negative controls. (C) MCF-7/ADR cells with knockdown of GALNT14 (ADR/shRNA-T14) are more sensitive to the Adriamycin treatment. The parental MCF-7/ARD or MCF-7 with control shRNA treatment (ADR/shRNA-NC) was used as negative controls. (D, E) The IC50 of Adriamycin was measured from triplicates of MTT assays with SPSS 22.0. Bar graphs represent mean ± SEM. **P < .01.
The association of Expression of GALNT14 with that of P-gp in Breast Cancer Cells
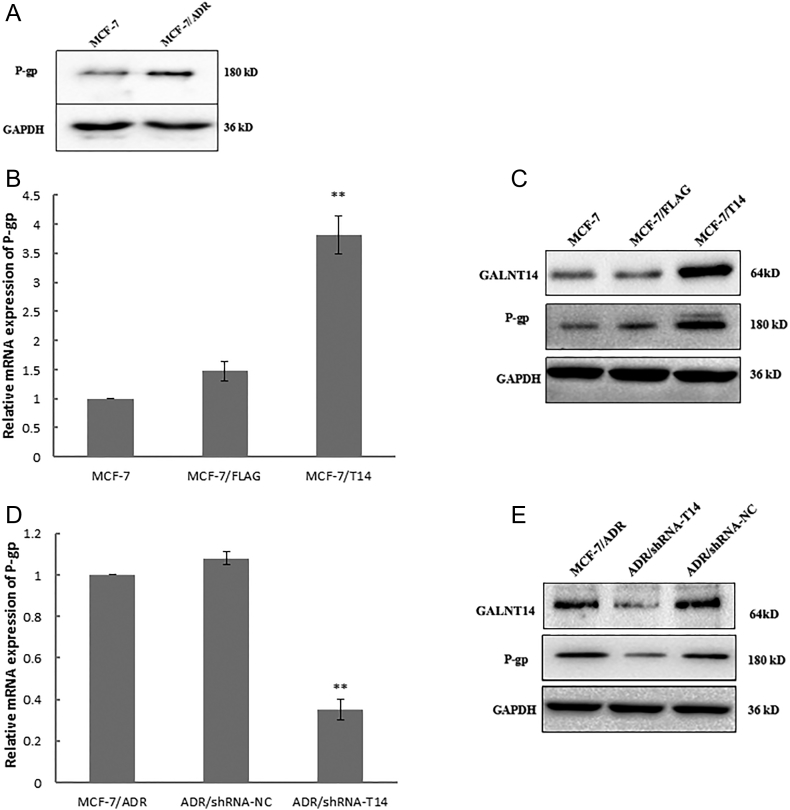
As overexpression of P-gp is one of the major causes to induce chemoresistance in cancer cells, we examine the expression level of P-gp in both MCF-7 cells and MCF-7/ADR cells. As shown in Figure 3A, similar to GALNT14, P-gp was also upregulated in the Adriamycin-resistant MCF-7 cells. Moreover, when we upregulated the expression of GALNT14 in the MCF-7 cells, both the mRNA and the protein levels of P-gp were increased (Figure 3, B and C). In contrast, when we downregulated GALNT14 by shRNA in the MCF-7/ADR cells, the protein level of P-gp was remarkably reduced (Figure 3, D and E). Taken together, these results indicate that the expression of GALNT14 associates with the protein level of P-gp in MCF7 cells.
Figure 3.
GALNT14 regulates the expression of P-gp in breast cancer cells. (A) The protein level P-gp is upregulated in MCF-7/ADR cells. Western blot was performed. GAPDH was used as the protein loading control. (B, C) The mRNA and the protein levels of P-gp were increased in MCF-7 cells expressing GALNT14. The parental MCF-7 or MCF-7 expressing FLAG tag empty vector was used as the negative controls. (D, E) Downregulated GALNT14 by shRNA in the MCF-7/ADR cells remarkably reduces the protein level of P-gp. The parental MCF-7/ARD or MCF-7 with control shRNA treatment (ADR/shRNA-NC) was used as negative controls. GAPDH mRNA and GAPDH protein served as loading controls. Bar graphs represent mean ± SEM of three independent experiments. **P < .01.
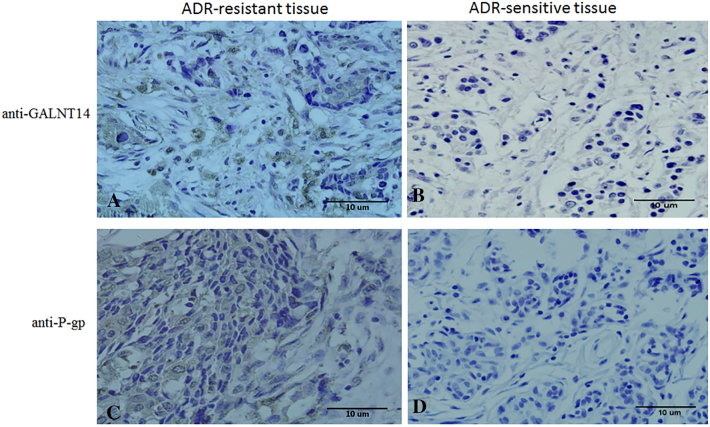
Next, we examined the expression of both GALNT14 and P-gp in human breast cancer samples. Microarrays containing 30 Adriamycin-sensitive cancer tissues and 30 Adriamycin-resistant cancer tissues were used. Five different visual fields were randomly selected and observed under the optical microscope (Figure 4). The results of the immunohistochemical study of total 60 breast tissues are summarized in Table 1. The expression of GLANT14 and P-gp was observed in 27/30 (90%) and 23/30 (77%) Adriamycin-resistant samples, respectively. Moreover, the level of the expression varies from moderate to strong. In contrast, the expressions of GALNT14 and P-gp were negative in 67% and 60% of chemosensitive cancer tissues. The remaining positive staining is weak, indicating the weak or little expression of GLANT14 and P-gp in Adriamycin-sensitive samples.
Figure 4.
The expression of GALNT14 and P-gp is associated with Adriamycin resistance in human breast cancer tissues. (A) The positive immunohistochemistry staining of GALNT14 in Adriamycin-resistant breast cancer samples. (B) GALNT14 expression was negative in Adriamycin-sensitive breast cancer tissues. (C) P-gp expression was positive in Adriamycin-resistant breast cancer tissues. (D) P-gp expression was negative in Adriamycin-sensitive breast cancer tissues. Magnification: ×400.
Table 1.
The Expressions of GALNT14 and P-gp in Human Breast Cancer
| Breast Cancer Tissue Type | Total Number | GALNT14 Expression |
P-gp Expression |
||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| ADR sensitive | 30 | 10 + (33%) | 20 (67%) | 12 + (40%) | 18 (60%) |
| ADR resistant | 30 | 27 ++ (90%) | 3 (10%) | 23 ++ (77%) | 7 (23%) |
| P value | <.01 | <.01 | |||
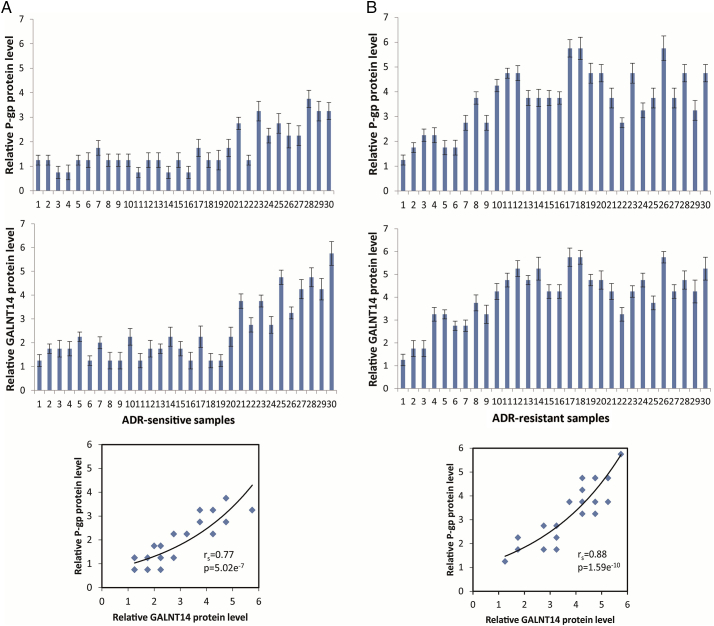
Following this line of evidence, we further examined individual tumor samples and found that although the average levels of GALNT14 and P-gp expression in the drug-resistant samples are relatively higher than those in the drug-sensitive samples, the relatively strong correlations were observed between the levels of GALNT14 and P-gp with rs= 0.77, P = 5.02e−7 in drug-sensitive group and with rs= 0.88, P = 1.59e−10 in drug-resistant group (Figure 5, A and B). It suggests that the expression levels of GALNT14 are highly associated with those of P-gp in both Adriamycin-sensitive and Adriamycin-resistant breast cancer samples (Figure 5). Thus, there is a significant correlation between the expressions of GALNT14 and P-gp and breast cancer chemoresistance. It has been shown that the expression of P-gp is considered as diagnostic marker for MDR. Thus, our results indicate that GALNT14 could also serve a biomarker for future clinical treatment for breast cancer.
Figure 5.
Correlation analysis of GALNT14 and P-gp expression levels. The protein expression levels of GALNT14 and P-gp were examined in 60 Adriamycin-resistant and Adriamycin-sensitive breast cancer samples using immunohistochemistry. GALNT14 expression level is highly associated the P-gp expression in both drug-resistant and drug-sensitive samples according to total immunostaining score. The data were summarized from three independent experiments. Data were presented as mean ± SD.
The Regulation of the Glycosylation and Stability of P-gp by GALNT14 in Breast Cancer Cells
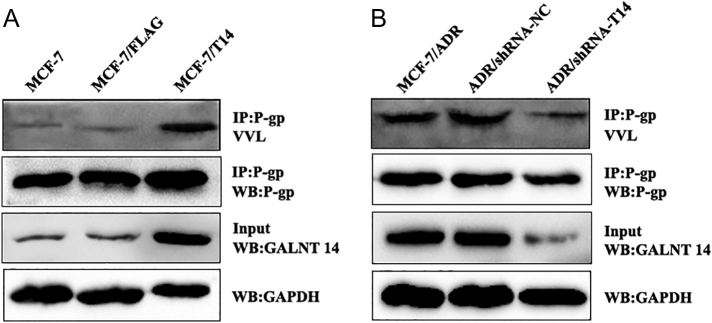
Since GALNT14 is a member of N-acetylgalactosaminyltransferase enzyme family and P-gp is a highly glycosylated protein, we asked if GALNT14 mediates P-gp glycosylation. The glycosylation status of P-gp protein in GALNT14-overexpressed and knockdown cells was examined by IP with anti-P-gp and lectin blot (Figure 6). The glycosylation of P-gp was reduced when GALNT14 was knocked down, while the glycosylation of P-gp was increased when GALNT14 was overexpressed. Thus, the results indicate that GALNT14 regulates the glycosylation status of P-gp.
Figure 6.
GALNT14 regulates the glycosylation level of P-gp. P-gp was IPed from GALNT14-overexpressed (FLAG-GALNT14) MCF-7 cells (A) or GALNT14 knockdown (shRNA-T14) MCF-7/ADR cells (B). The glycosylation of P-gp was examined with lectin lot, and cell lysates were blotted with the indicated antibodies.
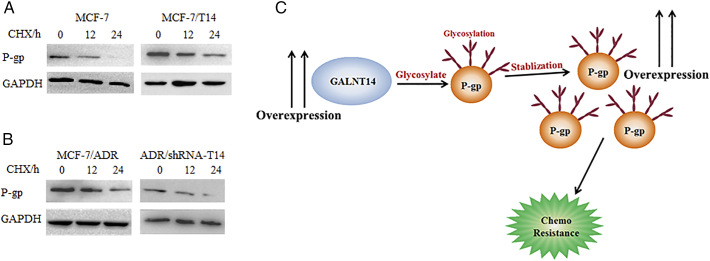
It has been shown that protein glycosylation regulates the protein stability [22]. Next, we examined whether GALNT14 regulates the stability of P-gp. We treated MCF7 cells and MCF-7/T14 with 50 μg/ml cycloheximide (CHX) to suppress protein synthesis. The protein level of P-gp was degraded in MCF-7 cells in 24 hours. In contrast, P-gp was relatively stable in the MCF-7/T14 (Figure 7A). Moreover, we performed the similar assays using both the MCF-7/ADR cells and ADR/shRNA-T14 cells. When GALNT14 was downregulated, P-gp was much less stable (Figure 7B). Taken together, our results suggest that GALNT14 facilitates P-gp protein stability in MCF-7 cells. As the high expression of P-gp is one of the major reasons for chemodrug resistance, our study proposes a novel pathway for understanding chemodrug resistance (Figure 7C).
Figure 7.
GALNT14 regulates the stability of P-gp. MCF-7 expressing GALNT14 (A) or MCF-7/ADR cells treated with shRNA-GALNT14 (B) were further treated with 50 μg/ml of cycloheximide (CHX) for indicated hours. Western blots were performed with anti–P-gp antibody to examine the stability of P-gp. GAPDH served as loading controls. (C) A model to show the mechanism by which the upregulated GALNT14 promotes MDR.
Discussion
MDR is a major obstacle to successful treatment of breast cancer; overexpression of P-gp occurs in almost 50% of human cancers [23]. Here, our studies reveal a novel pathway that GALNT14 regulates the stability of P-gp for MDR. Overexpression of GALNT14 promotes the glycosylation of P-gp, which in turn suppresses the degradation of P-gp in tumor cells. Elevated level of P-gp is likely to result in an increase in extracellular efflux and a decrease in the efficacy of chemotherapeutic agents [24].
GALNT14 mediates protein glycosylation that is one of the most common posttranslational modifications, and is often associated with tumor development and malignant transformation [25], [26]. Here our studies provide the first evidence that GALNT14 participates in MDR, another avenue in cancer research field. Our results further show that GALNT14 promotes the glycosylation of P-gp. It has been reported that the absence of oligosaccharides from P-gp decreases the efficiency of drug efflux due to misfolding of the protein that also increases the susceptibility of P-gp to degradation [27]. Loo and Clarke [28] also reported that the drug efflux activity of mutant MDR cell lines could be reduced by endoglycosidase-H treatment. We further determine that GALNT14-mediated glycosylation on P-gp plays a key role for its stability. Due to limited approach, we currently could not examine the functional significance of site-specific glycosylation. Future in-depth analysis on glycosylation of P-gp may reveal the detailed molecular mechanism by which GALNT14-mediated glycosylation facilitates MDR. Nevertheless, our studies not only uncover a novel role of GALNT14 in MDR but also suggest that GALNT14 could be a diagnostic and therapeutic target for breast cancer treatment.
In this study, our research focuses on breast cancer. It is likely that GALNT14-mediated P-gp glycosylation and stability are general phenomena in chemoresistance in other types of cancer. Thus, our study may open a new avenue in chemoresistance analysis, which should be further examined in other types of cancer in future.
Methods
Cell Culture
Human breast cancer cell lines were routinely cultured in DMEM (GIBCO) supplemented with 10% FBS (TransGenBiotech) at 37°C in an atmosphere containing 5% CO2. MCF-7/ADR was cultured in DMEM containing 10% FBS and 0.5 g/ml Adriamycin. All cultures were fed with fresh medium every 2 to 3 days.
Real-Time RT-PCR
Total RNA extraction from selected cultured cells. RNA from MCF-7 cells was used for detecting the expression levels of GALNT14 and GAPDH. Four micrograms (μg) of total RNA from each sample was used to detect real-time RT-PCR (QRT-PCR) products. PCR cycling conditions for all of the samples were as follows: 60 minutes at 42°C for reverse transcription, 3 minutes at 95°C for SYBR Premix Ex Taq II activation, and 40 cycles for the melting (95°C, 15 seconds) and annealing/extension (60°C, 1 minute) steps. GALNT14 and GAPDH primers for QRT-PCR were designed using the Beacon Designer. The sequences of the GALNT14 primers were as follows: forward, 5′-TAGCATCATCATCACCTTCCAC-3′; reverse, 5′-TTACAGTCATCAGGGTCAT TGC-3′. The sequences of the P-gp primers were as follows: forward, 5′-CCGTGGCAAACTGGTACTTT-3′; reverse, 5′-GACGCCAACATAGACCAC CT-3′. The sequences of the GAPDH primers were as follows: forward, 5′-GGTCGGAGTCAACGGATTTG-3′; reverse, 5′-ATGAGCCCCAGCCTTCTCC AT-3′. All QRT-PCR experiments were performed three times in duplicate in one 96-well plate. Using the comparative CT method, the resulting Ct values were converted to picogram quantities according to each standard curve. Then, the quantity of GALNT14 was normalized to GAPDH and subtracted from no reverse transcriptase controls. This value was then averaged for each duplicate.
Cytotoxicity Assay for Cell Viability Analysis
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma, USA) assay was used to assess the effect of GALNT14 silencing on the chemosensitivity of MDR cells and GALNT14 exogenous overexpressionon sensitive cells to Adriamycin. Cells were plated in a 96-well plate at 5×105 cells per well and then incubated with different concentrations of Adriamycin (Sigma, USA) for another 48 hours. The MTT assay was performed following the instructions. Optical densities value (ODs) were measured using a spectrometric absorbance of 570 nm on a Thermo Fisher Scientific microplatereader (USA). Dose-response curves were plotted, and IC50 values were calculated from three independent experiments.
Western Blot Assay
The cells were grown to 80% confluence in culture dishes containing 10% FBS media. The cells were then lysed in NP-40 and protease inhibitors. Twenty micrograms of total cell lysates was loaded onto a 10% Bis-Tris gel and separated using the electroporation system (BIO-RAD). After the proteins were transferred to a NC membrane, blocked with 5% fat-free milk for 1 hour, incubated the relevant primary antibodies (rabbit polyclonal antibody GALNT14: ab196245, Abcam, diluted 1:500); anti–P-glycoprotein (rabbit monoclonal antibody: ab170904, Abcam, diluted 1:1000); overnight at 4°C, followed by incubation with rabbit peroxidase-conjugated secondary antibodies. GAPDH was used as an internal control. The blots were developed by the enhanced chemiluminescence (ECL) detection system (Beyotime).
Immunohistochemistry
Adriamycin (doxorubicin)-resistant and -sensitive breast cancer tissue microarrays were obtained from Xinchao Biological Technology Co., Ltd., Shanghai. The thickness of each slice is 5 μm. All tumors were formalin fixed and paraffin embedded. After deparaffinizing the tissues, slides were microwaved for 15 minutes with 0.01 M citrate buffer (pH 6.0) to retrieve the antigens. Then, endogenous peroxidase was removed by the treatment of the tissues with 3% H2O2 followed by avidin-biotin blocking. To inhibit nonspecific binding of antibodies, the slides were treated with 10% normal goat serum. Incubation of the slides with anti-GALNT14 antibodies (bs-11018R, Bioss) and anti–P-gp antibodies (ab170904, Abcam), respectively overnight, add the corresponding secondary antibodies. After washing with PBS, staining was performed by twostep system (Zhongshan Bio, China). Reactions were revealed with 3,3'-diaminobenzidine (DAB, Sigma). The immunostaining frequency for each tumor was scored as follows: 0, for negative samples or 10% stained tumor tissue; 1, for samples stained between 10% and 39% of tumor tissue; 2, for samples stained between 40% and 79% of tumor tissue; and 3, for tumors with 80% of stained tumor tissue. Signal intensity was scored as strong (3, dark brown color), moderate (2, medium brown color), weak (1, light brown color), and null (0, no immunostaining). Total immunostaining score results from the multiplication of both parameters. Samples were scored totally as follows: strong (+++, total immunostaining score = 6-9), moderate (++, total immunostaining score = 3-4), weak (+, total immunostaining score = 1-2), and null (−, total immunostaining score = 0). Scores were established jointly by four observers under a multihead microscope.
Lectin Blot
Total protein was extracted from indicated cells. P-gp was immunoprecipitated from the cell lysates and subjected to Vicia Villosa Lectin (VVL) blot. After separation by 10% SDSPAGE, samples were transferred to NC membrane that was blocked in blocking solution (5% BSA in TBST). Membrane was incubated with a 0.2 μg/ml biotinylated VVL solution (B-1235, VECTOR laboratories) and then incubated with Streptavidin-HRP (S911, Thermo Scientific). The result was visualized by an ECL kit (Thermo Scientific).
Statistical Analysis
Statistical analysis was performed using SPSS 22.0. and Origin 7.5 (Origin Lab). All data were presented as mean±SD, and the Student’s t test was used to determine statistical significance. Pathological data were analyzed using the ANOVA test. P < .05 was considered statistically significant.
Spearman’s correlation was performed to determine the correlation between the expression level of GALNT14 and that of P-gp using an online software (Free Statistics Software, Office for Research Development and Education, version 1.1.23-r7, URL http://www.wessa.net/). A difference with a P < .05 was considered statistically significant.
Authors’ Contributions
C. W. and J. S. designed the experiments. J. S., Y. L., and Y. W. performed the experiments. J. S., Y. L.,Y. W., and X. Y. analyzed data. C.W. and J. S. wrote the manuscript. C. W. and X. Y. revised the manuscript. All authors read and approved the final manuscript.
Footnotes
This research was supported by National Natural Science Foundation of China (grant no. 31670812, no. 81672794), a grant for Returned Overseas Chinese Scholars of Hebei Province (no. CY201602), and Hundreds of outstanding talent innovation projects in Hebei Province (no. SLRC2017023), Natural Science Foundation of Hebei province (No. C2018201171).
Conflicts of interest: The authors declare that they have no conflicts of interest.
Contributor Information
Xiaochun Yu, Email: xyu@coh.org.
Chen Wu, Email: dawnwuchen@163.com.
References
- 1.Kunjachan S, Rychlik B, Storm G, Kiessling F, Lammers T. Multidrug resistance: physiological principles and nanomedical solutions. Adv Drug Deliv Rev. 2013;65(13-14):1852–1865. doi: 10.1016/j.addr.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo MT. Roles of multidrug resistance genes in breast cancer chemoresistance. Adv Exp Med Biol. 2007;608:23–30. doi: 10.1007/978-0-387-74039-3_2. [DOI] [PubMed] [Google Scholar]
- 3.Taheri M, Mahjoubi F, Omranipour R. Effect of MDR1 polymorphism on multidrug resistance expression in breast cancer patients. Genet Mol Res. 2010;9(1):34–40. doi: 10.4238/vol9-1gmr669. [DOI] [PubMed] [Google Scholar]
- 4.Patwardhan G, Gupta V, Huang J, Gu X, Liu YY. Direct assessment of P-glycoprotein efflux to determine tumor response to chemotherapy. Biochem Pharmacol. 2010;80(1):72–79. doi: 10.1016/j.bcp.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donmez Y, Gunduz U. Reversal of multidrug resistance by small interfering RNA (siRNA) in doxorubicin-resistant MCF-7 breast cancer cells. Biomed Pharmacother. 2011;65(2):85–89. doi: 10.1016/j.biopha.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 7.Yousefi B, Samadi N, Baradaran B, Shafiei-Irannejad V, Zarghami N. Peroxisome proliferator-activated receptor ligands and their role in chronic myeloid leukemia: therapeutic strategies. Chem Biol Drug Des. 2016;88(1):17–25. doi: 10.1111/cbdd.12737. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa S. Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol Pharm Bull. 2006;29(1):1–6. doi: 10.1248/bpb.29.1. [DOI] [PubMed] [Google Scholar]
- 9.Holland ML, Panetta JA, Hoskins JM, Bebawy M, Roufogalis BD, Allen JD, Arnold JC. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol. 2006;71(8):1146–1154. doi: 10.1016/j.bcp.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Haber M, Bordow SB, Haber PS, Marshall GM, Stewart BW, Norris MD. The prognostic value of MDR1 gene expression in primary untreated neuroblastoma. Eur J Cancer. 1997;33(12):2031–2036. doi: 10.1016/s0959-8049(97)00229-3. [DOI] [PubMed] [Google Scholar]
- 11.Keppler D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb Exp Pharmacol. 2011;201:299–323. doi: 10.1007/978-3-642-14541-4_8. [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y, Morita SY, Matsuo M, Ueda K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 2007;98(9):1303–1310. doi: 10.1111/j.1349-7006.2007.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18(10):750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 14.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaman EM, Brooks SA. The extended ppGalNAc-T family and their functional involvement in the metastatic cascade. Histol Histopathol. 2014;29(3):293–304. doi: 10.14670/HH-29.293. [DOI] [PubMed] [Google Scholar]
- 16.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13(9):1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 17.Wu YM, Liu CH, Hu RH, Huang MJ, Lee JJ, Chen CH, Huang J, Lai HS, Lee PH, Hsu WM. Mucin glycosylating enzyme GALNT2 regulates the malignant character of hepatocellular carcinoma by modifying the EGF receptor. Cancer Res. 2011;71(23):7270–7279. doi: 10.1158/0008-5472.CAN-11-1161. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, Fujikane T, Hirata K, Nakamura Y, Katagiri T. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70(7):2759–2769. doi: 10.1158/0008-5472.CAN-09-3911. [DOI] [PubMed] [Google Scholar]
- 19.Tarhan YE, Kato T, Jang M, Haga Y, Ueda K, Nakamura Y, Park JH. Morphological changes, cadherin switching, and growth suppression in pancreatic cancer by GALNT6 knockdown. Neoplasia. 2016;18(5):265–272. doi: 10.1016/j.neo.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin TC, Chen ST, Huang MC, Huang J, Hsu CL, Juan HF, Lin HH, Chen CH. GALNT6 expression enhances aggressive phenotypes of ovarian cancer cells by regulating EGFR activity. Oncotarget. 2017;8(26):42588–42601. doi: 10.18632/oncotarget.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huanna T, Tao Z, Xiangfei W, Longfei A, Yuanyuan X, Jianhua W, Cuifang Z, Manjing J, Wenjing C, Shaochuan Q. GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol Carcinog. 2015;54(10):1159–1171. doi: 10.1002/mc.22186. [DOI] [PubMed] [Google Scholar]
- 22.Sola RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 2009;98(4):1223–1245. doi: 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Wu X, Li J, Sun Y, Gao P, Zhang C, Zhang H, Zhou G. MDR1 (multidrug resistence 1) can regulate GCS (glucosylceramide synthase) in breast cancer cells. J Surg Oncol. 2011;104(5):466–471. doi: 10.1002/jso.21958. [DOI] [PubMed] [Google Scholar]
- 24.Chen T. Overcoming drug resistance by regulating nuclear receptors. Adv Drug Deliv Rev. 2010;62(13):1257–1264. doi: 10.1016/j.addr.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lattova E, Bartusik D, Spicer V, Jellusova J, Perreault H, Tomanek B. Alterations in glycopeptides associated with herceptin treatment of human breast carcinoma mcf-7 and T-lymphoblastoid cells. Mol Cell Proteomics. 2011;10(9) doi: 10.1074/mcp.M111.007765. [M111.007765-1-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Michele M, Marcone S, Cicchillitti L, Della Corte A, Ferlini C, Scambia G, Donati MB, Rotilio D. Glycoproteomics of paclitaxel resistance in human epithelial ovarian cancer cell lines: towards the identification of putative biomarkers. J Proteome. 2010;73(5):879–898. doi: 10.1016/j.jprot.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Fakla I, Hever A, Molnar J, Fischer J. Tomato lectin labels the 180 kD glycoform of P-glycoprotein in rat brain capillary endothelia and mdr tumor cells. Anticancer Res. 1998;18(4c):3107–3111. [PubMed] [Google Scholar]
- 28.Loo TW, Clarke DM. Reconstitution of drug-stimulated ATPase activity following co-expression of each half of human P-glycoprotein as separate polypeptides. J Biol Chem. 1994;269(10):7750–7755. [PubMed] [Google Scholar]