Abstract
Background
HIV-infected subjects with suboptimal CD4 restoration despite suppressive combined antiretroviral treatment (cART) (immunodiscordant subjects) have been classically characterized after a variable period of time under cART. Recently, we have reported that an increased frequency of proliferating CD4 T-cells in these subjects is already present before the cART onset. The potential contribution of peripheral compensatory homeostatic proliferation (HP) is yet unknown. We aimed to analyze the expression of HP-related cellular markers on CD4 T-cells of immunodiscordant subjects before cART.
Methods
We analyzed the expression of OX40 and α4β7 on peripheral CD4 T-cells from immunodiscordant and control subjects (n = 21 each group) before cART initiation, and also on available follow-up samples (after 24 month of suppressive cART). Additionally, we tested the expression of these markers in an in vitro system for the study of human HP processes.
Results
Immunodiscordant subjects showed increased levels of OX40 and α4β7 on CD4 T-cells before cART initiation. While the cART tended to reduce these levels, immunodiscordant subjects still maintained comparatively higher levels of OX40 and α4β7 after 24 months under suppressive cART. These HP-related markers were upregulated in vitro during the human HP, especially during the fast HP.
Conclusion
Our results are compatible with exacerbated HP processes in immunodiscordant subjects, already before the cART onset.
Keywords: immunodiscordant response to combined antiretroviral treatment, low CD4 recovery, homeostatic parameters, homeostatic proliferation, OX40, α4β7, CD4 T-cell homeostasis, HIV
Introduction
Those subjects starting combined antiretroviral treatment (cART) with low CD4 counts and maintaining such low levels despite persistent suppression of viremia (immunodiscordant subjects with low-level CD4) constitute a particularly relevant population among HIV-infected subjects. They show increased rates of clinical complications than those subjects having a subsequent proper CD4-cell recovery (1, 2). Unfortunately, the mechanisms triggering such response still remain unknown.
These immunodiscordant subjects have been exhaustively studied after cART onset, when immunodiscordant response to cART has already taken place (3, 4), but less is known about immune alterations preexisting before cART initiation and, therefore, preceding such anomalous response to the treatment. Our group has been a pioneer in reporting immune alterations preceding the low CD4 recovery in this scenario (5, 6). Interestingly, these immunodiscordant subjects showed increased frequencies of proliferating CD4 T-cells already before cART initiation (5), denoting an early impairment of CD4 T-cell homeostasis, which deserves further research.
Globally, in T-cell lymphopenia conditions, a peripheral compensatory homeostatic proliferation (HP) of preexisting T-cells occurs (7). Two different types of HP have been extensively described: a commensal/self-antigen-driven fast HP and a cytokine-driven slow HP (mainly IL7 and IL15) (8), although both types of stimuli can synergize impacting each type of HP (9, 10). Recent works have found that fast HP specifically upregulates the tumor necrosis factor receptor superfamily member 4 (OX40) (11, 12), and that the blockade of OX40–OX40L interaction hamper the fast HP, taking place mainly in mesenteric lymph nodes (11). In this sense, a gut-homing imprinting (α4β7-integrins) has also been associated with the fast HP (11), but also with the slow HP in HIV-infected subjects after IL7 administration (13).
Our aim was to analyze the expression of OX40 and α4β7 on CD4 T-cells of immunodiscordant subjects before the cART onset. Additionally, in an in vitro model for the study the human HP we explored the modulation of these markers during such processes.
Materials and Methods
Subjects and Samples
Samples were selected from the Spanish AIDS Research Network Cohort (CoRIS) (14) and kindly provided by its HIV BioBank (15). Samples were processed following current procedures and frozen immediately after their reception. All patients participating in the study gave their informed consent and institutional ethical committees approved protocols. We selected pre-cART samples (up to 6 months before cART onset) of peripheral blood mononuclear cells from antiretroviral-naïve subjects who had started cART with <200 CD4/mm3 and did not reach 250 CD4/mm3 after 24 months of suppressive treatment (Low CD4 recovery subjects; LR-subjects), and subjects who also started cART with <200 CD4/mm3 but achieved more than 250 CD4/mm3 after 24-months of suppressive treatment (High CD4 recovery subjects, HR-subjects). Both groups of subjects were matched by sex, age, viral load, and baseline CD4 counts. Finally, 21 subjects per group were included (a flow chart of subject’s selection is shown in Figure S1 in Supplementary Material). Depending on sample availability, additional post-cART follow-up samples (24 ± 6 months on cART) were also analyzed.
For the in vitro T-cell culture experiments, buffy coats from HIV-1 and Hepatitis C Virus (HCV) seronegative anonymous donors from the Centro Regional de Transfusión Sanguínea de Sevilla-Huelva y Banco de Tejidos (Seville, Spain) were obtained. The study was approved by the local Ethical Committee and was performed according to the European Union guidelines and the Declaration of Helsinki.
Immunophenotyping
After thawing, cells were stained with surface antibodies (detailed in Supplemental Methods in Supplementary Material). Viable cells were identified using LIVE/DEAD fixable Aqua Blue Dead Cell Stain (Life Technologies, USA). The homing-related molecules α4-integrin and β7-integrin, and the TNFR-related molecule OX40 (CD134) were analyzed. A schematic diagram of the gating strategy used is shown in Figure S2 in Supplementary Material. Flow cytometry was performed on a LSR Fortessa (BD, USA). The analysis was performed using FlowJo version 9.2 (Tree Star) and data are expressed as frequencies (%).
In Vitro Culture of Human Lymphocytes
The in vitro culture used for the study of HP was performed as previously described (10). Briefly, untouched CFSE-dyed naïve CD4 T-cells were seeded in supplemented medium (RPMI 1640 supplemented with 10% fetal bovine serum, 1.7 mM glutamine, 100 µl/ml streptomicine and 100 U/ml peniciline), and stimulated with irradiated autologous aAPCs (1/1 ratio aAPCs/Naïve) and rIL7 (10 ng/ml). Finally, after 10 days of culture, cells were collected and immunophenotyped.
Statistical Analyses
Variables from in vivo analyses were expressed as median values and interquartile range. Mann–Whitney U and Wilcoxon rank test were used to analyze unpaired and paired comparisons, respectively. For analyses of cellular markers in the culture, the variables were expressed as mean and SD. A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for the Social Sciences software (SPSS 22.0, USA). Graphs were created using Prism version 5.0 (GraphPad Software, USA).
Results
Higher Levels of OX40 and α4β7 on CD4 T-Cells of LR-Subjects Before and After cART Instauration
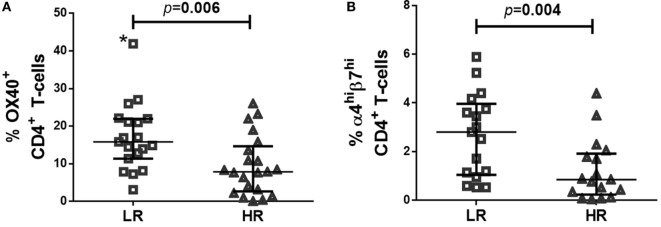
The baseline clinical characteristics of LR- and HR-subjects are described elsewhere (5). Most importantly, groups did not differ statistically in matched variables, nor did in other clinical variables as time from HIV diagnosis or route of transmission. Before cART initiation, LR-subjects showed higher frequencies of OX40+ CD4+ T-cells (Figure 1A), and a trend to higher frequencies of α4+β7+ CD4+ T-cells (LR = 9.2 [5.2–12.1]; HR = 5.0 [2.5–9.0]; p = 0.051), that reached statistical significance when comparing frequencies of α4hiβ7hi CD4+ T-cells (Figure 1B).
Figure 1.
Expression of OX40 and α4β7 in CD4 T-cells of LR-subjects at combined antiretroviral treatment onset. (A) Frequency of OX40+CD4+ T-cells; (B) Frequency of α4hiβ7hi CD4+ T-cells. After excluding outlier value (*) statistical significance was p = 0.009. Data were determined using multiparametric flow cytometry and are expressed as median value and interquartile range (IQR). Mann–Whitney test was used for the statistical significance calculation.
According to the classification criteria, after 24 months under cART, LR-, and HR-subjects had achieved 208 [141–234] and 429 [372–524] CD4 T-cells/mm3, respectively. Additionally, we longitudinally compared samples of 10 LR and 8 HR-subjects. Suppressive cART tended to reduce levels of OX40 (p = 0.069 and p = 0.036 for longitudinal comparisons of LR- and HR-subjects, respectively) and α4hiβ7hi (p = 0.093 and p = 0.116 for longitudinal comparisons of LR- and HR-subjects, respectively). However, LR-subjects still sustained higher levels than HR-subjects for both markers, OX40 (LR = 10.0 [11.3–7.1]; HR = 5.8 [7.4–5.0]; p = 0.034) and α4hiβ7hi (LR = 4.9 [6.4–2.7]; HR = 1.5 [3.7–1.0]; p = 0.025).
OX40 and α4β7-Integrins Are Upregulated During HP of Human CD4 T-Cells
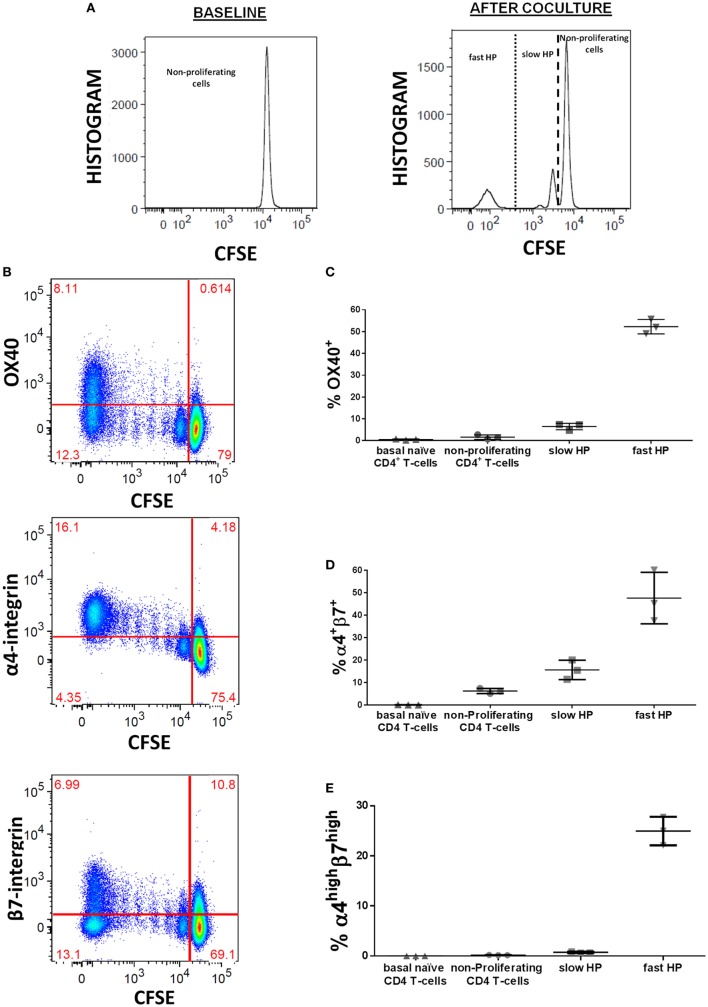
We explored the modulation of these HP-related markers previously associated with the HP using an in vitro cell culture specifically designed to study such processes in human T-lymphocytes (10). This culture system allowed us analyzing the expression of such markers as a consequence of each type of HP, the slow and the fast HP. Dilution of CFSE was used for identification of different types of HP (Figure 2A). Especifically, fast HP was defined as viable CD4 T-cells which fully diluted CFSE (and acquired memory phenotype), while slow HP was defined as viable CD4 T-cells which partially diluted CFSE (but maintained naïve phenotype) (10). We observed that OX40, α4-integrin, and β7-integrin were upregulated on human lymphocytes during the HP induced by homeostatic stimuli (Figure 2B). OX40 was mainly expressed in cells undergoing fast HP (Figure 2C). Similarly, when considering the coexpression of α4 and β7 as a single marker, a greater specificity in discriminating fast and slow HP was observed for α4highβ7high compared with α4+β7+ (Figures 2D,E). As it can be observed, the percentages of OX40+ and α4highβ7high cells were around the 50 and 30%, respectively, among those generated during the fast HP, whereas this percentage was lower than 10 and 2%, respectively, among those generated by slow HP.
Figure 2.
OX40 and α4β7 expression during the homeostatic proliferation (HP) process of human naïve CD4 T-cells. Untouched CFSE-stained naïve CD4 T-cells were culture in presence of rIL7 (10 ng/ml) and with irradiated autologous APCs (1/1 ratio): (A) representative CFSE histograms used for identification of different types of HP at baseline (0 day) and after 10 days of culture, (B) OX40, α4-integrin, and β7-integrin upregulation during HP, (C) frequencies of OX40 in the different types of HP subsets, (D) frequencies of α4+β7+ in the different types of HP subsets, (E) frequencies of α4highβ7high in the different types of HP subsets. Frequencies of both OX40 and α4β7 were calculated after background substation from baseline condition. Data are expressed as mean value and SD.
Discussion
We report here that HIV-infected subjects with low CD4 T-cell counts and immunodiscordant response to cART present higher expression of OX40 and α4β7 on CD4 T-cells before and after suppressive cART than their control group. These markers, which have been related to HP mainly in animal models, were also upregulated during HP of human T-lymphocytes, mostly during fast HP, similarly as it occurs in vivo.
While a higher expression of markers of activation, senescence, exhaustion, and proliferation on CD4 T-cells has been extensively reported in immunodiscordant subjects after cART (16–19), they only differed in a higher expression of Ki67 on CD4 T-cells when all these markers were compared before the cART onset (5). Interestingly, in this comparative study, microbial translocation, HCV coinfection, baseline viral load, or viral tropism did not differ either between groups (5). Since HP is an additional potential cause of a higher proliferative status, especially in lymphopenia scenarios (7) such as HIV-infection (20), we aimed here to explore the expression of HP-related markers in this particular context.
It is well-known that immunodiscordant subjects present higher frequencies of Treg (21, 22) and Th17 (23, 24) after cART initiation. Additionally, we have recently reported that increased frequencies of both Treg and Th17 are already present before cART initiation (5, 6) in these subjects. Interestingly, the fast HP is able to generate Treg (10, 25) and Th17 (11), and the costimulatory molecule OX40 is a key mediator in such process (11, 12). Moreover, we report here that OX40 is also upregulated specifically during the human fast HP. Thus, the higher frequency of OX40 + CD4 T-cells found now in immunodiscordants, together with the previous data, point to an exacerbation of the fast HP process in this scenario. Importantly, the OX40–OX40L axis has been associated with the production of proinflammatory cytokines such as IL6 by dendritic cells (26) and with inflammatory disorders (27), suggesting that HP, through OX40 upregulation, could also contribute to the higher inflammation observed in this scenario (5). Also of relevance, inflammatory cytokines such as IL6 have been reported to increase proliferation of memory CD4 T-cells but also lead to downregulation of CD127 (IL7 receptor-α) (28). Therefore, inflammatory cytokines induced by the OX40–OX40L interaction could theoretically exacerbate fast HP and likewise hamper slow HP. However, further studies are necessary to a better understanding of the specific relationship between inflammatory cytokines and HP.
The T-cell trafficking from blood to the gut-associated lymphoid tissue (GALT) is mediated by the β7- and α4-integrins among other homing-molecules (29, 30). Here, we have found a higher frequency of α4highβ7high CD4 T-cells in immunodiscordant subjects before and after suppressive cART. Using an in vitro system, we previously reported that both, fast and slow human HP upregulate β7-integrin (10). Additionally, we have now observed that human HP also upregulates α4-integrin and that α4+β7+ CD4 T-cells, but especially α4highβ7high CD4 T-cells, are mainly generated during fast HP. Also relevant, immunodiscordant subjects before cART initiation did not show higher levels of peripheral IL7 (5), the main cytokine driving slow HP. Altogether is consistent with the fast HP as the more plausible HP mechanism leading to the increased expression of α4β7 on CD4 T-cells in this particular context. Interestingly, α4β7-integrins favor the HIV colonization of GALT and the establishment of mucosal HIV reservoir (31). In this line, the higher expression of α4β7 in CD4 T-cells recently reported in a different cohort of immunodiscordant subjects under suppressive cART, was correlated with a higher proviral HIV load (24). Additionally, the expression of OX40 on CD4 T-cells has been associated with a high metabolic activity which is ultimately associated with a higher permissibility to HIV infection (32), and HP process itself has been also associated with higher amounts of HIV provirus (33). Therefore, all these data suggest that the higher HP before of cART onset in immunodiscordant subjects could imply a higher proviral HIV load, but additional studies are necessary to clarify this topic.
Our cohort study has a limited number of subjects, but it is counterbalanced by a clean case:control design allowing the adjustment by the main associated risk factors to the immunodiscordant response to cART. On the other hand, an in vivo human model for the study of HP, including anatomical support, would have been preferable but is not easily affordable. However, the in vitro model was useful to corroborate the upregulation of studied markers during human HP.
In summary, we have found that immunodiscordant subjects with low-level CD4 show an early and specific increase in the expression of OX40 and α4β7 on CD4 T-cells. The longitudinal exploration allowed proving that such alteration persists after suppressive cART, despite some extent of improvement. These novel features and our previous related data are all consistent with a promptly enhanced HP in this scenario.
Ethics Statement
The name of the ethics committee that approved the study is “CEI de los hospitales universitarios Vírgen Macarena-Virgen del Rocío.” All studied subjects gave a written informed consent before donate their samples. The study was approved by the local Ethical Committee and was performed according to the European Union guidelines and the Declaration of Helsinki.
Author Contributions
IR-S contributed to the design, performed research, analyzed, and interpreted data. IR-S and YP wrote the manuscript. IH-F and MG contributed to several analyses and/or experiments. JR, MR, DP, and JO represent contribution to sample and data collection by RIS BB/CoRIS. MM-F and FV critically reviewed the manuscript. ML and YP designed the study and contributed to data interpretation. YP conceived the study.
Conflict of Interest Statement
Part of the methods used in this article, specifically the culture system, is pending to be patented. However, the markers studied in this work are not included in such patent. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Mª Antonia Abad, Mª Mar Rodriguez, Marta de Luna, and Cytometry Facility of IBiS, especially Mª José Castro, for their technical assistance and to Juan Manuel Praena for statistical assistance. The authors also acknowledge the HIV BioBank integrated into the Spanish AIDS Research Network and collaborating Centers for the generous gifts of clinical samples used in this work. This study (RIS_EPICLIN 20/2015) would not have been possible without the collaboration of all the patients, medical and nursery staff, and data managers who have taken part in the project. The HIV BioBank, integrated in the Spanish AIDs Research Network, is supported by Instituto de Salud Carlos III, Spanish Healt Ministry (Grant no RD06/0006/0035 and RD12/0017/0037) as part of the Plan Nacional R + D + I and cofinanced by ISCIII- Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER) and Fundación para la investigación y prevención del SIDA en España (FIPSE). The RIS Cohort (CoRIS) is funded by the Instituto de Salud Carlos III through the Red Temática de Investigación Cooperativa en SIDA by the RD12/0017/0018 project, as part of the Plan Nacional R + D + I and cofinanced by ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER). The authors also thank Manuel Moyano from Centro Regional de Transfusión Sanguínea de Sevilla-Huelva y Banco de Tejidos (Seville, Spain) for the kind gift of samples.
Footnotes
Funding. This work was supported by grants from the Fondo de Investigación Sanitaria [FIS; PI14/01693, PI13/0796, PI16/0503], co-funded by Fondos Europeos para el Desarrollo Regional (FEDER), the Junta de Andalucía, Consejería de Economía, Innovación, Ciencia y Empleo [Proyecto de Investigación de Excelencia; CTS2593], AGAUR (2017SGR948) and GILEAD (GLD14/293). The Spanish AIDS Research Network of Excellence also supported this study (RD12/0017/0029 for I. Rosado, RD16/0025/0019 and RD16/0025/0006). YP was supported by the Consejería de Salud y Bienestar Social of Junta de Andalucía through the “‘Nicolás Monardes’” program [C-0013-2017].
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01673/full#supplementary-material.
References
- 1.van Lelyveld SFL, Gras L, Kesselring A, Zhang S, De Wolf F, Wensing AMJ, et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS (2012) 26:465–74. 10.1097/QAD.0b013e32834f32f8 [DOI] [PubMed] [Google Scholar]
- 2.Pacheco YM, Jarrin I, Rosado I, Campins AA, Berenguer J, Iribarren JA, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res (2015) 117:69–74. 10.1016/j.antiviral.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Massanella M, Negredo E, Clotet B, Blanco J. Immunodiscordant responses to HAART – mechanisms and consequences. Expert Rev Clin Immunol (2013) 9:1135–49. 10.1586/1744666X.2013.842897 [DOI] [PubMed] [Google Scholar]
- 4.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol (2012) 2012:670957. 10.1155/2012/670957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosado-Sánchez I, Jarrín I, Pozo-Balado MM, de Pablo-Bernal RS, Herrero-Fernández I, Alvarez-Ríos AI, et al. Higher levels of IL-6, CD4 turnover and Treg frequency are already present before cART in HIV-infected subjects with later low CD4 recovery. Antiviral Res (2017) 142:76–82. 10.1016/j.antiviral.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 6.Rosado-Sánchez I, Herrero-Fernández I, Tarancon-Diez L, Moreno S, Iribarren JA, Dalmau D, et al. Increased frequencies of Th17 cells and IL17a-producing regulatory T-cells preceding the immunodiscordant response to antiretroviral treatment. J Infect (2018) 76:86–92. 10.1016/j.jinf.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 7.Williams KM, Hakim FT, Gress RE. T cell immune roconstitution following lymphodepletion. Semin Immunol (2007) 19:318–30. 10.1016/j.smim.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol (2005) 174:6039–44. 10.4049/jimmunol.174.10.6039 [DOI] [PubMed] [Google Scholar]
- 9.Hennion-Tscheltzoff O, Leboeuf D, Gauthier SD, Dupuis M, Assouline B, Grégoire A, et al. TCR triggering modulates the responsiveness and homeostatic proliferation of CD4+ thymic emigrants to IL-7 therapy. Blood (2013) 121:4684–93. 10.1182/blood-2012-09-458174 [DOI] [PubMed] [Google Scholar]
- 10.Rosado-Sánchez I, González-Magaña A, Pozo-Balado MM, Herrero-Fernández I, Polaino MJ, Rodríguez MM, et al. An in vitro system of autologous lymphocytes culture that allows studying the homeostatic proliferation mechanisms of human naïve CD4 T-cells. Lab Invest (2018) 98:500–11. 10.1038/s41374-017-0006-3 [DOI] [PubMed] [Google Scholar]
- 11.Kawabe T, Sun S-L, Fujita T, Yamaki S, Asao A, Takahashi T, et al. Homeostatic proliferation of naive CD4+ T cells in mesenteric lymph nodes generates gut-tropic Th17 cells. J Immunol (2013) 190:5788–98. 10.4049/jimmunol.1203111 [DOI] [PubMed] [Google Scholar]
- 12.Yamaki S, Ine S, Kawabe T, Okuyama Y, Suzuki N, Soroosh P, et al. OX40 and IL-7 play synergistic roles in the homeostatic proliferation of effector memory CD4+T cells. Eur J Immunol (2014) 44:3015–25. 10.1002/eji.201444701 [DOI] [PubMed] [Google Scholar]
- 13.Cimbro R, Vassena L, Arthos J, Cicala C, Kehrl JH, Park C, et al. Interleukin-7 induces expression and activation of integrin α4β7 promoting naïve T-cell homing to the intestinal mucosa. Blood (2012) 120:2610–20. 10.1182/blood-2012-06-434779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caro-Murillo AM, Castilla J, Pérez-Hoyos S, Miró JM, Podzamczer D, Rubio R, et al. Cohorte RIS de pacientes con infección por VIH sin tratamiento antirretroviral previo (CoRIS): metodología y primeros resultados. Enferm Infecc Microbiol Clin (2007) 25:23–31. 10.1157/13096749 [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Merino I, de las Cuevas N, Jimenez JL, Gallego J, Gomez C, Prieto C, et al. The Spanish HIV BioBank: a model of cooperative HIV research. Retrovirology (2009) 6:27. 10.1186/1742-4690-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina-Pinelo S, Vallejo A, Diaz L, Soriano-Sarabia N, Ferrando-Martinez S, Resino S, et al. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J Antimicrob Chemother (2009) 64:579–88. 10.1093/jac/dkp248 [DOI] [PubMed] [Google Scholar]
- 17.Massanella M, Negredo E, Pérez-Alvarez N, Puig J, Ruiz-Hernández R, Bofill M, et al. CD4 T-cell hyperactivation and susceptibility to cell death determine poor CD4 T-cell recovery during suppressive HAART. AIDS (2010) 24:959–68. 10.1097/QAD.0b013e328337b957 [DOI] [PubMed] [Google Scholar]
- 18.Marchetti G, Gori A, Casabianca A, Magnani M, Franzetti F, Clerici M, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS (2006) 20:1727–36. 10.1097/01.aids.0000242819.72839.db [DOI] [PubMed] [Google Scholar]
- 19.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J, Kohrgruber N. Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. J Acquir Immune Defic Syndr (2011) 56:118–24. 10.1097/QAI.0b013e3181fbab9f [DOI] [PubMed] [Google Scholar]
- 20.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A (2008) 105:19851–6. 10.1073/pnas.0810032105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Méndez-Lagares G, Pozo-Balado MM, Genebat M, Garcia-Perganeda A, Leal M, Pacheco YM. Severe immune dysregulation affects CD4+CD25hiFoxP3+ regulatory T cells in HIV-infected patients with low-level CD4 T-cell repopulation despite suppressive highly active antiretroviral therapy. J Infect Dis (2012) 205:1501–9. 10.1093/infdis/jis230 [DOI] [PubMed] [Google Scholar]
- 22.Suy F, Botelho-Nevers E, Gagneux-Brunon A, Frésard A, Paul S, Lambert C, et al. Immunologic nonresponder and T-regulatory cells in HIV-1 infection. AIDS (2013) 27:2968–70. 10.1097/QAD.0000000000000022 [DOI] [PubMed] [Google Scholar]
- 23.Valiathan R, Asthana D. Increase in frequencies of circulating Th-17 cells correlates with microbial translocation, immune activation and exhaustion in HIV-1 infected patients with poor CD4 T-cell reconstitution. Immunobiology (2016) 221:670–8. 10.1016/j.imbio.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 24.Girard A, Vergnon D, Depincé-Berger A-E, Roblin X, Lutch F, Lambert C, et al. A high rate of β7+ gut homing lymphocytes in HIV infected immunogical non responders is associated with poor CD4 T cell recovery during suppressive HAART. J Acquir Immune Defic Syndr (2016) 72:259–65. 10.1097/QAI.0000000000000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol (2004) 173:7259–68. 10.4049/jimmunol.173.12.7259 [DOI] [PubMed] [Google Scholar]
- 26.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol (1997) 159:3838–48. [PubMed] [Google Scholar]
- 27.Malmström V, Shipton D, Singh B, Al-shamkhani A, Puklavec MJ, Barclay AN, et al. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol (2001) 166:6972–81. 10.4049/jimmunol.166.11.6972 [DOI] [PubMed] [Google Scholar]
- 28.Shive CL, Mudd JC, Funderburg NT, Sieg SF, Kyi B, Bazdar DA, et al. Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis (2014) 210:619–29. 10.1093/infdis/jiu125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science (1996) 272:60–6. 10.1126/science.272.5258.60 [DOI] [PubMed] [Google Scholar]
- 30.Gorfu G, Rivera-Nieves J, Ley K. Role of β7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med (2009) 9:837–50. 10.2174/156652409789105525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol (2008) 9:301–9. 10.1038/ni1566 [DOI] [PubMed] [Google Scholar]
- 32.Palmer CS, Duette GA, Wagner MCE, Henstridge DC, Saleh S, Pereira C, et al. Metabolically active CD4 + T cells expressing Glut1 and OX40 preferentially harbor HIV during in vitro infection. FEBS Lett (2017) 591:3319–32. 10.1002/1873-3468.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chomont N, El-far M, Ancuta P, Trautmann L, Boulassel M, Procopio FA, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med (2009) 15:893–900. 10.1038/nm.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.