Abstract
Perforation of the gall bladder can occur due to a complication of acute (in 3%–10%) or chronic cholecystitis, presenting with or without gallstones. Other causes include trauma, neoplasms, steroid therapy or vascular compromise. In 1934, Niemeier classified the condition into three types: type I, acute perforation into the free peritoneal cavity; type II, subacute perforation with abscess formation; and type III, chronic perforation with fistula formation between the gall bladder and another viscus with type I experiencing the highest mortality rate. In particular, there are very few cases of gall bladder perforation associated with ischaemic bowel disease. We present a case of type I gall bladder perforation in a 70-year-old woman, without any apparent comorbidities, presenting with acute abdomen consistent with perforated duodenal ulcer with pneumoperitoneum on a plain abdominal radiograph and contrast-enhanced CT with eventual discovery of fundal perforation and ischaemic small bowel at laparotomy.
Keywords: gastrointestinal surgery, pancreas and biliary tract
Background
Acute calculous cholecystitis can present without classic signs and symptoms in the elderly (>65 years old), especially with risk factors for atherosclerosis. Gall bladder (GB) perforation is a rare but life-threatening complication of acute (in 3%-10%)1 cholecystitis, with a reported mortality rate of 12%–42%. Niemeier2 classified it into three types: type I, acute perforation into the peritoneal cavity; type II, subacute perforation with abscess formation; and type III, chronic perforation with fistula formation3 with type I experiencing the highest mortality rate. Vascular compromise of the GB wall is usually secondary to GB distension leading to gangrene, necrosis and perforation.1 The association of GB perforation with ischaemic small bowel, as in the current case, is uncommon and emphasises the effects of age-associated widespread atherosclerosis and its role in the complications of acute cholecystitis as well as effect on the rest of the gastrointestinal system.
Case presentation
A 70-year-old woman from a poor socioeconomic background was referred to our emergency department with a chief complaint of abdominal pain. It started in the epigastric region 3 days ago, progressing to involve the entire abdomen in 24–48 hours prior to presentation. It was associated with obstipation and three to four episodes of vomiting, which did not contain any blood. There was no history of fever, jaundice, loss of weight or appetite, indigestion, trauma and chronic non-steroidal anti-inflammatory drug use. There was a history of self-limiting episodic epigastric pain in the past 3 years for which the patient did not seek any healthcare advice. She had no history of diabetes, hypertension, asthma, tuberculosis or any surgical interventions.
On examination, the patient was disoriented. She was hypotensive (blood pressure (BP) 100/70 mm Hg) and had tachycardia (~102/min), but was afebrile. Her abdomen was distended with diffuse tenderness and rigidity with absent bowel sounds on auscultation. Late inspiratory crackles could be appreciated on auscultation of the right lung base. The remainder of the systemic examination was within normal limits. An abdominal radiograph identified 3 mm of air under the right hemidiaphragm. Her contrast-enhanced CT (CECT)—performed at another institution—showed pneumoperitoneum with mildly thickened proximal small bowel loops, mild ascites and diffuse mesenteric thickening and stranding along with evidence of thickened and oedematous GB and atrophic pancreas with few calcific foci in the body suggestive of chronic pancreatitis. Routine investigations—complete blood count, coagulation profile, kidney function test and liver function test—were within normal limits, except her total leucocyte count was low at 2.5 × 10∧9/L. With the aforementioned clinical and radiological findings, presumptive diagnosis of generalised peritonitis secondary to perforated duodenal ulcer, with quick Sequential (Sepsis-related) Organ Failure Assessment (qSOFA) score of 2, was made.
Immediate management included resuscitation with intravenous fluids, intravenous antibiotics, intravenous analgesics, intravenous proton pump inhibitor along with vital monitoring. After stabilisation, she was proceeded to emergency laparotomy. Approximately 1500 mL of bilious, purulent fluid together with biliary calculi was present in the peritoneal cavity with gangrenous patches all over the omentum and areas of pus on the small bowel and mesenteric surface (figure 1). The entire bowel was examined, but no perforation or obstruction was present. The small intestine showed signs of compromised blood supply with bluish discolouration and absent peristalsis (figure 2). It was covered with hot packs for 10 min and 100% oxygen which resulted in a recovery of the small bowel appearance with a normal colour and sheen. On examination of the GB, 0.5 cm diameter defect was present in the fundus with multiple calculi in the lumen (figure 3). Cholecystectomy was performed, and the abdomen was closed after thorough peritoneal lavage and a right pelvic drain. Histopathology of the GB revealed acute calculous cholecystitis superimposed on chronic cholecystitis. Postoperatively, she was placed on ventilator in the intensive care unit (ICU). However, she developed cardiorespiratory arrest a few hours later and could not be resuscitated despite best efforts.
Figure 1.

Bile-stained omentum and bowel seen after emergency laparotomy.
Figure 2.
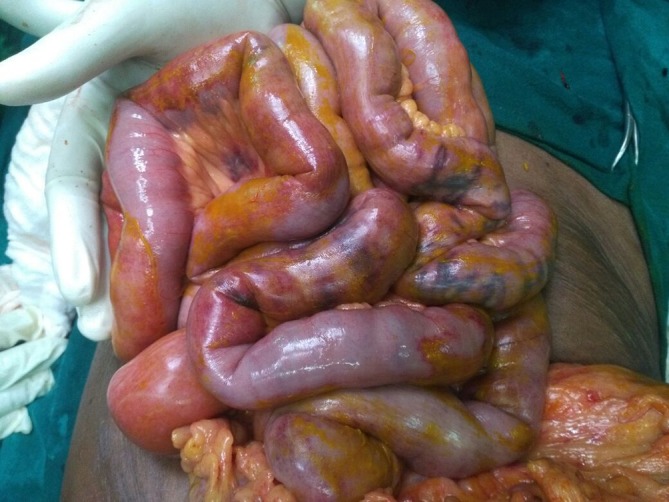
Small bowel showing signs of ischaemia-bluish discolouration and absent peristalsis.
Figure 3.

Intraoperative view of the gall bladder perforation (pictured above). The picture below shows the postcholecystectomy gall bladder specimen with forceps in the perforated fundus. Multiple calculi of varying sizes recovered from the lumen of the gall bladder and the peritoneal cavity.
Differential diagnosis
Perforated duodenal ulcer.
Gastric ulcer perforation.
Small bowel ischaemia with or without perforation.
Large bowel perforation.
Discussion
Perforation of the GB usually occurs within 3–4 days after the onset of acute cholecystitis. There is no specific symptom complex diagnostic of GB perforation.4 Risk factors include old age, diabetes, atherosclerosis and immunosuppression.5 Type I perforation presents as acute abdomen with signs of peritonitis or rapidly deteriorating clinical course of acute cholecystitis. The fundus of the GB is the most common site of perforation owing to its poor vascular supply.4 If the perforation is located at the fundus, it is less likely to be covered by the omentum, thus bile and stones are likely to drain into the peritoneal space, causing peritonitis.6 Most of the type I perforations are reported in elderly population, especially women5 7 8 and middle-aged men with a history of liver disease.5 6 9
In our patient, diagnostic imaging showed pneumoperitoneum which is a hallmark sign of hollow viscus perforation. This led us to suggest that a perforated duodenal ulcer was a possible preoperative diagnosis. We excluded the possibility of emphysematous cholecystitis, given the absence of diabetes in our patient, normal blood sugar levels on presentation and lack of intramural air on CT. In these circumstances, we hypothesised that the free air was due to passage of air from duodenum into the biliary tree because of delay in presentation to hospital and then further delay in presentation to the institution of treatment. There was presence of bowel ischaemia on laparotomy, which might be explained by shock. However, the preoperative BP was 100/70 mm Hg, making it unlikely to be the sole cause. However, given the patient’s age, widespread atherosclerotic changes in the abdominal aorta and/or its branches1 could also contribute to ischaemia of the bowel, this superimposed on low normal systolic BP may have contributed to the patchy small bowel ischaemia and also GB causing perforation at the poorly vascularised fundus. The history of episodic epigastric pain could have reflected mesenteric angina, previous attacks of cholecystitis or chronic pancreatitis, identified on CECT. However, due to the urgency of the clinical situation, non-invasive and/or invasive vascular studies were not indicated. This finding and the case described by Vancauwenberghe et al1 emphasise a causative role of diffuse atherosclerosis associated with old age in the pathogenesis of GB perforation, even in those without any apparent medical morbidity.
The common associated findings on imaging that accompany GB perforation are a pericholecystic fluid collection and layering of GB wall on ultrasound, and pericholecystic fluid collection, streaky omentum or mesentery, and GB wall defect on CT; with CT being superior to ultrasound for diagnosis of GB perforation.10 Although ultrasound remains the preferred initial examination for the evaluation of suspected GB perforation, unfortunately, it often fails to demonstrate the perforation because of increased intestinal gas and pain.6 CT scan findings can be divided into primary GB changes, wall thickening, wall enhancement, wall defect, intramural abscess, intramural gas, mural haemorrhage, presence of gallstones; pericholecystic changes and findings of extra GB organs, pericholecystic liver enhancement, liver abscess, portal vein thrombosis, reactive mural thickening of adjacent hollow organ (hepatic flexure of colon and duodenum), presence of lymph nodes, intraperitoneal free air, ascites, ileus and Mirizzi syndrome.6 The value of radiological modalities for the detection of GB perforation can be appreciated for preoperative diagnosis of type II and III perforations. However, their value seems limited in type I perforation presenting acutely because of non-specific findings, with the majority being diagnosed only at surgery by observing the bile-coloured ascites,7 as in our current case.
In conclusion, acute calculous cholecystitis can present without classic signs and symptoms in the elderly (>65 years old), especially with risk factors for atherosclerosis. This leads to preoperative diagnostic dilemma with eventual worsening of the condition leading to unique clinical presentation including type I perforation (like in the present case), type III perforation and gallstone ileus,11 12 GB torsion13–15 and haemobilia.9 16 Most of these are diagnosed during laparotomy and present as a challenging modality for surgeons and therefore should be kept in mind as one of the differential diagnoses when dealing with acute abdomen in the elderly population. Preoperative stabilisation is essential, with a special attention to the fluid and electrolytes balance and the management of comorbid conditions.11 The key is to institute surgical management as soon as possible after appropriate resuscitation as laparotomy still plays a decisive role in confirmation of the diagnosis as well as therapy, which includes cholecystectomy.1 However, in patients with biliary-enteric fistula, that is, type III perforation, choice of the most appropriate surgical approach—enterolithotomy with cholecystectomy performed later (two-stage surgery) or a one-stage procedure with enterolithotomy, cholecystectomy and fistula repair—is dependent on factors related to the patient and the clinical presentation.11 The prognosis is dependent on the time elapsing between the onset of symptoms and the institution of definitive treatment.5 The mortality in our patient can be attributed to the delayed presentation and the severity of illness marked by a qSOFA score ≥2.17
Futhermore, guidelines for performing cholecystectomy in elderly patients presenting with acute cholecystitis in elderly patients, especially with risk factors like atherosclerosis, diabetes or liver disease, should be reviewed, possibly shortening the time between onset of the symptoms and the procedure and hence preventing the progression to development of these complications as delayed diagnosis, concomitant comorbidity and advanced age are the causes of high mortality rate in such cases.11
Learning points.
Gall bladder perforation should be kept in mind as one of the differential diagnoses when dealing with acute abdomen, particularly as it can resemble perforated duodenal ulcer, in the elderly population.
The key is prompt recognition as well as instituting surgical management as soon as possible after appropriate resuscitation, as laparotomy still plays a decisive role in confirming the diagnosis and therapy, which includes cholecystectomy.
The prognosis is dependent to a large degree on the time elapsing between the onset of symptoms and the institution of definitive treatment.
Additionally, guidelines for performing cholecystectomy in elderly patients presenting with acute cholecystitis in elderly patients, especially with risk factors including atherosclerosis, diabetes or liver disease should be reviewed, possibly shortening the time between onset of the disease and the procedure; hence, preventing the progression to development of complications such as perforation as they are highly susceptible and have a bad prognosis.
Footnotes
Contributors: NW, OKM and VV contributed equally to the manuscript formation.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vancauwenberghe T, Vanhoenacker FM, Verheyen L. Ischemic gallbladder perforation. JBR-BTR 2011;94:152–3. 10.5334/jbr-btr.562 [DOI] [PubMed] [Google Scholar]
- 2.Niemeier OW. Acute Free Perforation of the Gall-Bladder. Ann Surg 1934;99:922–4. 10.1097/00000658-193499060-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Date RS, Thrumurthy SG, Whiteside S, et al. Gallbladder perforation: case series and systematic review. Int J Surg 2012;10:63–8. 10.1016/j.ijsu.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 4.Pines B, Rabinovitch J. Perforation of the gallbladder in acute cholecystitis. Ann Surg 1954;140:170–9. 10.1097/00000658-195408000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong CL, Wong TH, Rauff A. Acute gall bladder perforation--a dilemma in early diagnosis. Gut 1991;32:956–8. 10.1136/gut.32.8.956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiapponi C, Wirth S, Siebeck M. Acute gallbladder perforation with gallstones spillage in a cirrhotic patient. World J Emerg Surg 2010;5:11 10.1186/1749-7922-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HJ, Park SJ, Lee SB, et al. A case of spontaneous gallbladder perforation. Korean J Intern Med 2004;19:128–31. 10.3904/kjim.2004.19.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan SA, Arshad Z, Anwer AW, et al. Gallbladder perforation: a rare complication of acute cholecystitis. J Pak Med Assoc 2010;60:228–9. [PubMed] [Google Scholar]
- 9.Aljiffry MM, Almulhim AN, Jamal MH, et al. Acute cholecystitis presenting with massive intra-abdominal haemorrhage. J Surg Case Rep 2014;2014:rju019 10.1093/jscr/rju019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim PN, Lee KS, Kim IY, et al. Gallbladder perforation: comparison of US findings with CT. Abdom Imaging 1994;19:239–42. 10.1007/BF00203516 [DOI] [PubMed] [Google Scholar]
- 11.Conzo G, Mauriello C, Gambardella C, et al. Gallstone ileus: One-stage surgery in an elderly patient: One-stage surgery in gallstone ileus. Int J Surg Case Rep 2013;4:316–8. 10.1016/j.ijscr.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Monaco P, Migliaccio C, La Mura F, et al. Report of two cases of gallstone ileus and literature review. BMC Geriatr 2009;9(Suppl 1):A32 10.1186/1471-2318-9-S1-A32 [DOI] [Google Scholar]
- 13.Alkhalili E, Bencsath K. Gallbladder torsion with acute cholecystitis and gross necrosis. BMJ Case Rep 2014;2014:bcr2014204917 10.1136/bcr-2014-204917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boer J, Boerma D, de Vries Reilingh TS. A gallbladder torsion presenting as acute cholecystitis in an elderly woman: A case report. J Med Case Rep 2011;5:588 10.1186/1752-1947-5-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabizon S, Bradshaw K, Jeyarajan E, et al. Gallbladder torsion: a diagnostic challenge. Case Rep Surg 2014;2014:1–2. 10.1155/2014/902814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Ortiz DI, Toro DH, Vega W. Hemobilia: a rare presentation of acute cholecystitis. Clin Gastroenterol Hepatol 2008;6:e36–7. 10.1016/j.cgh.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]


