Abstract
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are two related mucocutaneous disorders with different severities. Although the incidence is low, SJS and TEN are life-threatening and predominantly drug-induced conditions. There is a strong relationship between the HLA-B*1502 allele and carbamazepine-induced SJS and TEN in different Southeast Asian populations. Here, we report a case of Filipino with SJS/TEN overlap probably induced by carbamazepine. The condition was treated with hydrocortisone followed by prednisone. The HLA-B*1502 allele was not found in this case. The patient tested positive for the HLA-B75 serotype, suggesting that carbamazepine-induced SJS/TEN may be serotype specific. Establishing the genotype before initiation of the drug may be advantageous for some patients and will aid physicians in determining the optimal drug therapy. Prevention of adverse drug reactions (ADR) may be done if pharmacists and other healthcare professionals work as a multidisciplinary ADR team to ensure that safe medication practices are realised.
Keywords: pharmacology and therapeutics, genetics
Background
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare and severe conditions that affect both the skin and mucous membranes. Both SJS and TEN are characterised by blisters arising on purple macules. Lesions are widespread and usually predominate on the trunk of the body. The percentage of skin detachment on body surface area differentiates SJS and TEN. Cases with skin detachment of less than 10% are classified as SJS, while those with detachment of more than 30% are classified as TEN. Cases are considered as SJS/TEN overlap when the detachment affects 10% to 30% of the body surface area. Although the incidence of SJS and TEN is low, mortality rates are high. The incidence of SJS and TEN ranges from 1.2 to 6 and 0.4 to 1.2 per million person-years, respectively.1 The incidence of SJS in the Philippines, Malaysia and Taiwan is about 10 times higher than those reported in Caucasian countries.2 In a 7-year retrospective study conducted in the Philippine General Hospital, SJS and TEN accounted for 0.03% of total admissions. The mortality rate was found to be 4.7% for SJS and 16.7% for TEN.3
SJS and TEN are manifestations of a drug reaction with carbamazepine as the frequent culprit. Carbamazepine ranked first among the most common implicated drugs in Southeast Asia.4 In the Philippines, data obtained from the Food and Drug Administration showed that carbamazepine and allopurinol ranked second following phenytoin. A high percentage (71.9%) of skin and subcutaneous disorders with carbamazepine treatment were reported. Approximately 16% of individual case safety reports have documented SJS cases.5 Here, we report a case of SJS/TEN overlap in a Filipino patient treated with carbamazepine.
Case presentation
A 40-year old Filipino man with a chief complaint of rashes was admitted at the emergency room of the Philippine General Hospital. The patient had seasonal outbursts at home and episodes of violence towards his mother. No medications were given and no consultations were done as outbursts were controlled. One year prior to admission, the patient had more frequent outbursts after his father’s death. The increasing frequency of outbursts prompted consultation at the National Centre for Mental Health for which the following medications were given: carbamazepine 200 mg tablet twice daily, quetiapine 25 mg tablet HS and risperidone 2 mg tablet HS.
The patient had no known vices nor drug allergies. After 12 days of taking medications, the patient developed periorbital and lip swelling, followed by onset of erythematous petechial rashes over the chest, the extremities spreading to the abdomen and the face. All medications were discontinued the following day on consultation at a local health centre. Cetirizine was given but no improvement was observed. The persistence of symptoms prompted admission at the Philippine General Hospital.
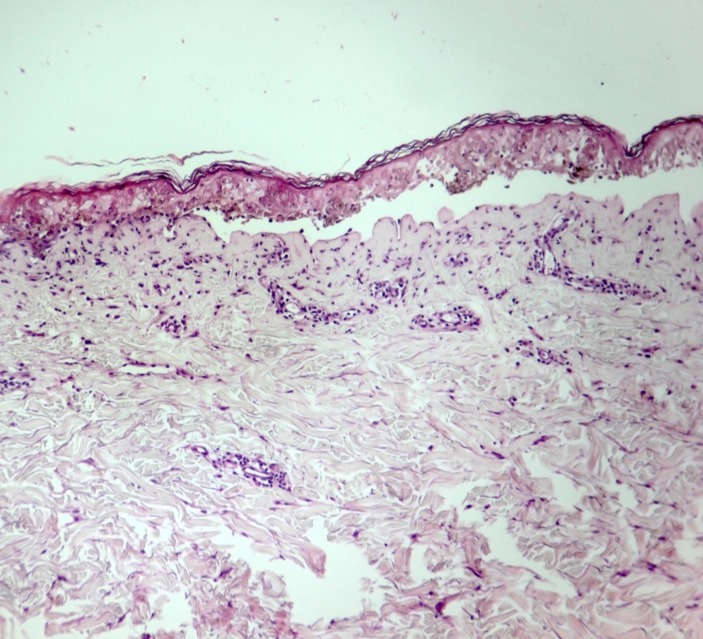
Clinical examination of the patient revealed matting of eyelashes with purulent discharge, oral ulcers, lip ulcers and swelling with dried purulent and haemorrhagic crusts. The patient was febrile (up to 38.7°C). Dysphagia was present. The skin lesions characterised as violaceous dusky red macules and patches with areas with flaccid bullae were present in the face, the trunk and the extremities. The scrotal area was also affected. The Nikolsky sign was found to be positive. Skin detachment of approximately 15% of the body surface area was reported, suggesting a case of SJS/TEN overlap (figure 1). Skin biopsy was done and the results showed full thickness split with necrotic keratinocytes which were consistent with SJS or TEN (figure 2).
Figure 1.

Clinical presentation of Stevens-Johnson syndrome/toxic epidermal necrolysis overlap. (A) Periorbital swelling, conjunctivitis and matting of eyelashes with mucopurulent discharge on both eyes and swelling of lips with dried purulent and haemorrhagic crusts; (B) multiple violaceous dusky red macules and patches with areas of bullae.
Figure 2.
Histopathological findings. The skin biopsy section shows basketweave orthokeratosis, necrotic keratinocytes at all epidermal levels with full-thickness skin necrosis, focal vacuolar interface change, pigment incontinence and mild superficial perivascular mixed cell infiltrates consisting of lymphocytes, neutrophils, plasma cells and rare eosinophils.
The SJS/TEN overlap was probably induced by carbamazepine based on the results of a drug causality assessment using the Naranjo algorithm. The total score was 8. The patient was treated with hydrocortisone followed by prednisone. Other treatments were given which included paracetamol, antacids, antihistamines, mouth gargles, nasal sprays and topical preparations. The severity-of-illness score for toxic epidermal necrolysis (SCORTEN) was 1 (mortality rate: 3.2%).
Outcome and follow-up
After 13 days of hospitalisation, the patient was discharged with signs of improvement. One year posthospitalisation, DNA was extracted from the patient’s saliva samples. Genetic testing for HLA was found to be positive for the HLA-B75 serotype. Specifically, the patient tested positive for HLA-B*1521 and was negative for HLA-B*1502.
Discussion
Carbamazepine is used for the treatment of epilepsy, bipolar disorder and neuropathic pain.6 In this case, carbamazepine was prescribed to manage behavioural changes as manifested by increased irritability. As a mood stabiliser, carbamazepine was given in combination with atypical antipsychotics namely quetiapine and risperidone. Meta-analyses showed that the combination of mood stabilisers and antipsychotics is more effective than a mood stabiliser alone, but at the expense of more adverse effects.7 8
The use of carbamazepine is associated with high risk of severe cutaneous adverse reactions such as SJS and TEN. This strong association was confirmed in the European Study of Severe Cutaneous Adverse Reaction(EuroSCAR).9 Quetiapine fumarate (Seroquel) also can cause SJS and TEN but these adverse reactions were temporally related to quetiapine treatment.10 To determine which drug caused SJS/TEN in this case, a drug causality assessment was performed using the Naranjo algorithm. The drug with the highest score of 8 was carbamazepine, suggesting that SJS/TEN was probably due to carbamazepine treatment. However, the proponents could not discount the possibility that quetiapine may further increase the risk of SJS/TEN due to possible additive effects.
Studies show that the association of carbamazepine with SJS/TEN has genetic predisposition. An association between HLA-B*1502 and carbamazepine-induced SJS/TEN was found in Han Chinese, Thais, Indians, Malays, Vietnamese and Canadians of Asian origin.11–28 No association was observed among Koreans, Japanese and Caucasians.29–33 To date, the association of HLA-B*1502 with carbamazepine-induced SJS/TEN in the Filipino population has not been investigated. In this case, the HLA-B75 serotype was found to be present. Specifically, the patient tested positive for HLA-B*1521 but negative for HLA-B*1502. Some reports have shown cases of patients who developed carbamazepine-induced SJS and were negative for HLA-B*1502. Kaniwa et al did not detect the HLA-B*1502 allele in Japanese patients with carbamazepine-induced SJS/TEN; instead, they detected the HLA-B*1511 allele.34 In India, Mehta et al found that six out of eight patients with carbamazepine-induced SJS had the HLA-B*1502 allele. One patient was found to have the HLA-B*1508 allele.16 In the study of Tassaneeyakul et al in a Thai population, 37 of 42 patients with carbamazepine-induced SJS/TEN carried the HLA-B*1502 allele. Both HLA-B*1521 and HLA-B*1511 were detected in some patients.17 HLA-B*1502, HLA-B*1508, HLA-B*1511 and HLA-B*1521 are members of the HLA-B75 serotype. The results of the study of Yuliwulandari et al in the Javanese and Sundanese population of Indonesia suggest that carbamazepine-induced SJS/TEN may be serotype specific. They also identified HLA-B*1521 in patients who tested negative for HLA-B*1502.35
The occurrence of carbamazepine-induced SJS/TEN in this case could have been prevented through genetic testing prior to carbamazepine prescription. This is to determine the risk of developing SJS/TEN when carbamazepine therapy is initiated. If the patient tested positive for the HLA-B*1502 allele, carbamazepine is avoided and an alternative drug is prescribed to the patient. The cost-effectiveness of genetic testing to prevent life-threatening SJS/TEN induced by carbamazepine has been demonstrated in Asian countries such as Taiwan, Singapore, Malaysia and Thailand.36–40 Successful clinical implementation of pharmacogenetic testing to reduce the risk of SJS/TEN has been reported. A decade ago, 10–20 Taiwanese with SJS/TEN were admitted yearly, but nowadays, there are only a few cases admitted. In Thailand, the incidence of SJS/TEN has reduced sharply. The country is now in the phase of eradicating these life-threatening severe cutaneous adverse reactions.41 42 Unfortunately, the practice of biomarker testing before prescribing a drug is not yet realised in the Philippines. Local researches including an association study and pharmacoeconomic evaluation must be done. The case presented here is part of an ongoing study of the association of the HLA-A and HLA-B alleles with carbamazepine-induced SJS/TEN in the Filipino population. To the best of our knowledge, this is the first study investigating the HLA alleles that are associated with SJS/TEN due to carbamazepine treatment among the Filipinos. Once a strong association between genotype and phenotype is found, new therapy or a screening test should be developed. Developing new therapy takes time and requires a lot of resources so we believe that developing and implementing a screening test in order to predict the risk of carbamazepine-induced SJS/TEN is more feasible in the near future.
Furthermore, the adverse drug reaction (ADR) reported here could have been prevented if the pharmacists and other healthcare professionals work together as a multidisciplinary ADR team to ensure that safe medication practices are realised. As drug experts, pharmacists play a vital role in the prevention, identification, assessment, treatment, monitoring and reporting of ADRs.43
Learning points.
The Stevens-Johnson syndrome/toxic epidermal necrolysis induced by carbamazepine maybe serotype specific.
It is suggested that the patients who will receive carbamazepine therapy be screened for the HLA-B75 serotype instead of the HLA-B*1502 allele only.
Establishing the genotype before initiation of the drug will aid physicians in determining the optimal drug therapy.
Acknowledgments
The authors would like to thank the Department of Medical Sciences, Ministry of Public Health, Thailand for the genotyping done in this patient and the Office of Vice President for Academic Affairs, University of the Philippines and the National Institutes of Health, University of the Philippines Manila for financial support.
Footnotes
Contributors: All authors contributed equally to drafting, revision, and preparation of the manuscript. All authors read and approved the final version. FC is the principal investigator. He initiated the collaborative research, conceptualised and designed the study with coauthors, monitored all research activities and analysed the data. PT, SM, NW, and JJ provided academic guidance, technical assistance and practical support in all matters concerning research; supervised the genotyping, quality control procedures and proper disposal of unused DNA samples after genotyping; and analysed the data. LTAA, JJB, JCG, LCL, JPBR, FR, and KFSG provided technical assistance in all matters concerning research, most especially in the clinical aspect of the study. They were involved in data collection and analysis, photos and biopsy slide retrieval, patient recruitment, informed consent taking, patient interview, and saliva sample collection. CLS provided technical assistance in all matters concerning research, most especially in the laboratory work of the study. She supervised the DNA extraction protocol optimization, DNA extraction, quality control procedures, and sample storage.
Funding: This study was funded by National Institutes of Health, University of the Philippines Manila (grant no: NIH 2017-001).
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Ethics approval: This study was approved by the University of the Philippines Manila Research Ethics Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272–85. 10.1056/NEJM199411103311906 [DOI] [PubMed] [Google Scholar]
- 2.Farkas R. Clinical review, adverse events of Carbamazepine. 2007. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/016608s098,020712s029,021710_clinrev.pdf (cited 2017 Oct 12).
- 3.Onishi ECC, Roa FDC. Stevens-Johnson syndrome and toxic epidermal necrolysis at the Philippine General Hospital: A seven-year retrospective study (January 2004 to December 2010). 2013. JPDS.
- 4.Lee HY, Martanto W, Thirumoorthy T. Epidemiology of Stevens–Johnson syndrome and toxic epidermal necrolysis in Southeast Asia. Dermatologica Sinica 2013;31:217–20. 10.1016/j.dsi.2013.09.007 [DOI] [Google Scholar]
- 5.Uppsala Monitoring Centre. Carbamazepine. 2016. updated 1 Aug 2016, cited 12 Oct 2017 https://vigilyze.who-umc.org/#/.
- 6.U.S. Food and Drug Administration. Information for Healthcare Professionals: Dangerous or Even Fatal Skin Reactions - Carbamazepine (marketed as Carbatrol, Equetro, Tegretol, and generics). 2007. updated 14 August 2013; cited 5 October 2017 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124718.htm.
- 7.Scherk H, Pajonk FG, Leucht S. Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and meta-analysis of randomized controlled trials. Arch Gen Psychiatry 2007;64:442–55. 10.1001/archpsyc.64.4.442 [DOI] [PubMed] [Google Scholar]
- 8.Smith LA, Cornelius V, Warnock A, et al. Acute bipolar mania: a systematic review and meta-analysis of co-therapy vs. monotherapy. Acta Psychiatr Scand 2007;115:12–20. 10.1111/j.1600-0447.2006.00912.x [DOI] [PubMed] [Google Scholar]
- 9.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128:35–44. 10.1038/sj.jid.5701033 [DOI] [PubMed] [Google Scholar]
- 10.Quetiapine fumarate (Seroquel®). Wilmington, DE: AstraZeneca Pharmaceuticals LP, 2010. [Google Scholar]
- 11.Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature 2004;428:486 10.1038/428486a [DOI] [PubMed] [Google Scholar]
- 12.Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics 2006;16:297–306. 10.1097/01.fpc.0000199500.46842.4a [DOI] [PubMed] [Google Scholar]
- 13.Man CB, Kwan P, Baum L, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia 2007;48:1015–8. 10.1111/j.1528-1167.2007.01022.x [DOI] [PubMed] [Google Scholar]
- 14.Locharernkul C, Loplumlert J, Limotai C, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia 2008;49:2087–91. 10.1111/j.1528-1167.2008.01719.x [DOI] [PubMed] [Google Scholar]
- 15.Liao WP, Shi YW, Cheng SH, et al. Association between HLA-B*1502 allele and cutaneous reactions induced by carbamazepine or lamotrigine in Han Chinese. Epilepsia 2009;50:252–3. [Google Scholar]
- 16.Mehta TY, Prajapati LM, Mittal B, et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol 2009;75:579–82. 10.4103/0378-6323.57718 [DOI] [PubMed] [Google Scholar]
- 17.Tassaneeyakul W, Tiamkao S, Jantararoungtong T, et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia 2010;51:926–30. 10.1111/j.1528-1167.2010.02533.x [DOI] [PubMed] [Google Scholar]
- 18.Wu XT, Hu FY, An DM, Xt W, Fy H, Dm A, et al. Association between carbamazepine-induced cutaneous adverse drug reactions and the HLA-B*1502 allele among patients in central China. Epilepsy Behav 2010;19:405–8. 10.1016/j.yebeh.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Too CL, Murad S, et al. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int J Dermatol 2011;50:221–4. 10.1111/j.1365-4632.2010.04745.x [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Wang J, Zhao LM, et al. Strong association between HLA-B*1502 and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. Eur J Clin Pharmacol 2011;67:885–7. 10.1007/s00228-011-1009-4 [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Zhou JQ, Zhou LM, et al. Association between HLA-B*1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure 2011;20:446–8. 10.1016/j.seizure.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 22.Then SM, Rani ZZ, Raymond AA, et al. Frequency of the HLA-B*1502 allele contributing to carbamazepine-induced hypersensitivity reactions in a cohort of Malaysian epilepsy patients. Asian Pac J Allergy Immunol 2011;29:290–3. [PubMed] [Google Scholar]
- 23.Kulkantrakorn K, Tassaneeyakul W, Tiamkao S, et al. HLA-B*1502 strongly predicts carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Thai patients with neuropathic pain. Pain Pract 2012;12:202–8. 10.1111/j.1533-2500.2011.00479.x [DOI] [PubMed] [Google Scholar]
- 24.Shi YW, Min FL, Qin B, et al. Association between HLA and Stevens-Johnson syndrome induced by carbamazepine in Southern Han Chinese: genetic markers besides B*1502? Basic Clin Pharmacol Toxicol 2012;111:n/a–64. 10.1111/j.1742-7843.2012.00868.x [DOI] [PubMed] [Google Scholar]
- 25.Amstutz U, Ross CJ, Castro-Pastrana LI, et al. HLA-A 31:01 and HLA-B 15:02 as genetic markers for carbamazepine hypersensitivity in children. Clin Pharmacol Ther 2013;94:142–9. 10.1038/clpt.2013.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khor AH, Lim KS, Tan CT, et al. HLA-B*15:02 association with carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in an Indian population: a pooled-data analysis and meta-analysis. Epilepsia 2014;55:e120–e124. 10.1111/epi.12802 [DOI] [PubMed] [Google Scholar]
- 27.Kwan PKL, Mhl N, Sv L. Association between HLA-B*15:02 allele and antiepileptic drug-induced severe cutaneous reactions in Hong Kong Chinese: a population-based study. Hong Kong Med J 2014;20:S16–18. [PubMed] [Google Scholar]
- 28.Nguyen DV, Chu HC, Nguyen DV, et al. HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in Vietnamese. Asia Pac Allergy 2015;5:68–77. 10.5415/apallergy.2015.5.2.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SH, Lee KW, Song WJ, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res 2011;97:190–7. 10.1016/j.eplepsyres.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 30.Kaniwa N, Saito Y, Aihara M, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics 2008;9:1617–22. 10.2217/14622416.9.11.1617 [DOI] [PubMed] [Google Scholar]
- 31.Kashiwagi M, Aihara M, Takahashi Y, et al. Human leukocyte antigen genotypes in carbamazepine-induced severe cutaneous adverse drug response in Japanese patients. J Dermatol 2008;35:683–5. 10.1111/j.1346-8138.2008.00548.x [DOI] [PubMed] [Google Scholar]
- 32.Niihara H, Kakamu T, Fujita Y, et al. HLA-A31 strongly associates with carbamazepine-induced adverse drug reactions but not with carbamazepine-induced lymphocyte proliferation in a Japanese population. J Dermatol 2012;39:594–601. 10.1111/j.1346-8138.2011.01457.x [DOI] [PubMed] [Google Scholar]
- 33.Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 2008;18:99–107. 10.1097/FPC.0b013e3282f3ef9c [DOI] [PubMed] [Google Scholar]
- 34.Kaniwa N, Saito Y, Aihara M, et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia 2010;51:2461–5. 10.1111/j.1528-1167.2010.02766.x [DOI] [PubMed] [Google Scholar]
- 35.Yuliwulandari R, Kristin E, Prayuni K, et al. Association of the HLA-B alleles with carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis in the Javanese and Sundanese population of Indonesia: the important role of the HLA-B75 serotype. Pharmacogenomics 2017;18:1643–8. 10.2217/pgs-2017-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Lin JJ, Lu CS, Cs L, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 2011;364:1126–33. 10.1056/NEJMoa1009717 [DOI] [PubMed] [Google Scholar]
- 37.Dong D, Sung C, Finkelstein EA. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 2012;79:1259–67. 10.1212/WNL.0b013e31826aac73 [DOI] [PubMed] [Google Scholar]
- 38.Then SM, Rani ZZM, Raymond AA, et al. Pharmacogenomics screening of HLA-B*1502 in epilepsy patients: How we do it in the UKM Medical Centre, Malaysia. Neurology Asia 2013;18:27–9. [Google Scholar]
- 39.Tiamkao S, Jitpimolmard J, Sawanyawisuth K, et al. Cost minimization of HLA-B*1502 screening before prescribing carbamazepine in Thailand. Int J Clin Pharm 2013;35:608–12. 10.1007/s11096-013-9777-9 [DOI] [PubMed] [Google Scholar]
- 40.Rattanavipapong W, Koopitakkajorn T, Praditsitthikorn N, et al. Economic evaluation of HLA-B*15:02 screening for carbamazepine-induced severe adverse drug reactions in Thailand. Epilepsia 2013;54:1628–38. 10.1111/epi.12325 [DOI] [PubMed] [Google Scholar]
- 41.Sukasem C, Chantratita W. A success story in pharmacogenomics: genetic ID card for SJS/TEN. Pharmacogenomics 2016;17:455–8. 10.2217/pgs-2015-0009 [DOI] [PubMed] [Google Scholar]
- 42.Mitropoulos K, Al Jaibeji H, Forero DA, et al. Success stories in genomic medicine from resource-limited countries. Hum Genomics 2015;9:1–7. 10.1186/s40246-015-0033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Society of Health-System Pharmacists. ASHP guidelines on adverse drug reaction monitoring and reporting. Am J Health-Syst Pharm 1995;52:417–9. [DOI] [PubMed] [Google Scholar]