Abstract
Background
Recommended therapeutic options for the management of venous thromboembolism (VTE) in patients with cancer are burdensome, and compliance with guidelines is unknown.
Objectives
To describe current treatment patterns and to evaluate patient persistence on various anticoagulants.
Patients/Methods
Medical and pharmacy claims from the Humana Database were analyzed (01/2007‐12/2014). Newly diagnosed cancer patients treated with anticoagulants were categorized into one of the following cohorts: low–molecular‐weight heparin (LMWH), warfarin, and rivaroxaban. Discontinuation, switching, and persistence with the index therapy were analyzed.
Results
A total of 2941 newly diagnosed patients with cancer who developed VTE and received anticoagulation in outpatient settings were identified. Of these, 97% initiated anticoagulation with LMWH (n=735; 25%), warfarin (n=1403; 47.7%), or rivaroxaban (n=709; 24.1%). Median treatment durations for the LMWH, warfarin, and rivaroxaban cohorts were 3.3, 7.9, and 7.9 months, respectively; Kaplan‐Meier rates of persistence to the initial therapy were 37%, 61%, and 61% at 6 months. Warfarin and rivaroxaban users were significantly more likely to remain on initial therapy compared to LMWH (adjusted hazard ratios [HRs; 95% CI]: warfarin, 0.33 [0.28‐0.38]; rivaroxaban, 0.38 [0.32‐0.46]). The proportion of patients that switched from their initial treatment to another anticoagulation treatment was 22.9%, 7.9%, and 4.7% in the LMWH, warfarin, and rivaroxaban cohorts, respectively.
Conclusions
This real‐world analysis showed that, despite guideline recommendations, warfarin and rivaroxaban are at least as equally utilized as LMWH for the treatment of cancer‐associated thrombosis. LMWH was associated with significantly lower persistence, shorter duration of treatment, and more switching than warfarin and rivaroxaban.
Keywords: anticoagulant, cancer, deep vein thrombosis, pulmonary embolism, thrombosis, venous thromboembolism
Essentials.
Therapeutic options for the management of VTE in patients with cancer remain limited.
Medical and pharmacy claims from the Humana Database were analyzed.
LMWH was associated with lower persistence and more switching than warfarin and rivaroxaban.
LMWH is at significantly higher risk to discontinue early relative to warfarin and rivaroxaban.
1. INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading cause of death for cancer patients.1 When compared to the general population, patients with cancer are associated with a 4.1‐fold risk of thrombosis, and the risk increases by 6.5‐fold for those undergoing chemotherapy.2, 3 Anticoagulation is key for the treatment and prevention of VTE recurrence. Current guidelines recommend anticoagulation with low–molecular‐weight heparin (LMWH) monotherapy for at least 3‐6 months for treatment and secondary prophylaxis in patients with cancer.4, 5 Treatment beyond the initial 6 months should also be considered for patients with metastatic disease or those receiving chemotherapy. These recommendations are based on clinical trials that show superiority of LMWH therapies over vitamin K antagonists (VKAs); however, efficacy superiority reached statistical significance only in one of the clinical trials (the CLOT trial).6, 7, 8
Vitamin K antagonists, primarily warfarin, are still widely used in clinical practice, but these drugs are difficult to manage in oncology patients: VKAs are associated with many interactions with other drugs and food. These interactions can lead to fluctuations in the international normalized ratio (INR), and despite the need for frequent monitoring, VKAs are associated with more bleeding complications than LMWH therapies.9, 10, 11
A recent meta‐analysis of randomized controlled trials also found that direct oral anticoagulants (DOACs) were as effective and safe as warfarin for the treatment of VTE among patients with cancer.12 In this population, a recent subgroup analysis of the EINSTEIN‐DVT and PE randomized clinical trial comparing rivaroxaban to VKAs showed that a single‐drug approach with the DOAC rivaroxaban resulted in similar efficacy and safety to VKAs.13
Little is known about the current utilization of anticoagulant agents in patients with cancer after the approval of DOAC treatments in recent years. The aim of the current study was to describe current treatment patterns and to evaluate patients’ persistence to anticoagulant agents.
2. METHODS
2.1. Data source
Medical and pharmacy claims from the Humana Database from January 2007 to December 2014 were used to conduct the analysis. The Humana database includes over 18 million covered lives of commercial and Medicare members in all census regions in the United States, but predominantly in the Midwest and South regions. Over 9 million members have both medical and pharmacy coverage. The present study used data elements from commercial and Medicare Advantage Part D (MA‐PD) members, such as demographics, enrollment history, inpatient and outpatient claims, emergency department visits, and pharmacy claims. Data were de‐identified and data collection complied with the requirements of the Health Insurance Portability and Accountability Act (HIPAA).
2.2. Study design
A retrospective cohort design was used to describe current treatment patterns and to evaluate persistence to therapy on various anticoagulants. Newly diagnosed patients with cancer were identified with at least 1 inpatient stay or 2 outpatient visits with a diagnosis of cancer during the study period (from January 2007 to December 2014). Patients with a first VTE diagnosis (index VTE) in 2013 or 2014 were identified to reflect current prescribing patterns of anticoagulant treatments (the approval date of rivaroxaban for treatment of VTE was November 2012).14 The index VTE had to occur 30 days before or any time after first cancer diagnosis. A 30‐day window before the cancer diagnosis was allowed to account for VTE as an early sign of cancer. Patients who received one or more anticoagulant prescription claims within 30 days after their VTE diagnosis were selected. Patients were required to have at least 6 months of baseline data prior to the index VTE. Patients with a prior history of VTE or anticoagulation before cancer and patients with a prior dispensing of an anticoagulant before the index VTE were excluded from the study.
Focusing on the most commonly prescribed anticoagulants, patients were classified into LMWH, warfarin, and rivaroxaban cohorts based on the first anticoagulant received. The only exception is the warfarin cohort, which represents patients who either initiated on warfarin alone or who received LMWH during a short duration, as a bridging agent. Use of other anticoagulants, including fondaparinux, heparin, apixaban, or dabigatran, was low and could not be analyzed due to the small sample size.
The observation period (follow‐up) spanned from the date of the first anticoagulant dispensing to the end of insurance eligibility, death, or the end of data availability (December 2014), whichever occurred earlier.
2.3. Study endpoints
Persistence on therapy was evaluated as continuous treatment on the index therapy, defined as no gap of more than 60 days between the end of a dispensing days of supply and the start date of the next dispensing, if any. The treatment duration was therefore calculated from the start date of the first dispensing of the index therapy until treatment non‐persistence, (not including the 60‐day gap) or the end of the follow‐up period.
2.4. Statistical analysis
Patient characteristics were summarized by treatment cohorts (ie, LMWH, warfarin, and rivaroxaban users). Descriptive statistics, including mean (standard deviation [SD]) for continuous data and relative frequency for categorical data, were generated to describe the baseline characteristics of the different treatment cohorts. Statistical differences between cohorts were assessed using Chi‐square tests (categorical variables) and Student's t tests (continuous variables). Patient characteristics were assessed during the 6‐month baseline period prior to the index VTE. The type of index cancer was also reported and may have occurred before the 6‐month baseline period.
Kaplan‐Meier survival analyses at 6 and 12 months were performed to evaluate and compare time to discontinuation between groups. Multivariate Cox proportional hazard models (ie, time to event analysis) were used to compare the time to discontinuation between cohorts. Covariates for the adjusted models included age, sex, cancer type, region, race, calendar quarter of first VTE (eg, 2013Q1), setting in which the VTE was diagnosed (inpatient, outpatient, or emergency department), type of VTE (DVT, PE, or both), Charlson comorbidity index (CCI), and health care costs during the 6‐month baseline period before the index VTE. In addition, surgeries (ie, major surgery,15, 16, 17 abdominal surgery, and surgery‐provoked VTE [ie, neurosurgery or orthopedic surgery]) and other type of provoked VTEs (ie, trauma, acute spinal cord injury, fracture, estrogen therapy, pregnancy/postpartum state, or oral contraceptive use)15 in the 30 days prior to the index date were also included as covariates in the model.
Since the number of days of supply of the first refill for oral anticoagulants is usually longer than for injectable anticoagulants, a sensitivity analysis was performed on the subset of patients treated for at least 30 days, thus excluding patients who received an initial treatment of a short duration. This sensitivity analysis assessed whether the differences in persistence between patients treated with injectable anticoagulants vs oral anticoagulants may have been inflated by the higher number of days of supplies of oral anticoagulant dispensings.
All statistical analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA).
3. RESULTS
3.1. Patients characteristics
A total of 2941 newly diagnosed VTE patients with cancer who were treated with anticoagulant agents were identified. Of these, 97% received anticoagulation with either LMWH (n = 735; 25%), warfarin (n = 1403; 47.7%), or rivaroxaban (n = 709; 24.1%; Table 1). Mean age and gender were similar across treatment cohorts. Approximately 90% of patients had primary diagnoses of solid tumors. Diagnoses for DVT, PE, and both DVT and PE (DVT/PE) were 55%, 27%, and 18%, respectively, and were similar across the drug cohorts. Patients with cancers associated with very high VTE risk (ie, stomach, pancreas, and brain) ranged from 15% in LMWH to 7.8% in rivaroxaban cohorts, while cancers associated with high VTE risk (ie, lung, lymphoma, gynecologic, bladder, testicular, and renal) ranged from 38% in LMWH to 28% in warfarin cohort.
Table 1.
Patient Characteristics
| LMWH [A] | Warfarin [B] | Rivaroxaban [C] | P‐valuesa | ||
|---|---|---|---|---|---|
| (N=735) | (N=1403) | (N=709) | [A] vs [B] | [A] vs [C] | |
| Age, mean (SD) [median] | 71.2 (10.4) [71.0] | 73.3 (10.5) [73.0] | 73.3 (9.5) [73.0] | <.001 | <.001 |
| Gender, female, n (%) | 389 (52.9) | 699 (49.8) | 353 (49.8) | .173 | .233 |
| Race/Ethnicity, n (%) | |||||
| White | 534 (72.7) | 1092 (77.8) | 567 (80.0) | .008 | .001 |
| Black | 101 (13.7) | 202 (14.4) | 79 (11.1) | .679 | .135 |
| Hispanic | 7 (1.0) | 9 (0.6) | 6 (0.8) | .428 | .831 |
| Other | 17 (2.3) | 21 (1.5) | 10 (1.4) | .175 | .206 |
| Unknown | 76 (10.3) | 79 (5.6) | 47 (6.6) | <.001 | .012 |
| Region,b n (%) | |||||
| South | 406 (55.2) | 779 (55.5) | 483 (68.1) | .900 | <.001 |
| Midwest | 227 (30.9) | 414 (29.5) | 151 (21.3) | .509 | <.001 |
| Northeast | 26 (3.5) | 33 (2.4) | 11 (1.6) | .122 | .399 |
| West | 76 (10.3) | 177 (12.6) | 64 (9.0) | .112 | .017 |
| Type of primary cancer,b n (%) | |||||
| Solid cancer | 675 (91.8) | 1,244 (88.7) | 633 (89.3) | .022 | .096 |
| Lung | 154 (21.0) | 216 (15.4) | 139 (19.6) | .001 | .525 |
| Prostate | 44 (6.0) | 191 (13.6) | 91 (12.8) | <.001 | <.001 |
| Breast | 50 (6.8) | 181 (12.9) | 87 (12.3) | <.001 | <.001 |
| Colorectal | 98 (13.3) | 166 (11.8) | 84 (11.8) | .316 | .395 |
| Other solid cancer | 329 (44.8) | 490 (34.9) | 232 (32.7) | <.001 | <.001 |
| Hematologic cancer | 70 (9.5) | 161 (11.5) | 93 (10.9) | .167 | .823 |
| Time from cancer to first VTE, n (%) | |||||
| Less than 6 months | 496 (67.5) | 736 (52.5) | 350 (49.4) | <.001 | <.001 |
| 6 months to 1 year | 79 (10.7) | 175 (12.5) | 89 (12.6) | .242 | .285 |
| More than 1 year | 160 (21.8) | 492 (35.1) | 270 (38.1) | <.001 | <.001 |
| Type of index VTE, n (%) | |||||
| PE | 200 (27.2) | 367 (26.2) | 200 (28.2) | .600 | .672 |
| DVT | 395 (53.7) | 782 (55.7) | 393 (55.4) | .378 | .519 |
| PE and DVT | 140 (19.0) | 254 (18.1) | 116 (16.4) | .593 | .181 |
| Index VTE | |||||
| Hospitalization, n (%) | 459 (62.4) | 950 (67.7) | 424 (59.8) | .015 | .302 |
| LOS (days), mean (SD) [median] | 6.4 (4.9) [5.0] | 7.6 (5.9) [6.0] | 5.9 (4.8) [5.0] | <.001 | .099 |
| Outpatient, n (%) | 194 (26.4) | 320 (22.8) | 189 (26.7) | .065 | .910 |
| Emergency department, n (%) | 82 (11.2) | 133 (9.5) | 96 (13.5) | .221 | .168 |
| VTE risk by cancer type at baseline,c n (%) | |||||
| Very high riskd | 110 (15.0) | 133 (9.5) | 55 (7.8) | <.001 | <.001 |
| High riske | 282 (38.4) | 387 (27.6) | 226 (31.9) | <.001 | .010 |
| Antineoplastic use at baseline,c n (%) | 92 (12.5) | 186 (13.3) | 104 (14.7) | .629 | .233 |
| Quan‐Charlson comorbidity index,c mean (SD) [median] | 5.0 (3.1) [6.0] | 4.6 (3.0) [4.0] | 4.2 (2.9) [4.0] | .001 | <.001 |
| Selected baseline comorbidities,c n (%) | |||||
| Hypertension | 486 (66.1) | 1,057 (75.3) | 503 (70.9) | <.001 | .143 |
| COPD | 181 (24.6) | 435 (31.0) | 220 (31.0) | .008 | .025 |
| Diabetes | 202 (27.5) | 438 (31.2) | 207 (29.2) | .199 | .733 |
| Congestive heart failure | 80 (10.9) | 221 (15.8) | 106 (15.0) | .009 | .068 |
| Liver diseases | 161 (21.9) | 179 (12.8) | 90 (12.7) | <.001 | <.001 |
| Obesity | 77 (10.5) | 172 (12.3) | 73 (10.3) | .474 | .927 |
| Atrial fibrillation/flutter | 43 (5.9) | 128 (9.1) | 69 (9.7) | .030 | .022 |
| Stroke/TIA | 42 (5.7) | 103 (7.3) | 36 (5.1) | .364 | .805 |
| Provoked VTEf , g, n (%) | 79 (10.7) | 180 (12.8) | 74 (10.4) | .161 | .848 |
| Prior surgeryf, n (%) | |||||
| Major surgery | 138 (18.8) | 279 (19.9) | 108 (15.2) | .538 | .073 |
| Abdominal | 190 (25.9) | 296 (21.1) | 113 (15.9) | .013 | <.001 |
| Surgery‐provoked VTEh | 13 (1.8) | 47 (3.3) | 20 (2.8) | .035 | .181 |
COPD = chronic obstructive pulmonary disease; DVT = deep vein thrombosis; LMWH = low–molecular‐weight heparin; LOS = length of stay; PE = pulmonary embolism; SD = standard deviation; TIA = transient ischemic attack; VTE = venous thromboembolism.
P‐values were estimated using Student t tests for continuous variables and Chi‐squared tests for categorical variables.
Not mutually exclusive.
Evaluated during the 6‐month baseline period.
Stomach, pancreas, or brain tumor.
Lung, lymphoma, gynecologic, bladder, testicular, or renal cancer.
Evaluated during the 30‐day period prior to the index VTE.
Defined as an index VTE with trauma, acute spinal cord injury, fracture, estrogen therapy, pregnancy/postpartum state, oral contraceptive use, neurosurgery or orthopedic surgery.
Defined as an index VTE with neurosurgery or orthopedic surgery.
Approximately 68% (warfarin) to 58% (rivaroxaban) of VTE cases were diagnosed in the inpatient setting. Overall, patients treated with LMWH had significantly more comorbidities compared to warfarin and rivaroxaban cohorts as shown by the Quan‐Charlson comorbidity index scores (5.0 vs 4.6 and 4.2, respectively; P‐values: <.05). Furthermore, patients in the LMWH cohort had significantly more liver disease (21.9%; P‐values: <.05), and significantly less atrial fibrillation (5.9%; P‐values: <.05) compared to the warfarin and rivaroxaban cohorts (Table 1). A similar proportion of patients in the 3 cohorts had provoked VTEs (10‐13%) and major surgery (15‐20%) in the 30 days prior to the index date. LMWH users had significantly more abdominal surgeries (26%) compared to patients treated with warfarin (21%) or rivaroxaban (16%), and significantly less surgery provoked‐VTE (1.8%) compared to warfarin (3.3%; Table 1).
3.2. Treatment patterns and persistence on anticoagulant therapy
The median follow‐up period was relatively short in all cohorts, ranging from 4.7 months in the LMWH cohort to 7.1 months in the warfarin cohort. Around one‐third of the patients died during the follow‐up period: 331 (45.0%), 475 (33.9%), and 219 (30.9%) patients in the LMWH, warfarin, and rivaroxaban cohorts, respectively. Over 60% of patients received anticoagulant treatment in outpatient settings within 7 days post VTE diagnosis (Table 2). Approximately one‐quarter of patients who initiated LMWH switched to another anticoagulant therapy, while lower switching rates of 8% and 5% were observed for warfarin and rivaroxaban, respectively (Figure 1).
Table 2.
Treatment Patterns and Mortality During the Study Period
| Treatment patterns | LMWH | Warfarin | Rivaroxaban |
|---|---|---|---|
| (N=735) | (N=1403) | (N=709) | |
| Eligibility post VTE, months, mean [median] | 6.9 [4.7] | 8.9 [7.1] | 6.9 [5.2] |
| Time from first VTE to therapy, days, mean [median] | 6.6 [4.0] | 8.0 [6.0] | 6.1 [4.0] |
| 0‐7 days, n (%) | 516 (70.2) | 845 (60.2) | 526 (74.2) |
| 7‐14 days, n (%) | 137 (18.6) | 321 (22.9) | 101 (14.2) |
| 14‐30 days, n (%) | 80 (10.9) | 232 (16.5) | 77 (10.9) |
| Died during follow‐up, n (%) | 331 (45.0) | 475 (33.9) | 219 (30.9) |
| Initiated a new anticoagulant therapy after discontinuation,a n (%) | 71 (9.7) | 94 (6.7) | 39 (5.5) |
LMWH = low–molecular‐weight heparin; VTE = venous thromboembolism.
Discontinuation was defined as a gap of more than 60 days between the end of the days of supply of a dispensing and the start date of the next dispensing of the index therapy, if any.
Figure 1.
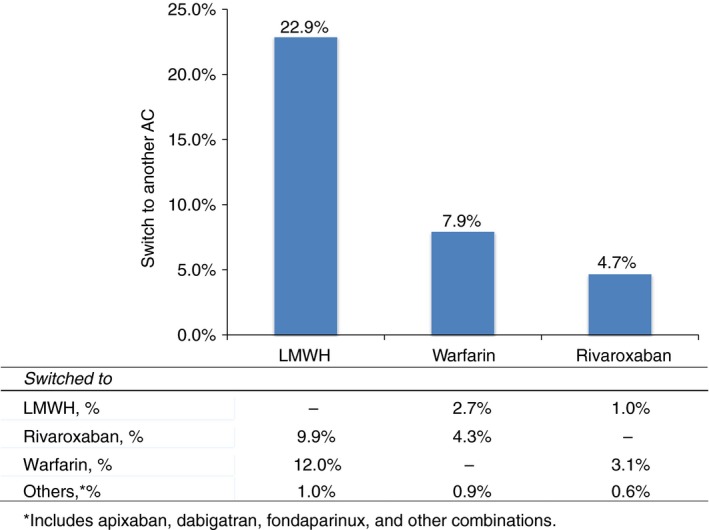
Patterns of anticoagulant transition. *Includes apixaban, dabigatran, fondaparinux, and other combinations
Patients treated with LMWH were less persistent on therapy compared to other treatment groups. Persistence with the initial therapy at 6 and 12 months, respectively, was 37% and 21% for LMWH users, 61% and 35% for warfarin users, and 61% and 36% for rivaroxaban users (Figure 2). The corresponding median treatment duration was 3.3 months for LMWH users and 7.9 months for warfarin and rivaroxaban users. Compared to LMWH users, users of oral agents were significantly less likely to discontinue therapy, with hazard ratios (HRs; 95% confidence interval [CI]) of 0.33 (0.28‐0.38) for warfarin users and 0.38 (0.32‐0.46) for rivaroxaban users (Figure 3).
Figure 2.
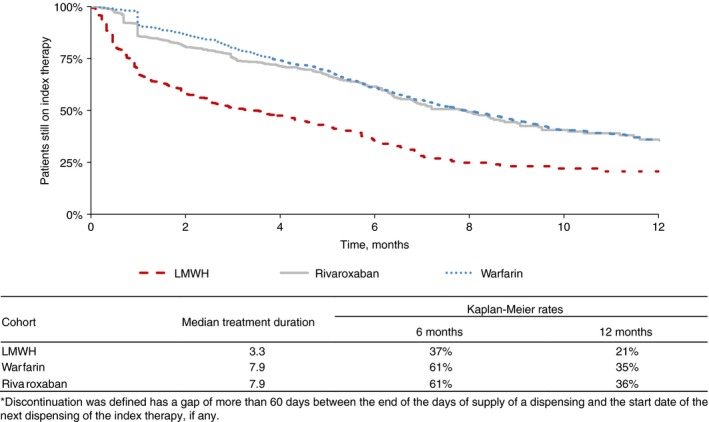
Persistence on index therapy. (Discontinuation was defined as a gap of more than 60 days between the end of the days of supply of a dispensing and the start date of the next dispensing of the index therapy, if any)
Figure 3.
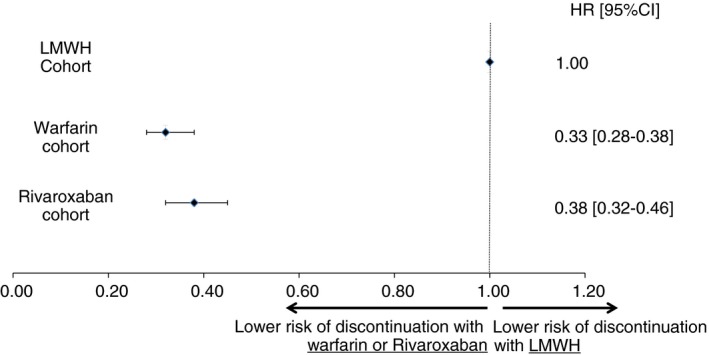
Adjusted risk of discontinuation of the index therapy.1
Note: Adjusted for age, sex, cancer type, region, race, calendar quarter of first venous thromboembolism (VTE) (eg, 2013Q1), setting in which the VTE was diagnosed (inpatient, outpatient, or emergency department), type of VTE (deep vein thrombosis, pulmonary embolism, or both), Charlson comorbidity index (CCI), and health care costs during the 6‐month period before the index VTE, and surgeries (ie, major surgery, abdominal surgery, and surgery‐provoked VTE) and other types of provoked VTEs in the 30 days prior to the index VTE
The sensitivity analysis performed on the subset of patients persistent for at least 30 days on the initial anticoagulant included a total of 453, 1268, and 597 patients in the LMWH, warfarin, and rivaroxaban cohorts, respectively. This sensitivity analysis only included patients treated for the standard refill of an oral warfarin prescription (ie, 30 days). The median duration of therapy was 6.0, 8.3, and 8.5 months in the LMWH, warfarin, and rivaroxaban cohorts respectively. In the sensitivity analysis (ie, patients treated for at least 30 days), LMWH/warfarin, warfarin, and rivaroxaban users were still significantly less likely to discontinue therapy compared to LMWH users, with adjusted HRs (95% CI) of 0.64 (0.53‐0.77) for the warfarin users and 0.65 (0.52‐0.82) for rivaroxaban users.
4. DISCUSSION
A large medical and pharmacy claims database was used to describe current treatment patterns and assess patient persistence on anticoagulant therapies for cancer‐associated thrombosis. Despite treatment guidelines, recommendations for the use of LMWH monotherapy for the treatment of and as secondary prophylaxis for VTE, over 70% of patients were treated with oral anticoagulants. Patients initiating with LMWH treatment persisted significantly shorter and were significantly more likely to discontinue the treatment relative to patients treated with warfarin or rivaroxaban. Approximately one‐quarter of patients who initiated on LMWH switched to other anticoagulant agents during the course of treatment compared to 8% and 5% patients observed with warfarin and rivaroxaban, respectively.
Since the publication of the CLOT study7 that demonstrated LMWH superiority over warfarin in a randomized control trial evaluating the risk of reoccurrence of VTE in cancer patients, guidelines started to recommend LMWH therapy over warfarin. Currently, all major guidelines, including the American College of Chest Physicians (ACCP),5 the American Society of Clinical Oncology (ASCO),4 the National Comprehensive Cancer Network (NCCN),18 and the European Society for Medical Oncology (ESMO),11 recommend LMWH for the treatment of VTE and as secondary prophylaxis in patients with cancer. Despite promising results for DOAC in cancer patients,12, 19 use of DOACs for either prevention or treatment of VTE in cancer patients is not recommended at the time4 based on a lack of evidence of efficacy and safety in patients with cancer.
In the current study, only 25% of patients were treated with a LMWH therapy as a single agent. The risk of discontinuation in these patients was significantly higher than in oral treatments. The low use of LMWH in cancer patients was observed in multiple other studies. Delate and colleagues20 observed that the use of LMWH only increased from 18% in 2000 to 31% in 2007 after the recommendations by the ACCP. Farge and colleagues21 reported that LMWH was used in 36% of patients in early maintenance (11‐90 days) and in 41% of patients for long‐term therapy (beyond 3 months). A few studies have identified reasons for the low utilization or discontinuation on LMWH. Even though physicians are aware of the guideline recommendations,22 reasons for noncompliance included: patients could not afford the out‐of‐pocket expense, the referring physician preferred that the patient received warfarin instead of LMWH therapies, or patients refused the long‐term use of injections.23
The current results showed that persistence on the initial anticoagulant therapy in cancer patients is very low in patients treated with initial LMWH. The persistence rate at 6 months was 37% for patients treated with LMWH and 61% for patients treated with oral therapies. Studies on persistence with anticoagulant therapy in cancer patients are limited, but similar real‐world studies also found that patients receiving injectable anticoagulants were less persistent than those treated with oral anticoagulants.24 This might also reflect the treatment patterns of physicians, prescribing LMWH for only short periods despite treatment recommendations among active cancer patients. Additionally, in patients with risk factors for VTE, including patients with cancer‐related VTE, adherence on warfarin was also reported to be low: 77% of patients treated with warfarin had a proportion of days covered lower than 0.8 during the year after initiation of therapy.25
The low persistence observed with LMWH is further reflected in the high‐switching rates from LMWH to other anticoagulants. In a previous study of 52,911 patients with cancer who were treated with an anticoagulant, 44% of the 21,164 patients treated with LMWH switched to another anticoagulant and 28% of the 26,456 patients treated with warfarin switched to another anticoagulant.24 The percentage of patients who switched in the current study was lower than what was reported in these other studies. This may be related to the shorter follow‐up in the current study due to our focus on a study population with VTE diagnosed after 2013.
This retrospective cohort analysis has certain limitations. First, in spite of information accuracy and completeness required by administrative databases for payment purposes, billing inaccuracies and missing data may still occur. Second, there were no data on patient adherence, and we assumed that medications supplied were actually used by the patients, which could have led to an overestimation of patients’ adherence. Third, the observational design was susceptible to biases such as information or classification bias (eg, identification of false‐positive VTE events). It is also possible that VTE events were under‐coded (ie, false‐negative). Finally, like all observational studies, adjustments in the multivariate analyses could only account for observable factors. For example, information on remission for patients who completed all cancer treatment was not available in the database which could impact the rate of adherence if anticoagulation therapy was discontinued due to remission. However, we do not have reasons to believe that this would have affected the findings since LMWH is frequently used in high‐risk settings24 and patients with lower risk for recurrent VTE may be less likely to receive LMWH, which suggests that the LMWH group may have had a longer duration of intended therapy if there was a difference in the remission status among the 3 groups. Despite these limitations, the current study has several strengths, including reliance on the real‐world utilization of LMWH, warfarin, and rivaroxaban use in patients with cancer, and it is consistent with prior similar analyses.
This real‐world analysis of current treatment patterns for cancer‐associated thrombosis showed that warfarin is still the most commonly used anticoagulant and that rivaroxaban is as commonly used as LMWH, despite guideline recommendations. Patients on LMWH had a significantly lower persistence and a shorter duration of treatment than patients on warfarin or rivaroxaban during the course of treatment. Patients initiating on these oral agents are at significantly lower risk to discontinue therapy relative to LMWH. Furthermore, more patients switched from LMWH to other anticoagulants compared with patients who had started on warfarin or rivaroxaban treatments.
AUTHOR CONTRIBUTIONS
A. A. Khorana, D. Milentijevic, J. Fortier, F. Laliberté, and P. Lefebvre contributed to study conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing/revisions. K. R. McCrae and D. Yannicelli contributed to data analysis and interpretation and manuscript writing/revisions. W. W. Nelson, C. Crivera, and J. Schein contributed to study conception and design, data analysis and interpretation, and manuscript writing/revisions. All authors provided final approval of the submitted manuscript.
RELATIONSHIP DISCLOSURE
Financial support for this research was provided by Janssen Scientific Affairs, LLC (JSA). Alok A. Khorana and Keith R. McCrae have received research grants from JSA. Jonathan Fortier, François Laliberté, and Patrick Lefebvre are employees of Groupe d'analyse, Ltée, a consulting company that has received research grants from JSA. Dejan Milentijevic, Winnie W. Nelson, Concetta Crivera, Daniel Yannicelli, and Jeff Schein are employees of JSA.
ACKNOWLEDGMENTS
Editorial support was provided by Alanna Franchetti, ELS, of MedErgy (Yardley, PA), and was funded by JSA.
Khorana AA, McCrae KR, Milentijevic D, et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost. 2017;1:14–22. 10.1002/rth2.12002
REFERENCES
- 1. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–34. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160:809–15. [DOI] [PubMed] [Google Scholar]
- 3. Gerotziafas GT, Mahé I, Elalamy I. New orally active anticoagulant agents for the prevention and treatment of venous thromboembolism in cancer patients. Ther Clin Risk Manag. 2014;10:423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lyman GH, Bohlke K, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract. 2015;11:e442–44. [DOI] [PubMed] [Google Scholar]
- 5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 6. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low‐molecular‐weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729–35. [DOI] [PubMed] [Google Scholar]
- 7. Lee AY, Levine MN, Baker RI, et al.; Randomized Comparison of Low‐Molecular‐Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. [DOI] [PubMed] [Google Scholar]
- 8. Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180‐day period. Clin Appl Thromb Hemost. 2006;12:389–96. [DOI] [PubMed] [Google Scholar]
- 9. Hull RD, Pineo GF, Brant RF, et al.; LITE Trial Investigators. Long‐term low‐molecular‐weight heparin versus usual care in proximal‐vein thrombosis patients with cancer. Am J Med. 2006;119:1062–72. [DOI] [PubMed] [Google Scholar]
- 10. Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11:56–70. [DOI] [PubMed] [Google Scholar]
- 11. Mandalà M, Falanga A, Roila F. Management of venous thromboembolism in cancer patients: ESMO clinical recommendations. Ann Oncol. 2008;19:ii 126–27. [DOI] [PubMed] [Google Scholar]
- 12. Van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2014;12:320–28. [DOI] [PubMed] [Google Scholar]
- 13. Prins MH, Lensing AW, Bauersachs R, et al.; EINSTEIN Investigators. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thromb J. 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Food and Drug Administration . FDA expands use of Xarelto to treat, reduce recurrence of blood clots [Internet]. [cited 2016 Mar 24]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm326654.htm
- 15. Laliberté F, Coleman CI, Bookhart B, et al. Weekly risk of venous thromboembolism recurrence in patients receiving oral anticoagulants. Curr Med Res Opin. 2014;30:1513–20. [DOI] [PubMed] [Google Scholar]
- 16. Merli GJ, Hollander JE, Lefebvre P, et al. Rates of hospitalization among patients with deep vein thrombosis before and after the introduction of rivaroxaban. Hospital Practice. 1995;2015(43):85–93. [DOI] [PubMed] [Google Scholar]
- 17. Merli GJ, Hollander JE, Lefebvre P, et al. Costs of hospital visits among patients with deep vein thrombosis treated with rivaroxaban and LMWH/warfarin. J Med Econ. 2016;19:84–90. [DOI] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network (NCCN) . Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Venous Thromboembolic Disease Version 2.2013. 2014.
- 19. Posch F, Königsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta‐analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delate T, Witt DM, Ritzwoller D, et al. Outpatient use of low molecular weight heparin monotherapy for first‐line treatment of venous thromboembolism in advanced cancer. Oncologist. 2012;17:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farge D, Trujillo‐Santos J, Debourdeau P, et al.; RIETE Investigators. Fatal events in cancer patients receiving anticoagulant therapy for venous thromboembolism. Medicine (Baltimore). 2015;94:e1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kleinjan A, Aggarwal A, Van de Geer A, et al. A worldwide survey to assess the current approach to the treatment of patients with cancer and venous thromboembolism. Thromb Haemost. 2013;110:959–65. [DOI] [PubMed] [Google Scholar]
- 23. Wittkowsky AK. Barriers to the long‐term use of low‐molecular weight heparins for treatment of cancer‐associated thrombosis. J Thromb Haemost. 2006;4:2090–91. [DOI] [PubMed] [Google Scholar]
- 24. Khorana AA, Yannicelli D, McCrae KR, et al. Evaluation of US prescription patterns: are treatment guidelines for cancer‐associated venous thromboembolism being followed? Thromb Res. 2016;145:51–53. [DOI] [PubMed] [Google Scholar]
- 25. Chen SY, Wu N, Gulseth M, et al. One‐year adherence to warfarin treatment for venous thromboembolism in high‐risk patients and its association with long‐term risk of recurrent events. J Manag Care Pharm. 2013;19:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]


