Abstract
Blockade of the programmed cell death 1-programmed cell death ligand 1 pathway is a new and promising therapeutic approach in Hodgkin lymphoma (HL). To our knowledge, the impact of soluble programmed cell death ligand 1 (sPD-L1) serum levels on HL patient prognosis has not yet been investigated. In this study, the prognostic value of sPD-L1 was assessed in patients with HL. We measured serum sPD-L1 levels and identified their prognostic value in 108 newly diagnosed HL patients using an enzyme-linked immunosorbent assay (ELISA). We found higher serum sPD-L1 concentrations in HL patients than in healthy controls. The best sPD-L1 cutoff value for predicting disease progression risk was 25.1674 ng/ml. The 4-year progression-free survival (PFS) rates for the high-sPD-L1 and low-sPD-L1 groups were 78.8% and 93.3%, respectively. Multivariate survival analysis showed that advanced stage and higher sPD-L1 levels (>25.1674 ng/ml) were independent prognostic factors for shorter PFS. In addition, higher sPD-L1 levels were positively correlated with advanced stage and negatively correlated with peripheral blood monocyte number. The serum sPD-L1 level is an independent prognostic factor for PFS in HL patients and may allow identification of a subgroup of patients who require more intensive therapy and who may benefit from anti-PD-1 agents.
Introduction
Hodgkin lymphoma (HL) is a rare cancer that originates from B lymphocytes and accounts for approximately 11% of all lymphoma cases and 0.5% of all cancers [1]. Standard treatment of newly diagnosed HL often involves a combination of multi-agent chemotherapy and radiotherapy, tailored to the stage of disease and the risk of relapse; this treatment can cure approximately 80% of patients [2]. Unfortunately, 20% of HL patients still relapse or develop refractory HL, for which effective treatment options are limited [3], [4]. Second-line salvage with high-dose chemotherapy (HDC) and autologous stem cell transplantation (auto-SCT) has become the standard care for refractory/relapsed HL, leading to long-lasting responses in approximately 50% of patients [5]. However, disease recurrence or progression after auto-SCT is associated with a very poor prognosis. Thus, alternative therapies, such as antibody-drug conjugates (anti-CD30) [6] and immune checkpoint blockade drugs (anti-PD-1 and anti-PD-L1) [7], [8] may be necessary. Furthermore, identification of patients with high risk of relapse is crucial in HL treatment.
Cancer cells have been shown to escape immune surveillance by up-regulating surface molecules that directly induce T-cell suppression [9]. These mechanisms are known as immune checkpoint pathways. Programmed cell death-1 (PD-1), an immune checkpoint expressed on the surface of T-, B- and natural killer (NK) cells, is indicative of this phenotype, and signaling through its ligands, programmed cell death ligand-1 (PD-L1) and programmed death ligand-2 (PD-L2), can attenuate signaling through the T-cell receptor (TCR) and lead to anergy/apoptosis and contribute to immune escape [10], [11]. Recent clinical trials have shown that PD-1-blocking antibodies can enhance immunity in solid tumors and several hematologic malignancies, resulting in durable clinical responses [12], [13], [14], [15], [16]. Nivolumab and pembrolizumab, PD-1-blocking antibodies, both received breakthrough therapy designation from the FDA for HL patients [17], [18], [19].
Previous studies have indicated that PD-L1 overexpression was associated with poor survival in most solid tumors and hematopoietic malignancies [20], [21]. However, the value of PD-L1 as a prognostic factor remains controversial [22]. There is an association between PD-L1 protein expression and relative genetic alterations in classical HL (cHL). For example, progression-free survival (PFS) has been shown to be significantly shorter for patients with 9p24.1 amplification, which up-regulates PD-L1 expression [23]. PD-L1 expression can be detected on the surface of tumor and immune cells by immunohistochemistry (IHC) [24] and in blood samples by enzyme-linked immunosorbent assay (ELISA) [25]. Serum sPD-L1 levels are reportedly higher in patients with malignant cancer than in healthy individuals, and high sPD-L1 was found to be a poor prognostic factor for hematopoietic malignancies in recent studies [26]. However, no investigations have assessed the relationship between serum sPD-L1 levels and HL patient prognosis. Therefore, the present study was conducted to address this issue. In addition, we also explored the correlation between serum sPD-L1 levels and the clinicopathological characteristics and immunologic features of HL patients.
Materials and Methods
Patients
In total, 108 consecutive patients diagnosed with HL and treated in Sun Yat-Sen University Cancer Center between May 2005 and April 2015 were enrolled in our study. The criteria included a primary diagnosis of HL, serum at diagnosis was available, and complete follow-up information. This study was approved by the Sun Yat-Sen University Cancer Center Research Ethics Board and informed consent for use of patient samples and publication was obtained from all patients.
Treatments and Response Evaluation
Patients were clinically staged according to the Ann Arbor staging system and treated with risk-adapted treatment strategies. First-line treatment involved ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) or COPP (cyclophosphamide, vincristine, procarbazine and prednisone) chemotherapy, and some advanced stage patients underwent BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) chemotherapy in standard doses. The treatment courses, which comprised four to eight cycles, were based on the chemotherapy response. Radiotherapy was conducted depending on patients’ age, risk group, residual tumor and response to chemotherapy. Treatment response was evaluated after every two cycles based on the World Health Organization (WHO) evaluation criteria. Routine follow-up imaging analyses were performed every 3 months for the first 2 years, every 6 months for the next 3 years, and annually (or whenever clinically indicated) thereafter.
Soluble PD-L1 Measurement
Patient serum was collected at diagnosis before treatment from all 108 patients and from 15 healthy individuals matched for sex and age with enrolled patients and stored as 500 ml aliquots at –80°C. sPD-L1 was measured using an enzyme-linked immunosorbent assay (PDCD1LG1 ELISA kit, USCN Life Science, catalogue: SEA788Hu) according to the manufacturer’s instructions. The minimum detectable concentration of sPD-L1 was 0.057 ng/ml. Each sample was analyzed in duplicate. The intra-assay and inter-assay coefficients of variation were below 20%. Briefly, samples and standards were added to a microplate precoated with a PD-L1-specific monoclonal antibody. After enzyme reagent and any unbound antibody were removed by washing, a substrate solution was added to the wells, Stop Solution was used to terminate color development, and the absorbance value was read at 450 nm using a spectrophotometer (Tecan, Mannedorf, Switzerland). The sPD-L1 concentrations were calculated using a standard curve, which was constructed using the standards provided in the kit.
Statistical Analysis
Receiver operating characteristic (ROC) curve analysis was performed to determine the best cutoff value for the sPD-L1 concentration [27]. In this ROC curve, the point with the maximum sensitivity and specificity was selected as the cutoff value. Correlations between sPD-L1 concentration and various clinicopathological parameters were assessed using a Mann-Whitney U test or Wilcoxon-matched test, and a chi-squared test or Fisher’s exact test was used for categorical values. Overall survival (OS) was defined as the time between the first day of diagnosis and the date of death from any cause; the follow-up of surviving patients was censored at their latest follow-up date. PFS was defined as the time between the first day of diagnosis and the date of disease relapse or progression; the follow-up of surviving patients was censored at their latest follow-up date. OS or PFS was analyzed using Kaplan-Meier curves, which were compared using log-rank tests. Multivariate prognostic analyses of OS or PFS were performed using the Cox proportional hazards regression model. All statistical analyses were performed using SPSS version 21.0. The results were considered statistically significant when P < .05.
Results
Patient Characteristics and Baseline Serum sPD-L1 Levels
Patient Characteristics
In total, 108 HL patients were enrolled in our study. The median age at diagnosis was 34.6 years of age (range, 4~76 years), and the study included more male patients (68 cases) than female patients (40 cases). Most patients were diagnosed with cHL, and 9 were diagnosed with nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL). According to the Ann Arbor stage, patients were divided into Stage I (7, 6.5%), Stage II (57, 52.8%), Stage III (24, 22.2%) and Stage IV (20, 18.5%). There were 44 patients with B symptoms and 11 with bulky disease (mediastinal mass ratio ≥10 cm or ≥0.33×). All patients received first-line chemotherapy or chemo-radiotherapy treatment.
Baseline Serum sPD-L1 Levels
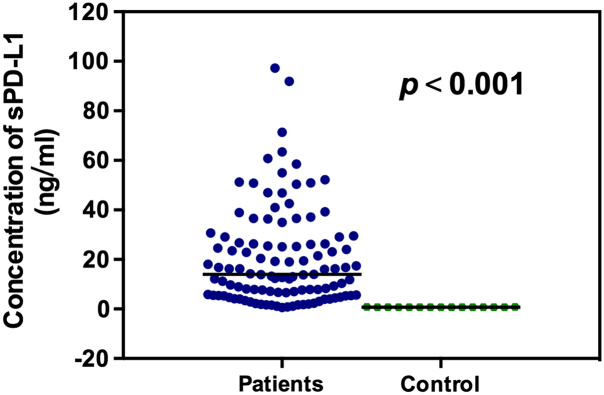
The mean sPD-L1 concentration for HL patients was 20.5039 ng/mL, which was much higher than that of healthy controls (0.722 ng/mL, P < .001; Figure 1).
Figure 1.
Serum sPD-L1 levels in patients with HL and in healthy controls.
Correlation of Serum sPD-L1 Levels with Clinical Characteristics and Inflammatory Markers
The Best Cutoff Value
An optimal cutoff value of 25.1674 ng/mL was defined by ROC curves for newly diagnosed HL patients, with an area above the curve (AUC) value of 0.643[95% confidence interval (CI) 0.473–0.814, P = .107] (Figure 2). According to this cutoff value, 33 patients (30.6%) were placed into the high-sPD-L1 group (>25.1674 ng/ml), and the remaining 75 patients (69.4%) were placed into the low-sPD-L1 group (≤25.1674 ng/ml). The sensitivity and specificity were 58.3% and 72.9%, respectively.
Figure 2.
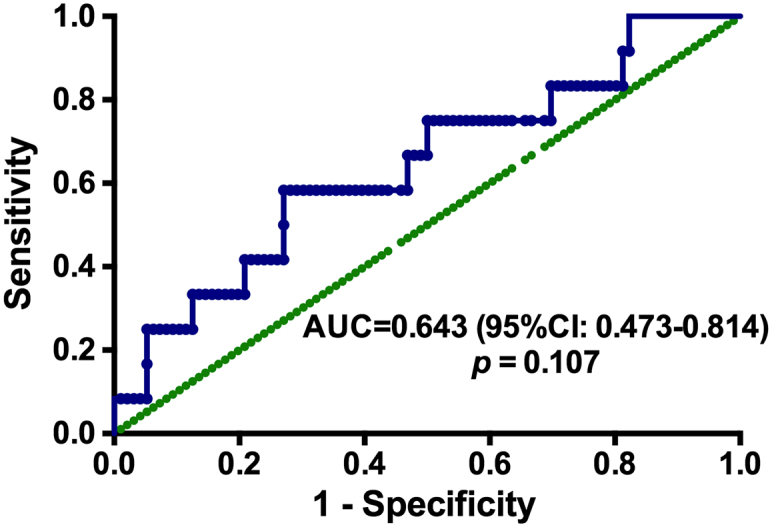
ROC curve analysis for the optimal cutoff point of serum sPD-L1 concentration.
Correlation Between Serum sPD-L1 Levels and Clinical Characteristics
There was no significant correlation between sPD-L1 level and gender, pathological type, clinical stage, bulky disease, age, a less than partial response (PR) after 2 cycles of chemotherapy or relapse rate(P > .05). However, higher sPD-L1 levels were positively correlated with advanced stage (P = .036) (Table 1).
Table 1.
Correlation Between Serum sPD-L1 Levels and HL Patients' Clinical Characteristics
| Characteristics | Serum sPD-L1 Level |
P | |
|---|---|---|---|
| High (>25.16 ng/ml) (n=33) |
Low (≤25.16 ng/ml) (n=75) |
||
| Age | .096 | ||
| ≤35 | 15 | 47 | |
| >35 | 18 | 28 | |
| Gender | .597 | ||
| Male | 22 | 46 | |
| Female | 11 | 29 | |
| Histological type | .345 | ||
| cHL | 29 | 70 | |
| NLPHL | 4 | 5 | |
| Ann Arbor Stage | .036 | ||
| Limited | 20 | 44 | |
| Advanced | 13 | 31 | |
| B symptoms | .258 | ||
| Yes | 17 | 27 | |
| No | 16 | 48 | |
| Bulky | .258 | ||
| Yes | 5 | 6 | |
| No | 28 | 69 | |
| TR after 2 cycles of chemotherapy | .091 | ||
| CR or PR | 14 | 45 | |
| Less than PR | 19 | 30 | |
| Relapse or not | .079 | ||
| Yes | 6 | 12 | |
| No | 27 | 63 | |
Abbreviations: cHL, classical Hodgkin lymphoma; NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; TR after 2 cycles of chemotherapy, treatment response after 2 cycles of chemotherapy; CR, complete response; PR, partial response; sPD-L1, soluble programmed death ligand-1.
The association between sPD-L1 levels and inflammatory markers was explored. As shown in Table 2, patients with a low peripheral blood monocyte number (P = .029) had higher serum sPD-L1 levels. There were no correlations between serum sPD-L1 levels and other inflammatory markers (P > .05).
Table 2.
Correlation Between Serum sPD-L1 Levels and Inflammatory Markers in HL Patients
| r | P | |
|---|---|---|
| WBC | 0.149 | .123 |
| LYM | 0.118 | .222 |
| NEU | 0.160 | .102 |
| MON | - 0.210 | .029 |
| HBG | 0.092 | .346 |
| PLT | 0.155 | .110 |
| Albumin | 0.125 | .210 |
Abbreviations: WBCs, peripheral blood white blood cells.
The Survival Rate of All HL Patients
The Survival Rate of All Newly Diagnosed HL Patients
The median follow-up time was 47 months (1-178 months). The 4-year PFS rate was 80%, with 12 patients relapsing with HL after treatment, and the estimated 4-year OS rate was 95%, with 4 patients dying of cancer. In addition, of all the patients, three developed a second cancer after HL treatment, including NK/T-cell lymphoma (1), infantile fibrosarcoma (1), and gastric carcinoma (1). With suitable and timely treatment, all 3 patients have survived thus far.
Correlation of Serum sPD-L1 Level and Other Clinical Factors With Survival
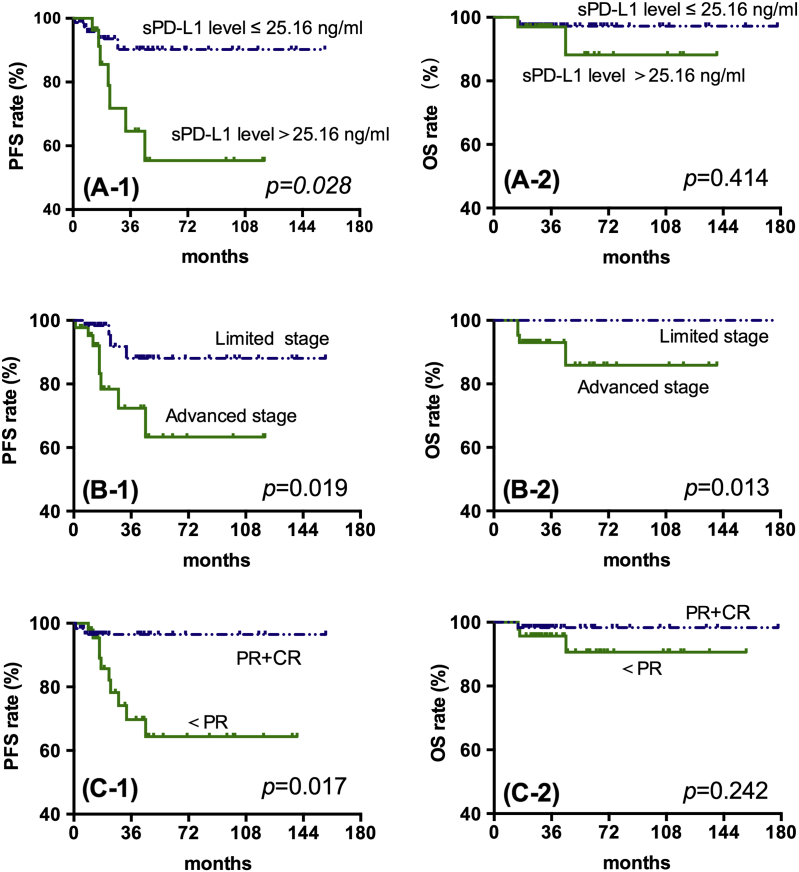
Patients with sPD-L1>25.1674 ng/ml had significantly lower 4-year-PFS compared with those with PD-L1≤25.1674 ng/ml (78.8% vs 93.3%, P = .028; Figure 3, A-1), but there was no difference in OS for different sPD-L1 levels (Figure 3, A-2). At the same time, advanced staged and<PR after two cycles of chemotherapy were also related to lower 4-year-PFS rates (Figure 3, B-1 and C-1). Specifically, the patients with lower sPD-L1 levels (≤25.1674 ng/mL), limited stage and complete response (CR) or PR after two cycles of chemotherapy had significantly longer PFS (P < .05). On the other hand, only patients with limited stage HL had a longer 4-year-OS (P = .013) (Figure 3, B-2).
Figure 3.
Kaplan-Meier survival analysis for all patients with Hodgkin Lymphoma.
A-1and A-2: PFS and OS of HL patients in low- or high-sPD-L1 groups.
B-1 and B-2: PFS and OS of HL patients with limited and advanced stages of disease.
C-1 and C2: PFS and OS of HL patients with different treatment responses.
Correlation Between Serum sPD-L1 Levels and Prognostic Factors
As shown in Table 3, patients with lower sPD-L1 levels (≤25.1674 ng/ml), limited stage and good treatment response (CR or PR) after 2cycles of chemotherapy had higher PFS rates (P < .05). However, histological type, B symptoms, bulky disease, and radiotherapy or not, did not affect long-term outcomes (P > .05). A multivariate survival analysis, including stage, treatment response, and sPD-L1 level, showed that limited stage, lower sPD-L1 levels (≤25.1674 ng/ml) and good treatment response (CR or PR) after 2cycles of chemotherapy were independent prognostic factors for longer PFS, but none were predictive of OS.
Table 3.
Univariate and Multivariate Analyses for PFS
| Parameters | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| Univariate Analysis |
Multivariate Analysis |
Univariate Analysis |
Multivariate Analysis |
|||
| P | HR (95%CI) | P | P | HR (95%CI) | P | |
| Histological type | .991 | .691 | 3.136 (3.058-3.255) | |||
| Stage | .013 | 1.284 (0.237-3.048) | .005 | .001 | 4.701 (4.163-5.449) | .182 |
| B symptoms | .171 | .105 | 1.304 (3.640-4.859) | |||
| Bulky | .859 | .192 | 0.895 (2.992-3.237) | |||
| Radiotherapy or not | .661 | .337 | 0.595 (3.643-3.993) | |||
| TR after 2 cycles of chemotherapy | .014 | 1.366(0.189-12.47) | .033 | .137 | 1.265 (0.954-4.722) | .174 |
| sPD-L1 level (>25.17ng/mL) |
.012 | 1.021(0.140-2.668) | .046 | .795 | 0.315 (3.636-5.784) | .182 |
Abbreviations: PFS, progression-free survival; TR after 2 cycles of chemotherapy, treatment response after 2 cycles of chemotherapy; sPD-L1, soluble programmed death ligand.
Discussion
We investigated serum levels of sPD-L1 in HL patients to identify any correlations with patient characteristics and survival outcomes. We found that serum sPD-L1 concentrations in HL patients were much higher than in healthy individuals. In HL patients, serum sPD-L1 levels were correlated with Ann Arbor stage and the immune-related factor peripheral blood monocyte number. Of note, PFS was significantly shorter for patients with an increased pretreatment serum sPD-L1 level. Furthermore, a Cox regression model, including serum sPD-L1 level, stage and treatment response after 2 cycles of chemotherapy, suggested that higher serum sPD-L1 level (>25.1674 ng/mL), advanced stage and poor early chemotherapy response were noteworthy adverse independent prognostic factors for PFS.
A prognostic role of serum sPD-L1 level had been reported for several tumor types, and the majority of data suggest that sPD-L1 is relevant to poor prognosis [28], [29], [30], [31], [32]. Among the studies, more than three directly compared sPD-L1 levels in patients with those in healthy controls and showed that cancer patients had significantly higher sPD-L1 levels [26], [33]. In addition, the work of Wang H et al. indicated that post-treatment sPD-L1levels were lower than pretreatment sPD-L1 levels in ENKTCL patients who achieved complete remission after standard treatment [34]. Here, we measured serum sPD-L1 levels in HL patients and in healthy volunteers and confirmed that serum sPD-L1 levels were higher in HL patients than in healthy controls.
In addition, we found that a higher sPD-L1 level was correlated with shorter PFS rate (78.8% vs. 93.3%, P = .028), but not correlated with OS rate, suggesting that sPD-L1 plays a key role in HL progression and chemotherapy resistance. The mechanisms by which elevated sPD-L1 levels contribute to poor prognosis in HL are not clear, but there are several possible explanations [35]. For example, activation of PD-1 impairs T-cell expansion and function by promoting IL-10 production [23]. PD-1 inhibits T-cell responses by promoting the induction and maintenance of Treg cells via down-regulation of phospho-Akt, mTOR, S6, and ERK2 and the concomitant up-regulation of PTEN, which induces drug resistance in HL cells [36], [37]. Further studies are needed to identify how PD1/PD-L1 signaling impacts HL prognosis.
Recent studies assessing the prognostic role of PD-L1 expression by IHC in various solid tumors and hematological malignancies have yielded mixed results [24], [38], [39], [40], [41], [42], [43], [44], [45], [46]. Pin wu et al. performed a meta-analysis of solid tumors and showed that PD-L1 overexpression was associated with poor 3-year OS in all studies analyzed, with the exception of one study on melanoma and lung cancer. Interestingly, few studies of the PD-1 and PD-L1 pathway in HL have been performed [47], [48]. HL usually exhibits diffuse and strong PD-L1 positivity in tumor (RS) cells based on IHC [49]. Paydas et al. found that PD-L1 was expressed on >5% of tumor cells in 70% of cHL and 54% of NLPHL, indicating that PD-L1 was not an independent risk factor for prognosis, but co-expression of PD-1 and PD-L1 was an independent risk factor [50]. In contrast, Young WhaKoh et al. reported that 75% of HL cases were positive for PD-L1 and identified significant adverse prognostic effects of PD-1 expression but not PD-1 and PD-L1 co-expression or PD-L1 expression alone [51]. Therefore, the prognostic value of PD-L1 overexpression in HL is still unclear. In addition, there are still some problems with the IHC method used to assess PD-L1 expression, resulting in a lack of clarity, including differences in the cutoff value of PD-L1 positivity (proportion of positive cells: 1, 5, 10%) and determination of positive cells (tumor cells and/or immune cells) [32]. Compared with IHC, there are many benefits to liquid biopsy, which is less invasive than tissue biopsy and may enable evaluation of immune status before and during treatment as opposed to assessing archival tissue samples [52], [53], [54], [55], [56]. Above all, we suggest that serum sPD-L1 level measured via ELISA rather than PD-L1 expression assessed with IHC may be a better prognostic factor for HL patients. However, the relationship between PD-L1 expression on IHC and serum sPD-L1 level still needs more research.
Additionally, we found that a higher sPD-L1 level is associated with advanced stage in HL patients, which may imply that sPD-L1 plays an important role or at least is a good cancer activity indicator. Generally, soluble forms of receptors are believed to typically be produced through proteolytic cleavage of membrane-bound proteins such as the sTNF and sB7-H3 or by translation of alternative spliced mRNA, as has been found with sCD86 and sCTLA-4 [57], [58], [59]. In addition, some research has found that addition of MMPI reduced the production of sPD-L1 on PD-L1-transfected cells [25]. Guangbo Zhang et al. observed that soluble B7-H3 binds to the B7-H3 receptor (B7-H3R) on activated T cells, which showed that sB7-H3 is a functionally active form [57]. These results indicate that the presence of sB7-H3 as an active form might significantly affect cell–cell interactions and responses to surface-bound B7-H3 [60]. However, the impact of the cleavage of membrane B7-H3 to its soluble form on cell–cell interactions and the role of B7-H3 in the pathological mechanisms of disease require further elucidation.
Our study also found that HL patients with lower peripheral blood monocyte number had a higher serum sPD-L1 level (P < .1), which is consistent with previous studies and further suggests that the presence of sB7-H1 may be one mechanism by which tumors compromise immune responses [25], [61], [62], [63]. Although the International Prognostic Score (IPS) remains a valid predictor of outcome for patients with advanced-stage disease, it is unsuccessful in stratifying patients with limited-stage cHL into subgroups with a poorer prognosis. Therefore, PD-L1 expression is a viable alternative prognostic factor to IPS in limited-stage cHL.
A previous study suggested that HL patients achieving CR after two courses of chemotherapy had a better prognosis, regardless of whether they were newly diagnosed or relapsed/refractory patients [64]. Our study suggested that good treatment response (CR or PR) was a favorable prognostic factor in HL but not an independent prognostic factor. These results illustrated that response-adapted strategies aiming to identify patients in whom therapy may be safely deescalated to minimize long-term toxicity are scientific and practical in clinical management.
Some limitations of our present study include its retrospective design, the short follow-up period for some recent cases, and the small sample size. Furthermore, there is a lack of cytogenetic and molecular abnormality analyses of patients in this study. Further investigations are warranted to clarify and understand the role of sPD-L1 as a prognostic or predictive biomarker of systemic chemotherapy as well as immune checkpoint inhibitors.
Conclusions
In summary, this is the first report measuring serum sPD-L1 protein levels in HL. Our results suggest that serum sPD-1, which can be easily measured in clinical practice, may be an important independent prognostic factor in cHL and useful for identifying a subgroup of patients with high risk for recurrence or progression. These results suggest a role of sPD-L1 in HL pathogenesis and offer new insights into potential therapeutic strategies.
Acknowledgements
We gratefully appreciate the participation of the patients in this study and we would like to thank all co-investigators for their contributions.
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the Sun Yat-Sen University Cancer Center Research Ethics Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Ansell SM. Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90:1574–1583. doi: 10.1016/j.mayocp.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Villasboas JC, Ansell SM. Recent advances in the management of Hodgkin lymphoma. F1000Res. 2016;5:768–776. doi: 10.12688/f1000research.8301.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baues C, Semrau R, Gaipl US, Brockelmann PJ, Rosenbrock J, Engert A, Marnitz S. Checkpoint inhibitors and radiation treatment in Hodgkin's lymphoma : New study concepts of the German Hodgkin Study Group. Strahlenther Onkol. 2017;193:95–99. doi: 10.1007/s00066-016-1050-4. [DOI] [PubMed] [Google Scholar]
- 4.Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, Vitolo U, Pulsoni A, Liberati AM, Specchia G. ABVD versus BEACOPP for Hodgkin's lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 5.Fedele R, Martino M, Recchia AG, Irrera G, Gentile M, Morabito F. Clinical options in relapsed or refractory Hodgkin lymphoma: An updated review. J Immunol Res. 2015;2015 doi: 10.1155/2015/968212. (1-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illes A, Jona A, Miltenyi Z. Brentuximab vedotin for treating Hodgkin’s lymphoma: an analysis of pharmacology and clinical efficacy. Expert Opin Drug Metab Toxicol. 2015;11:451–459. doi: 10.1517/17425255.2015.1007950. [DOI] [PubMed] [Google Scholar]
- 7.Villasboas JC, Ansell SM. Nivolumab for the treatment of classical Hodgkin lymphoma after failure of autologous stem cell transplant and brentuximab. Expert Rev Anticancer Ther. 2016;16:5–12. doi: 10.1586/14737140.2016.1121812. [DOI] [PubMed] [Google Scholar]
- 8.Onizuka M, Kojima M, Matsui K, Machida S, Toyosaki M, Aoyama Y, Kawai H, Amaki J, Hara R, Ichiki A. Successful treatment with low-dose nivolumab in refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Int J Hematol. 2017;106:141–145. doi: 10.1007/s12185-017-2181-9. [DOI] [PubMed] [Google Scholar]
- 9.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 11.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West H. Nivolumab as first line monotherapy for advanced non-small cell lung cancer: could we replace first line chemotherapy with immunotherapy? Transl Lung Cancer Res. 2014;3:400–402. doi: 10.3978/j.issn.2218-6751.2014.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rounds A, Kolesar J. Nivolumab for second-line treatment of metastatic squamous non-small-cell lung cancer. Am J Health Syst Pharm. 2015;72:1851–1855. doi: 10.2146/ajhp150235. [DOI] [PubMed] [Google Scholar]
- 14.Romero D. Nivolumab—an effective second-line treatment for NSCLC. Nat Rev Clin Oncol. 2015;12:685. doi: 10.1038/nrclinonc.2015.184. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and survival in solid tumors: A meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131403. (28478-28490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foran AE, Nadel HR, Lee AF, Savage KJ, Deyell RJ. Nivolumab in the treatment of refractory pediatric Hodgkin lymphoma. J Pediatr Hematol Oncol. 2017;39:e263–e266. doi: 10.1097/MPH.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 19.Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chin J Cancer. 2014;33:434–444. doi: 10.5732/cjc.014.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang D, Song Q, Wang H, Huang J, Wang H, Hou J, Li X, Xu Y, Sujie A, Zeng H. Independent prognostic role of PD-L1expression in patients with esophageal squamous cell carcinoma. Oncotarget. 2017;8:8315–8329. doi: 10.18632/oncotarget.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia H, Shen J, Hu F, Chen S, Huang H, Xu Y, Ma H. PD-L1 over-expression is associated with a poor prognosis in Asian non-small cell lung cancer patients. Clin Chim Acta. 2017;469:191–194. doi: 10.1016/j.cca.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Wang RX, Liu Y, Yang WT, Shao ZM. PD-L1 expression of the residual tumor serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Int J Cancer. 2017;140:1384–1395. doi: 10.1002/ijc.30552. [DOI] [PubMed] [Google Scholar]
- 23.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsao M.S., Le Teuff G., Shepherd F.A., Landais C., Hainaut P., Pirker R, Chevalier T, Graziano S, Kratze R. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol. 2017 Apr 1;28:882–889. doi: 10.1093/annonc/mdx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56:231–238. doi: 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Gouill S, Haioun C, Tarte K, Lamy T. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. 2014;28:2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 27.Mcneil BJ, Hanley JA. Statistical approaches to the analysis of receiver operating characteristic (ROC) curves. Med Decis Making. 1984;4:137–150. doi: 10.1177/0272989X8400400203. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, Wu X, Cheng X, Xing X, Du H. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelmeier F, Canli O, Tal A, Pleli T, Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–159. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Ouyang S, Wang J, Huang Z, Wang J, Liao l. Levels of programmed death-1 and programmed death ligand-1 in the peripheral blood of patients with oral squamous cell carcinoma and its clinical implications. Hua Xi Kou Qiang Yi Xue Za Zhi. 2015;33:529–533. doi: 10.7518/hxkq.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing YF, Zhang ZL, Shi MH, Ma Y, Chen YJ. The level of soluble programmed death-1 in peripheral blood of patients with lung cancer and its clinical implications. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:102–106. [PubMed] [Google Scholar]
- 32.Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, Okita NT, Kato K, Hamaguchi T, Yamada Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol. 2016;142:1727–1738. doi: 10.1007/s00432-016-2184-6. [DOI] [PubMed] [Google Scholar]
- 33.TOP . 2015. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Wang L, Liu WJ, Xia ZJ, Huang HQ, Jiang WQ, Li ZM, Lu Y. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget. 2016;7:33035–33045. doi: 10.18632/oncotarget.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roemer MG, Advani RH, Redd RA, Pinkus GS, Natkunam Y, Ligon AH, Connelly CF, Pak CJ, Carey CD, Daadi SE. Classical Hodgkin Lymphoma with reduced beta2M/MHC Class I expression is associated with inferior outcome independent of 9p24.1 Status. Cancer Immunol Res. 2016;4:910–916. doi: 10.1158/2326-6066.CIR-16-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, Apos O, Donnell E, Chapuy B, Takeyama K, Neuberg D. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Jiang J, Gao L, Wang X, Hu X. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res Ther. 2015;17:340. doi: 10.1186/s13075-015-0859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 39.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Fang YC, Chen XF, Liu GT. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droseser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Zhang SD, Mccrudden C, Chan KW, Lin Y, Kwok HF. The prognostic significance of PD-L1 in bladder cancer. Oncol Rep. 2015;33:3075–3084. doi: 10.3892/or.2015.3933. [DOI] [PubMed] [Google Scholar]
- 43.Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen M, Yip P, Yu B, Toole SA, Mccaughan BC. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 44.PD-L1 in renal cell cacinomon prognosis.pdf. 2016. pp. 1–9. [Google Scholar]
- 45.Qu HX, Zhao LP, Zhan SH, Geng CX, Xu L, Xin YN, Jiang XJ. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with esophageal squamous cell carcinoma: a meta-analysis. J Thorac Dis. 2016;8:3197–3204. doi: 10.21037/jtd.2016.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Y, Li S, Xu D, Chen S, Cai Y, Jiang W, Zhang X, Sun J, Wang K, Chang B. Prognostic value of programmed death-1, programmed death-ligand 1, programmed death-ligand 2 expression, and CD8(+) T cell density in primary tumors and metastatic lymph nodes from patients with stage T1-4N+M0 gastric adenocarcinoma. Chin J Cancer. 2017;36:61. doi: 10.1186/s40880-017-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh YW, Han JH, Park SY, Yoon DH, Suh C, Huh J. GLUT1 as a prognostic factor for classical Hodgkin's lymphoma: Correlation with PD-L1 and PD-L2 expression. J Pathol Transl Med. 2017;51:152–158. doi: 10.4132/jptm.2016.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vranic S, Ghosh N, Kimbrough J, Bilalovic N, Bender R, Arguello D, Veloso Y, Dizdarevic A, Gatalica Z. PD-L1 status in refractory lymphomas. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 50.Paydas S, Bağir E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94:1545–1552. doi: 10.1007/s00277-015-2403-2. [DOI] [PubMed] [Google Scholar]
- 51.Koh YW, Jeon YK, Yoon DH, Suh C, Huh J. Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumour Biol. 2016;37:7507–7514. doi: 10.1007/s13277-015-4622-5. [DOI] [PubMed] [Google Scholar]
- 52.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Publ Group. 2013:1–13. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 53.Xu LH, Chi XY, Li FY, Jia QT, Zha QB, He XH. Preparation and identification of human soluble sPD-L1 and its antibodies. Sheng Wu Gong Cheng Xue Bao. 2007;23:106–111. doi: 10.1016/s1872-2075(07)60008-9. [DOI] [PubMed] [Google Scholar]
- 54.Tu M, Chia D, Wei F, Wong D. Liquid biopsy for detection of actionable oncogenic mutations in human cancers and electric field induced release and measurement liquid biopsy (eLB) Analyst. 2016;141:393–402. doi: 10.1039/c5an01863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyun K-A, Kim J, Gwak H, Jung H-I. Isolation and enrichment of circulating biomarkers for cancer screening, detection, and diagnostics. Analyst. 2016;141:382–392. doi: 10.1039/c5an01762a. [DOI] [PubMed] [Google Scholar]
- 56.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 57.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao J, Pan X, Xing Y, Lu M, Chen Y, Shi M. Effects of soluble programmed death ligand 1 on regulating the proliferation of T lymphocytes and its mechanism. Zhonghua Yi Xue Za Zhi. 2015;95:449–452. [PubMed] [Google Scholar]
- 59.Cheng S, Zheng J, Zhu J, Xie C, Zhang X, Han X, Song B, Ma Y, Liu J. PD-L1 gene polymorphism and high level of plasma soluble PD-L1 protein may be associated with non-small cell lung cancer. Int J Biol Markers. 2015;30:e364–368. doi: 10.5301/jbm.5000170. [DOI] [PubMed] [Google Scholar]
- 60.Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peggs KS. Recent advances in antibody-based therapies for Hodgkin Lymphoma. Br J Haematol. 2015;171:171–178. doi: 10.1111/bjh.13578. [DOI] [PubMed] [Google Scholar]
- 62.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parsons K, Bernhardt B, Strickland B. Targeted immunotherapy for high-risk neuroblastoma—the role of monoclonal antibodies. Ann Pharmacother. 2013;47:210–218. doi: 10.1345/aph.1R353. [DOI] [PubMed] [Google Scholar]
- 64.Adams HJ, Nievelstein RA, Kwee TC. Systematic review and meta-analysis on the prognostic value of complete remission status at FDG-PET in Hodgkin lymphoma after completion of first-line therapy. Ann Hematol. 2016;95:1–9. doi: 10.1007/s00277-015-2529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]