Graphical abstract
Keywords: Cardiac resynchronization therapy, Echocardiography, Fabry disease
Highlights
-
•
Cardiac Fabry disease is rare, but often leads to a poor prognosis for patients with heart failure.
-
•
Echocardiography could reveal a subtle change in the left ventricular wall motion.
-
•
Combination therapy including cardiac resynchronization therapy improved the prognosis for patients with cardiac Fabry disease and heart failure.
Introduction
The cardiac variant of Fabry disease is associated with significant morbidity and early death. We report the case of a 60-year-old man who was admitted with severe dyspnea at rest. He developed heart failure with severe left ventricular (LV) systolic dysfunction, sustained atrial flutter, and frequent nonsustained ventricular tachycardia. He was diagnosed with the cardiac variant of Fabry disease and was treated with flutter ablation, optimal medical therapy, and cardiac resynchronization therapy (CRT) with a defibrillator. Three years after CRT, he is totally asymptomatic and is able to perform activities of daily living and work with no limitations. This report highlights the role of advanced echocardiography to evaluate LV function in patients with the cardiac variant of Fabry disease.
We previously reported a case of cardiac variant of Fabry disease treated with CRT.1 Three years of follow-up on this rare case will be detailed, as excellent images were successfully recorded along with the clinical course. This report highlights the role of advanced echocardiography to evaluate LV function in patients with cardiac variant of Fabry disease.
Case Presentation
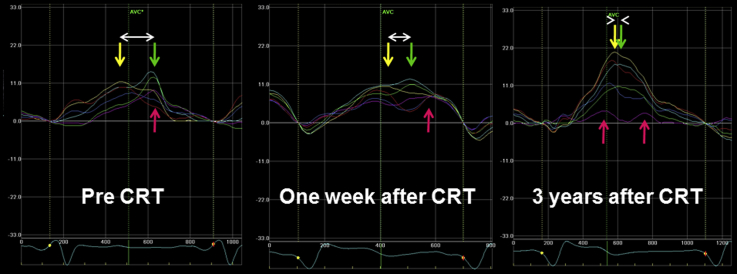
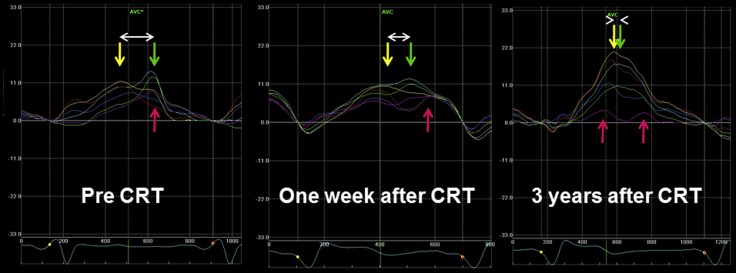
A 60-year-old man who had received a diagnosis of hypertrophic cardiomyopathy 13 years previously (Figure 1) was admitted to our hospital with severe dyspnea at rest (New York Heart Association class IV). He had developed heart failure with severe LV systolic dysfunction with an LV ejection fraction of 20% (Videos 1 and 2), sustained atrial flutter (Figure 2), and frequent nonsustained ventricular tachycardia. Dyssynchrony in the left ventricle was observed as a time delay of 164 msec between the anterior-septal and lateral wall peak strain before CRT2 (Figure 3, left). He was treated with atrial flutter ablation, optimal medical therapy, and CRT with a defibrillator. One week after CRT, the time difference had decreased to 89 msec, suggesting reduction of LV dyssynchrony (Figure 3, middle). We diagnosed the cardiac variant of Fabry disease by enzyme assay and endomyocardial biopsy, as there were no clinical signs of ocular, renal, or skin involvement. Single-photon emission computed tomography showed poor perfusion in the posterior wall of the left ventricle (Figure 4). On cardiac magnetic resonance imaging, a late-enhanced signal after gadolinium infusion was observed in the same lesion.1 After treatment of acute heart failure with optimal medication (spironolactone 25 mg, furosemide 20 mg, carvedilol 15 mg, candesartan 4 mg, and warfarin 3 mg), enzyme replacement therapy with agalsidase-β at 1 mg/kg every other week was instituted. Three years after CRT, echocardiography revealed improved LV wall motion (Videos 3 and 4), and no significant LV dyssynchrony (Figure 3, right) was observed. Meanwhile, peak systolic strain value in posterior segment (Figure 3, purple arrow) significantly decreased, suggesting deterioration of regional myocardial function, although peak systolic strain value in the anteroseptum (Figure 3, yellow), septum (Figure 3, red), and anterior (Figure 3, light blue) segments increased after CRT. At present, his condition is stable, and he has no functional limitations (New York Heart Association class I).
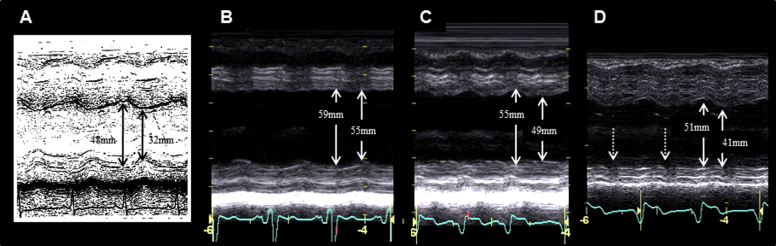
Figure 1.
M-mode echocardiograms. Arrows indicate LV end-diastolic and end-systolic dimensions. Normal contraction with LV hypertrophy 13 years before admission (A), dilated left ventricle with abnormal wall motion before CRT (B), decrease in LV dimensions and slight improvement in LV contraction 1 week after CRT (C), and normalized LV dimensions and further improvement in LV contraction 3 years after CRT (D) were seen (fractional shortening 33%, 7%, 11%, and 20%, respectively). Of note, posterior wall motion deteriorated (broken arrows).
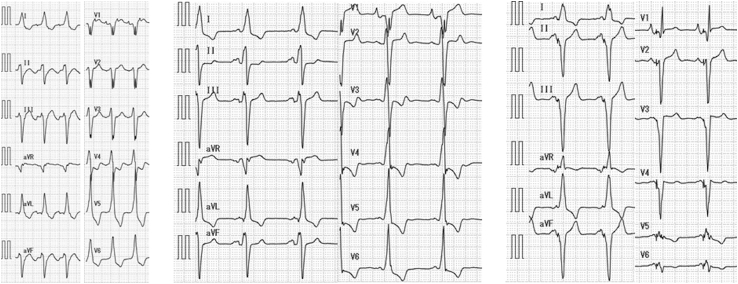
Figure 2.
Twelve-lead electrocardiograms. (Left) Typical atrial flutter and 2:1 atrioventricular conduction with left bundle branch block (QRS duration 160 msec) was observed on admission. (Middle) Sinus rhythm was maintained after ablation for atrial flutter (QRS duration 160 msec). (Right) Electrocardiogram of biventricular pacing shows the narrowing of QRS duration (100 msec).
Figure 3.
Speckle-tracking radial strain images in the midventricular short-axis view. The curves are color-coded by the defined myocardial regions as depicted in the figure (yellow, anterior septum; light blue, anterior segment; green, lateral; purple, posterior; dark blue, inferior; red, septum). Dyssynchrony is shown as the difference (white arrow) in the timing of peak systolic strain between the anteroseptal (yellow) and posterior wall (green).
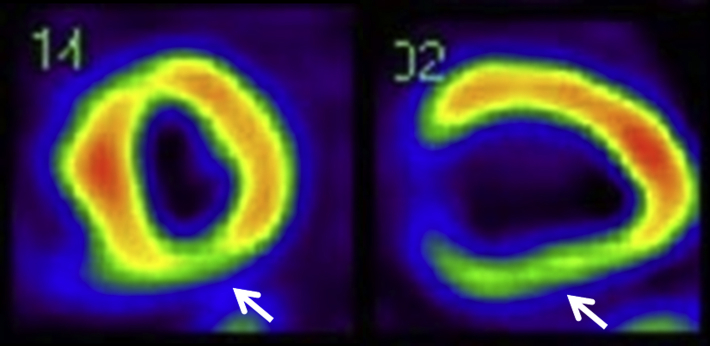
Figure 4.
Single-photon emission computed tomography. LV short-axis (left) and long-axis (right) images showed reduced uptake of 99mTc-tetrofosmin at the posterior wall (arrows).
Observing echocardiographic images along with the clinical course, we recognized valuable information about LV function, such as (1) decreased LV filling pressure immediately after CRT (Figure 5), (2) a decrease in LV dimensions and improvement in LV ejection fraction 1 week after CRT (Figure 1), (3) further improvement in LV ejection fraction during the long follow-up period after CRT (Table 1), (4) improvement in LV dyssynchrony (Figure 3); and (5) deterioration in posterior wall contraction (Figure 6).
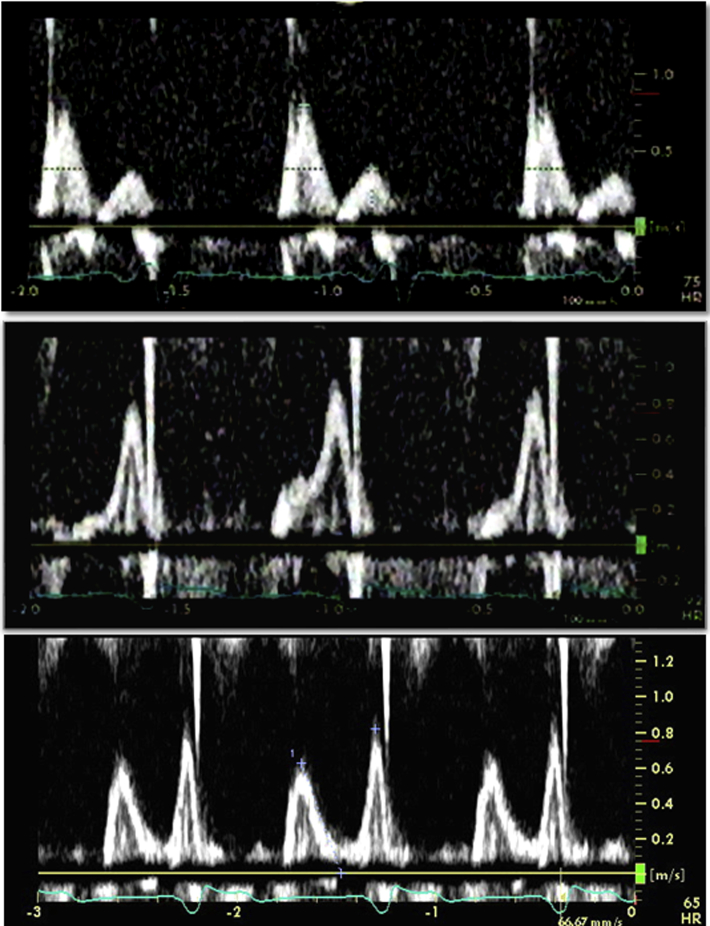
Figure 5.
Changes in transmitral flow velocity profile using pulsed Doppler imaging. A restrictive pattern with deceleration time of 126 msec before CRT (top) and an abnormal relaxation pattern with deceleration time of 148 msec immediately after CRT (middle) were demonstrated and suggested a decrease in LV filling pressure. An abnormal relaxation pattern with deceleration time of 200 msec was still evident 3 years after CRT (bottom).
Table 1.
Time courses of echocardiographic parameters
| Before CRT | 1 week after CRT | 3 years after CRT | |
|---|---|---|---|
| LVEDV (mL) | 130 | 126 | 92 |
| LVESV (mL) | 99 | 81 | 47 |
| LVEF (%) | 24 | 36 | 52 |
| E/A ratio | 2.07 | 0.47 | 0.76 |
| DT (msec) | 126 | 148 | 200 |
| E/e′ ratio | 21.6 | 16.8 | 16.3 |
| Tei index | 0.95 | 0.90 | 0.66 |
DT, Deceleration time in E wave; LVEDV, LV end-diastolic volume; LVESV, LV end-systolic volume; LVEF, LV ejection fraction.
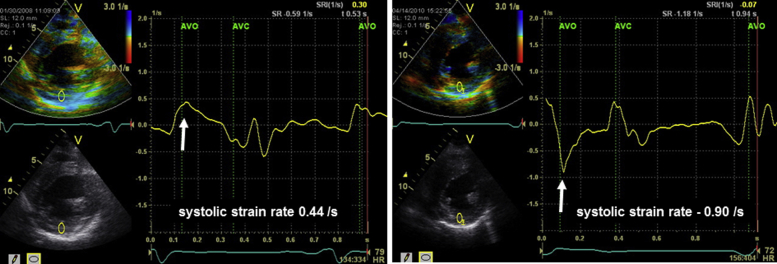
Figure 6.
Tissue Doppler radial strain images in the midventricular short-axis view. Before combination therapy (left), systolic strain rate in posterior wall was 0.44 sec−1 (white arrow), suggesting decreased myocardial function. On follow-up after combination therapy (right), it worsened to −0.90 sec−1 (white arrow), suggesting further deterioration of regional contractility.
Discussion
Fabry disease is an X-linked lysosomal storage disorder caused by deficient activity of the enzyme α-galactosidase A. The cardiac variant of Fabry disease is a relatively prevalent cause of hypertrophic cardiomyopathy and is associated with significant morbidity and early death due to heart failure or ventricular arrhythmias.3 In the case we present, however, LV function and performance status have remarkably improved. The Tei index, which is reported to be able to detect global LV dysfunction in patients with Fabry disease,4 also increased during follow-up after CRT (Table 1). Of course, ablation and optimal medical therapy additionally might improve LV function. As such, we cannot clearly assert the favorable effects of enzyme replacement therapy on myocardial damage, especially in the posterior wall, because the pathologic status had proceeded too far in this case. For confirmation, we have documented the decrease of systolic strain rate value in the posterior wall (Figure 6), as Weidemann et al.5 previously reported strain rate as the primary detector of LV myocardial damage in Fabry disease. This decrease might indicate gradual deterioration of regional myocardial function despite the combination therapy.
To the best of our knowledge, this is the first reported case of Fabry disease with heart failure successfully observed with a long course of combination therapy including CRT. In this rare case, echocardiographic images demonstrated a change for the better in LV dyssynchrony, revealed an improvement in global LV function, and showed a further deterioration of regional myocardial function in the posterior wall, suggesting that even optimal medical therapy may not be able to mitigate all of the pathologic effects of Fabry disease.
Conclusions
In conclusion, echocardiography was useful for sequential evaluation during treatment for cardiac Fabry disease with heart failure.
Footnotes
Conflicts of Interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2017.04.004.
Supplementary Data
Apical four-chamber view before CRT.
Parasternal short-axis view before CRT.
Apical four-chamber view during long follow-up after CRT.
Parasternal short-axis view during long follow-up after CRT.
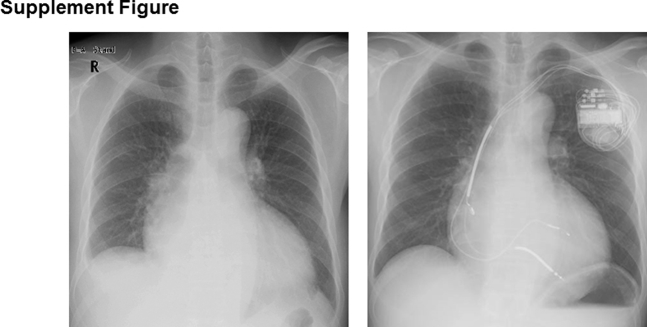
Supplemental Figure 1.
Chest x-ray before (left) and after (right) CRT.
References
- 1.Fukuzawa K., Yoshida A., Onishi T., Suzuki A., Kanda G., Takami K. Dilated phase of hypertrophic cardiomyopathy caused by Fabry disease with atrial flutter and ventricular tachycardia. J Cardiol. 2009;54:139–143. doi: 10.1016/j.jjcc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Suffoletto M.S., Dohi K., Cannesson M., Saba S., Gorcsan J., III Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–968. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 3.Takenaka T., Teraguchi H., Yoshida A., Taguchi S., Ninomiya K., Umekita Y. Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol. 2008;51:50–59. doi: 10.1016/j.jjcc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Niemann M., Breunig F., Beer M., Hu K., Liu D., Emmert A. Tei index in Fabry disease. J Am Soc Echocardiogr. 2011;24:1026–1032. doi: 10.1016/j.echo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Weidemann F., Breunig F., Beer M., Sandstede J., Turschner O., Voelker W. Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation. 2003;108:1299–1301. doi: 10.1161/01.CIR.0000091253.71282.04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical four-chamber view before CRT.
Parasternal short-axis view before CRT.
Apical four-chamber view during long follow-up after CRT.
Parasternal short-axis view during long follow-up after CRT.