Key Points
Gene therapy is cost-effective in severe hemophilia A compared with standard factor VIII prophylaxis.
Over a 10-year time horizon, gene therapy cost $1M and resulted in 8.33 QALYs gained, whereas prophylaxis cost $1.7M and resulted in 6.62 QALYs gained.
Abstract
Gene therapy provides a potential phenotypic cure for hemophilia, yet the cost of this novel treatment is high, tempering enthusiasm and raising questions regarding cost vs benefit. To evaluate the cost-effectiveness of gene therapy treatment of severe hemophilia A compared with prophylaxis with factor VIII (FVIII), we developed a Markov state–transition model to estimate the costs and effectiveness of severe hemophilia A treatment strategies from a United States health care system perspective. Quality-adjusted life-years (QALYs) were the effectiveness measure. In the base case, hypothetical cohorts of 30-year-old patients received gene therapy or FVIII prophylaxis. We obtained model probabilities and utilities from the literature and costs from Medicare reimbursement data. One-way and probabilistic sensitivity analyses were performed to test the robustness of results. Over a 10-year time horizon, total per-person gene therapy strategy costs were $1.0M and resulted in 8.33 QALYs, whereas prophylaxis cost $1.7M and resulted in 6.62 QALYs. Thus, gene therapy dominated prophylaxis (costs less and was more effective). Gene therapy remained dominant unless initial costs exceeded $1.6M and were <$100 000 per 1 QALY gained compared with prophylaxis if initial costs were <$1.7M. Results were not sensitive to variation of all other parameters over clinically plausible ranges. In a probabilistic sensitivity analysis simultaneously varying all parameters 3000 times over parameter distributions, gene therapy was dominant in 92% of model iterations. Treatment of severe hemophilia A with gene therapy is likely to be cost-saving or cost-effective compared with FVIII prophylaxis.
Visual Abstract
Introduction
Advances in gene therapy have placed a curative treatment of hemophilia A on the immediate horizon. Although not yet clinically available, there is significant concern and debate about the potential cost of such treatment. To appropriately evaluate the cost-effectiveness of gene therapy, the natural history of the disease and the current standards and cost of care need to be considered. In this study, we evaluated the cost-effectiveness of gene therapy treatment of patients with severe hemophilia A compared with current standard factor VIII (FVIII) for bleed prevention, prophylaxis, and bleed treatment.
Hemophilia A is an X-linked bleeding disorder caused by mutations in the gene coding for FVIII, resulting in deficient and/or defective coagulation, leading to spontaneous or traumatic bleeding into joints, muscles, or body cavities.1-4 When bleeds recur in the same joint, the synovial lining thins and joint arthropathy ensues, leading to disability, pain, and reduced quality of life.5 The disease affects ∼20 000 patients in the United States; of the estimated 400 000 affected globally, most have no access to clotting factor treatment, and many die of bleeding at a young age.2,3 In the United States, the standard of care is to prevent bleeds by the prophylactic administration of intravenous clotting FVIII 2-3 times weekly, to maintain a trough FVIII level >1% (0.01 IU/mL) to prevent spontaneous bleeding.4 Although prophylaxis reduces joint bleeding and joint damage and improves quality of life, the burden of treatment is high; as a result, most adults do not continue prophylaxis.5-7 Despite prophylaxis, breakthrough bleeding may occur with sports and other activities, further contributing to joint damage, pain, and disability and reducing quality of life.8
Although the benefit of FVIII prophylaxis in preventing joint damage and disability is well established, the cost of hemophilia treatment is among the highest for a single disease. An evaluation of Medicare Part B spending in 2010 identified hemophilia treatment as the most costly drug per average beneficiary.9 An uncomplicated severe hemophilia A patient costs >$140 000 per year based on private and government reimbursements.10,11 For the 30% of hemophilia patients who develop inhibitors, the cost exceeds $1M per year because more aggressive treatment is required, which is associated with poorer response to treatment than in a noninhibitor patient.12 In fact, clotting factor treatment accounts for >80% of the total health care expenditures for individuals with hemophilia.10,11 A typical hemophilia patient incurs inpatient costs 9 times higher than the average insured patient.13 In addition to the direct costs of treatment, patients experience many indirect costs of the disease related to loss of productivity, absenteeism, disability, and poor quality of life.14
Experimental gene therapy for hemophilia A uses a single infusion of an adeno-associated viral vector containing an optimized gene coding for human FVIII.15-17 The adeno-associated viral vector contains a liver-specific promoter that ensures liver-specific FVIII expression.15 Phase 1/2 dose-escalation trials have demonstrated sustained FVIII expression for >2 years, with marked reduction in bleeds and no inhibitor formation.16 Similarly, gene therapy trials in hemophilia B, which have been ongoing longer than those for hemophilia A, have resulted in stable factor expression for >7 years.18 Given the current burden of the disease and high cost of treatment of individuals with hemophilia, there is potential for gene therapy to offer a cost-effective therapeutic option.
Materials and methods
Overview of the model
We built a Markov state–transition model to evaluate the cost-effectiveness of gene therapy vs prophylaxis in a hypothetical cohort of 30- to 40-year-old male patients with uncomplicated severe hemophilia A. We defined uncomplicated hemophilia as patients without inhibitors, hepatitis C virus, or HIV. This age group was selected because of their wide representation in observational and clinical hemophilia trials. We did not include on-demand therapy in the base-case analysis, because this is not a chosen strategy; instead, it is a fallback option, with many patients using it secondary to compliance issues with prophylaxis. We took a third-party payer perspective, focusing on the direct medical costs of providing care. Effectiveness was measured using quality-adjusted life years (QALYs), the product of time spent in health states and the quality-of-life utility values of those states. We discounted future costs and effectiveness at an annual rate of 3%. We constructed our model using TreeAge Pro 2017 (TreeAge Software, Williamstown, MA).
Model assumptions
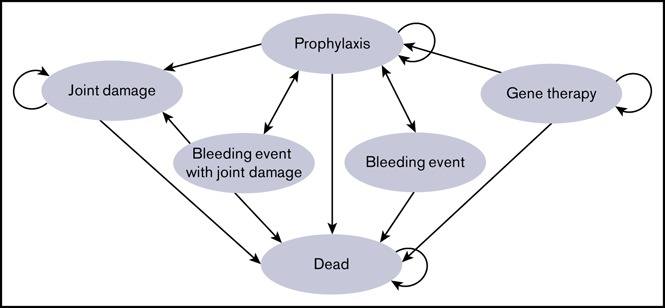
Cohorts entered the model assigned to prophylaxis or gene therapy strategies and cycled monthly through health states (Figure 1) over a 10-year time horizon. We chose 10 years because, although clinical data for the effectiveness of hemophilia A gene therapy are limited to 2 years,16 animal models have demonstrated the effectiveness of this treatment past 10 years,17 and similar gene therapy for hemophilia B has sustained results for >7 years.18 Patients undergoing prophylaxis each month could experience a bleeding event or joint damage, which could result in outpatient or inpatient therapy. Patients undergoing gene therapy had success, with transition to a postgene therapy state, or failed gene therapy, with transition to prophylaxis. Postgene therapy, patients could experience gene therapy–related complications during the first 12 months following gene therapy.
Figure 1.
Markov model. Patients remain in a health state (curved arrows) or move from 1 health state to another (straight arrows) based on transition probabilities. As patients cycle through the model, they accumulate costs and utilities expressed as QALYs. The Markov cycle length is 1 month and repeats over 10 years. During each cycle, patients are at risk for death from any cause, a bleeding event with or without joint damage. Gene therapy patients have an initial 1-year risk for failed therapy, resulting in transfer to the prophylaxis state.
We made several additional assumptions. First, patients undergoing gene therapy were assigned a success rate of 90% (varied from x-y% in sensitivity analysis), and patients who were successfully treated with gene therapy were assigned a utility of 1 (varied from x-1). This success rate is lower than what has been reported in phase 1/2 gene therapy trials in an attempt to capture some uncertainty for this currently unavailable therapy.16 Second, gene therapy was assigned a complication rate of 1% per month over the first year, and patients suffering a complication were assigned utility of 0.8 for that month. Third, gene therapy was assigned a cost of $850 000 in the base-case analysis, chosen based on the cost of the only commercially available gene therapy in the United States.19,20 Third, patients in the gene therapy and prophylaxis groups experience the same probability of death.21,22
Base-case estimates
The base-case estimates and ranges of all clinical probabilities, quality-of-life measures (utilities), and costs used in the model are displayed in Table 1. Ranges of utilities for health states from quality-of-life and cost-utility studies were used in the analysis, using an average for published values in the base-case analysis.
Table 1.
Base-case estimates and ranges used in sensitivity analysis
| Variables | Base-case estimate | Range | References | Notes |
|---|---|---|---|---|
| Clinical probabilities | ||||
| Bleeding events | ||||
| Prophylaxis | 0.3/mo | 2-4/y | 23 | |
| Hospitalization | 0.0175/mo | 0.01-0.02/mo | 25 | |
| Joint complications | ||||
| Joint bleed | 0.39/mo | 0.3-0.5/mo | 26 | |
| Hospitalization | 0.10/event | 0.05-0.15/mo | Range estimated | |
| Orthopedic procedure | 2/lifetime (0.0022/mo) | 1-3/lifetime | 27 | |
| Gene therapy | ||||
| Success | 0.90 | 0.8-1 | Range estimated | |
| Complications | 0.01 | 0.0005-0.002 | Range estimated | |
| Death | 0.00013/mo | 0.0001-0.0005/mo | 21 | Mean risk for death, 30-40-y-olds (CDC) |
| Time courses | ||||
| Bleeding event | 2 d | 2-4 d | 7 | |
| Hospitalization | 4 d | 2-6 d | 32 | Median hemophilia admission (HCUP) |
| Joint damage | 14 d | 7-21 d | ||
| Gene therapy complication | 4 d | — | ||
| Quality-of-life measures (utilities) | ||||
| Prophylaxis | 0.93 | 0.87-0.93 | 22, 28 | |
| Hospitalization | 0.66 | 0.5-0.7 | Based on bleed utility | |
| Bleed | 0.66 | 0.66-0.8 | 33 | |
| Joint damage | 0.64 | — | 30 | |
| Gene therapy | 1 | 0.9-1 | Assumed utility | |
| Gene therapy complication | 0.8 | 0.7-0.9 | Assumed utility | |
| Costs, US$ | ||||
| Prophylaxis | 37 759 | — | 7, 31 | Based on 88.7-kg male, 33 IU/kg 3 times weekly |
| Bleeding event | 8 870 | 4 435-17 740 | 7 | Based on 50 IU/kg/d for bleed duration |
| Hospitalization for bleeding event | 48 603 | 33 582-63 678 | 7, 32 | Average Medicare reimbursement (HCUP) for hospitalization plus factor at 50 IU/kg/d |
| Joint damage | 61 | 40-80 | 33 | |
| Orthopedic surgery (knee replacement) | 137 461 | 110 000-138 000 | 32 | Average Medicare reimbursement (HCUP) for knee replacement plus factor |
| Gene therapy | 850 000 | 10 000-2 100 000 | 20 | |
| Gene therapy failure | 800 | 500-1 000 | 20 |
The base-case estimates represent the best estimate for each value. Unless otherwise noted, ranges represent 95% confidence intervals.
CDC, Centers for Disease Control and Prevention.
Clinical probabilities
We derived probabilities for bleeding events for patients on prophylaxis from clinical trials evaluating novel treatment strategies.23,24 The risk of hospitalization was extrapolated from a hemophilia surveillance project.25 The probability of hemarthrosis was collected from a Center for Disease Control and Prevention 10-year hemophilia surveillance registry.26 The likelihood of requiring an orthopedic procedure was extrapolated from several trials evaluating prophylaxis vs on-demand therapy.24,26,27 To model mortality for prophylaxis and gene-therapy patients, we used data from United States standard life tables for the year 2014 for patients between the ages of 30 and 40 years.21 We assumed that the probability of death would be higher during months when bleeds/joint damage occurred.
Quality-of-life measures (utilities)
Utilities were scaled from 0 to 1, where 0 equals death, and 1 equals perfect health. These values represent preferences for a given health state. Ranges of utilities for health states therapy were collected from quality-of-life and cost-utility studies, and an average of these values was used in the base-case analysis.22,28 A study evaluating changes in quality of life for patients experiencing acute bleeding was used to determine the utility during a bleeding event.29 For patients with nonoperative joint damage, a utility from a survey of chronic osteoarthritis was used, and following an orthopedic procedure, a postoperative knee utility was used.30 During hospitalizations, patients were assigned the same utility as a severe bleeding event, because severe bleeds requiring longer duration or more intense treatment are common nonsurgical reasons for hospitalization. Patients undergoing successful gene therapy were assumed to have a utility of 1, and those suffering a complication from gene therapy were assigned a utility of 0.8 for the following month.
Costs
The pharmacy cost of FVIII concentrate was collected from FFF Enterprises’ wholesale price for recombinant FVIII. Prophylaxis cost was determined using the average weight for United States males older than 20 years of age (88.7 kg)31 following a typical dosing schedule of 3 times per week at 33 units per kilogram.7 The cost for a bleeding event was calculated using 50 units per kilogram over 2 days.7 The cost of a hemophilia bleeding event requiring hospitalization was determined using reimbursement data from the Healthcare Cost and Utilization Project (HCUP) 32 using an average 4-day inpatient stay. We used a cost for nonoperative osteoarthritis cost-effectiveness study to represent the cost of joint damage.33 We assumed all orthopedic procedures to be knee replacements and used HCUP data for this cost plus the additional cost of factor treatment postsurgery and during rehabilitation (region 3 surgical protocol). For the base case, gene therapy was assigned a cost of $850 000 based on the price of the only commercially available gene therapy in the United States.20 We assumed the cost of complications for gene therapy to be $800 based on expert opinion.
Analysis
For each treatment strategy, our model calculated the 10-year outcomes expressed as QALYs and costs. We compared the performance of strategies using the incremental cost-effectiveness ratio, which is the added cost of the more expensive strategy divided by its added clinical benefit in QALYs. We conducted 1-way sensitivity analyses to assess the impact of varying baseline variables over clinically plausible ranges on cost-effectiveness analysis results. In the probabilistic sensitivity analysis, all parameters were varied simultaneously over predefined probability distributions based on ranges listed in Table 1. Clinical probabilities and time durations were approximated by β distributions, and costs were approximated by γ distributions. Values from each probability distribution were randomly selected during each of 3000 iterations. We reported that the percentage of probabilistic iterations for which a given strategy was favored over a range of willingness-to-pay thresholds resulted in a net monetary benefit at various willingness-to-pay ceilings.
Results
Our base-case model, over a 10-year time frame, resulted in a total per-person gene therapy cost of $1.0M and 8.33 QALYs, whereas prophylaxis cost $1.7M and resulted in 6.62 QALYs. Gene therapy cost less and was more effective than prophylaxis, thus dominating it in the base case.
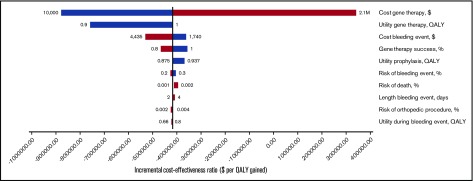
In 1-way sensitivity analyses varying all parameters individually over clinically plausible ranges (Table 1), only variation of gene therapy cost caused the gene therapy strategy to be no longer cost saving compared with prophylaxis. As shown in the tornado diagram (Figure 2), which depicts multiple 1-way sensitivity analyses, individual variation of gene therapy cost is the only parameter that results in incremental cost-effectiveness ratios >$0 per QALY gained. Gene therapy remained the dominant strategy unless its initial cost exceeded $1.6M and cost <$100 000 per 1 QALY gained compared with prophylaxis if initial costs were <$1.7M (Table 2). Results regarding gene therapy dominance were not sensitive to individual variation of all other parameter values. Gene therapy would remain cost saving if the monthly cost of prophylaxis is >$16 771 and would be favored at a $100 000 per 1 QALY threshold if monthly costs were >$12 963. Finally, in the probabilistic sensitivity analysis, gene therapy was the dominant strategy in 92% of model iterations.
Figure 2.
One-way sensitivity analysis. The results of 1-way sensitivity analysis are shown. Only variables whose variation caused a significant change in incremental cost-effectiveness (x-axis) are shown. Ranges of 1-way sensitivity analyses are shown next to each bar.
Table 2.
Base-case sensitivity analysis
| Variables | Prophylaxis | Gene therapy | Difference |
|---|---|---|---|
| Total cost | $1 693 630 | $1 022 249 | $671 381 |
| Effectiveness, QALYs | 6.62 | 8.33 | −1.71 |
| Incremental cost-effectiveness ratio, $/QALY | Dominated | Dominant |
Cost and QALYs are discounted at 3% per year.
Discussion
Our results demonstrate that, based on best available evidence, treatment with gene therapy is likely to be cost saving for the treatment of severe hemophilia A compared with the current standard of care with FVIII prophylaxis. Over the 10-year span of the model, gene therapy cost $1M compared with $1.7M for prophylaxis. Gene therapy also afforded increased benefit over this period, generating 8.33 QALYs compared with 6.66 QALYs for prophylaxis. With its decreased cost and increased benefit, gene therapy dominated prophylaxis in this analysis.
In sensitivity analysis, our results are highly robust over a wide range of values for all model parameters, with the results sensitive only to variation in gene therapy cost, with gene therapy remaining cost saving if initial costs are <$1.6M. Perceivably, results could be even more favorable if gene therapy effectiveness duration is >10 years or if the success rate is similar to what has been seen in clinical trials.16,18 Whether payers will change reimbursement approaches remains outside the scope of this analysis. Importantly we are not urging change in reimbursement policies but rather show what the results of current policies are. Oncology and solid organ transplantation, which struggle with the same issues, may represent potential benchmarks for future comparisons between costing and reimbursement strategies.
The age of patients at the time of potentially curative treatment is an important question from a cost-effectiveness standpoint. Because younger patients may reap the benefits of therapy over a longer period of time than their older counterparts, patient age at treatment is a significant variable. Currently, gene therapy for hemophilia is in clinical trials for adults only, making the age group for our model logical for this analysis. We also chose an initial age of 30-40 years for our cohort because this population is well represented in the literature used for reference values. Despite this restriction in the base case, we believe that our results are generalizable to a wider age group for the following reasons. In the 1-way sensitivity analysis, altering the probability of death, a good approximation for changes in age, did not significantly alter the cost-effectiveness of gene therapy. Additionally, the clinical probabilities, utilities, and costs are not specific to a particular age group, thereby making our results applicable over a wider age range.
Our assumption that successful gene therapy results in full quality of life could potentially bias results toward gene therapy. For example, this assignment may miss decreases in quality of life that already occurred in a patient before receiving gene therapy (eg, due to chronic joint damage). However, decreasing the quality of life utility from 1.0 to 0.9 with gene therapy still results in gene therapy as the dominant strategy. On the other hand, all hemophilia patients in this analysis have severe hemophilia without inhibitors, consistent with the exclusion of inhibitor patients from gene-transfer trials. Inhibitor patients cost significantly more to treat than standard hemophilia without inhibitors; omitting them from the model biased results toward prophylaxis.34
Our analysis has several limitations. First, the lack of commercially available gene therapy for hemophilia A and limited long-term experience in current clinical trials drives many of the assumptions in our model. We sought to limit the impact of these assumptions by biasing most model assumptions against gene therapy. We accomplished this in several ways, including lowering the success rate to 90% as opposed to the near 100% success rate seen in published trials.16 Similarly, the complication rate assumed in the base case was higher than available data suggest. Finally, the goal of gene therapy is to serve as a lifelong therapy. However, because no long-term data exist for hemophilia A, we selected a time horizon of 10 years based on data from hemophilia B and animal models.17,18 By limiting the time frame for what is believed to be lifelong treatment, the cost-effectiveness of gene therapy in this analysis is significantly reduced. Second, hemophilia patients receiving prophylaxis were assigned a time-dependent rate of chronic joint damage, independent of bleeding events. We selected this approach given the overwhelming prevalence of chronic arthropathy in this patient population.35 Third, the average weight of a hemophilia patient was estimated using Center for Disease Control statistics for an average 30-year-old male. However, hemophilia patients are limited in their mobility from chronic arthropathy and fear of hemarthrosis, resulting in higher rates of obesity.36 This could result in underestimation of factor cost for the prophylaxis group. Fourth, we assumed all failures in the gene therapy group occurred immediately after treatment. Currently, there are no published reports of late gene therapy failure, which could hypothetically occur in the setting of liver failure, because the liver is the target organ for FVIII expression.
Fifth, our model evaluated only the direct costs of hemophilia, including the costs of factor treatment, hospitalizations, bleeds, and orthopedic surgeries. Other costs of care, including office visits, physical therapy, and consumer costs were not evaluated for hemophilia patients in either cohort. Further, several novel hemophilia A treatments, including RNA interference (fitusiran) and a bispecific monoclonal antibody (emicizumab), have become or are poised to become commercially available.37 The impact of multiple competing novel therapies on the cost of gene therapy remains to be seen and is not broached in this analysis. Sixth, this model does not address the cost-effectiveness of gene therapy for mild and moderate hemophilia patients who are less likely to require prophylactic factor infusions given their less severe bleeding phenotype.7 Finally, our model evaluated the impact of gene therapy on patients 30-40 years of age, rather than assessing lifetime costs. However, because we used a 10-year time horizon, our results will be similar for other age groups to the extent that our parameter values over that 10-year period remain the same for other age groups in question or to the extent that age-specific differences in parameter values between gene therapy and prophylaxis groups remain the same. For example, if in 60-year-olds, the difference in mortality between treatment groups is less due to greater background mortality due to other causes in this age group (and hence greater mortality risk with gene therapy), the advantages of gene therapy could be less.
In conclusion, our model shows that gene therapy for the treatment of severe hemophilia A is likely to be cost saving or cost-effective compared with prophylaxis with FVIII. Gene therapy maintained superior cost-effectiveness, even in the setting of high upfront costs.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant 1-T32 110849-01-A1, Health Resources and Services Administration Federal Hemophilia Treatment Centers grant H30MC24050-04-00, and Pennsylvania Department of Health state support of the Hemophilia Center of Western PA (SAP 41000058531).
Authorship
Contribution: N.M., M.V.R., and K.J.S. designed the study, acquired and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret V. Ragni, University of Pittsburgh School of Medicine, Division Hematology/Oncology, Department of Medicine, Hemophilia Center of Western PA, 3636 Boulevard of the Allies, Pittsburgh, PA 15213; e-mail: ragni@pitt.edu.
References
- 1.Mannucci PM, Tuddenham EG. The hemophilias--from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773-1779. [DOI] [PubMed] [Google Scholar]
- 2.Soucie JM, Evatt B, Jackson D; The Hemophilia Surveillance System Project Investigators. Occurrence of hemophilia in the United States. Am J Hematol. 1998;59(4):288-294. [DOI] [PubMed] [Google Scholar]
- 3.Skinner MW. Haemophilia: provision of factors and novel therapies: World Federation of Hemophilia goals and achievements. Br J Haematol. 2011;154(6):704-714. [DOI] [PubMed] [Google Scholar]
- 4.Collins PW, Björkman S, Fischer K, et al. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thromb Haemost. 2010;8(2):269-275. [DOI] [PubMed] [Google Scholar]
- 5.Oladapo AO, Epstein JD, Williams E, Ito D, Gringeri A, Valentino LA. Health-related quality of life assessment in haemophilia patients on prophylaxis therapy: a systematic review of results from prospective clinical trials. Haemophilia. 2015;21(5):e344-e358. [DOI] [PubMed] [Google Scholar]
- 6.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535-544. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. ; Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-e47. [DOI] [PubMed] [Google Scholar]
- 8.Mazepa MA, Monahan PE, Baker JR, Riske BK, Soucie JM; US Hemophilia Treatment Center Network. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood. 2016;127(24):3073-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove J. Medicare: high-expenditure Part B drugs. 2012. Available at: http://www.gao.gov/assets/650/649459.pdf. Accessed 19 March 2018.
- 10.Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM. Health care expenditures for Medicaid-covered males with haemophilia in the United States, 2008. Haemophilia. 2012;18(2):276-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. 2012;18(2):268-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gringeri A, Mantovani LG, Scalone L, Mannucci PM; COCIS Study Group. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 2003;102(7):2358-2363. [DOI] [PubMed] [Google Scholar]
- 13.Fredericks M, Pyenson B, Iwasaki K. An actuarial study of hemophilia: implications for commercial and Medicaid managed care plans. Available at: http://www.milliman.com/insight/2014/An-actuarial-study-of-hemophilia-Implications-for-commercial-Medicaid-managed-care-plans/. Accessed 19 March 2018. [Google Scholar]
- 14.Escobar MA. Health economics in haemophilia: a review from the clinician’s perspective. Haemophilia. 2010;16(suppl 3):29-34. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh J, Lenting PJ, Rosales C, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121(17):3335-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangarajan S, Walsh L, Lester W, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519-2530. [DOI] [PubMed] [Google Scholar]
- 17.Sabatino DE, Lange AM, Altynova ES, et al. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther. 2011;19(3):442-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. FDA approval brings first gene therapy to the United States. FDA News Release. 30 August 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm. Accessed 12 April 2018.
- 20.Herper M. Spark Therapeutics sets price of blindness-treating gene therapy at $850,000. Available at: https://www.forbes.com/sites/matthewherper/2018/01/03/spark-therapeutics-sets-price-of-blindness-curing-gene-therapy-at-850000/#393ab1837dc3. Accessed 19 March 2018. [Google Scholar]
- 21.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65(4):1-122. [PubMed] [Google Scholar]
- 22.Farrugia A, Cassar J, Kimber MC, et al. Treatment for life for severe haemophilia A- A cost-utility model for prophylaxis vs. on-demand treatment. Haemophilia. 2013;19(4):e228-e238. [DOI] [PubMed] [Google Scholar]
- 23.Mahlangu J, Powell JS, Ragni MV, et al. ; A-LONG Investigators. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagliaferri A, Franchini M, Coppola A, et al. Effects of secondary prophylaxis started in adolescent and adult haemophiliacs. Haemophilia. 2008;14(5):945-951. [DOI] [PubMed] [Google Scholar]
- 25.Soucie JM, Symons J IV, Evatt B, Brettler D, Huszti H, Linden J; Hemophilia Surveillance System Project Investigators. Home-based factor infusion therapy and hospitalization for bleeding complications among males with haemophilia. Haemophilia. 2001;7(2):198-206. [DOI] [PubMed] [Google Scholar]
- 26.Manco-Johnson MJ, Soucie JM, Gill JC; Joint Outcomes Committee of the Universal Data Collection, US Hemophilia Treatment Center Network. Prophylaxis usage, bleeding rates, and joint outcomes of hemophilia, 1999 to 2010: a surveillance project. Blood. 2017;129(17):2368-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith PS, Teutsch SM, Shaffer PA, Rolka H, Evatt B. Episodic versus prophylactic infusions for hemophilia A: a cost-effectiveness analysis. J Pediatr. 1996;129(3):424-431. [DOI] [PubMed] [Google Scholar]
- 28.Risebrough N, Oh P, Blanchette V, Curtin J, Hitzler J, Feldman BM. Cost-utility analysis of Canadian tailored prophylaxis, primary prophylaxis and on-demand therapy in young children with severe haemophilia A. Haemophilia. 2008;14(4):743-752. [DOI] [PubMed] [Google Scholar]
- 29.Recht M, Neufeld EJ, Sharma VR, et al. Impact of acute bleeding on daily activities of patients with congenital hemophilia with inhibitors and their caregivers and families: observations from the Dosing Observational Study in Hemophilia (DOSE). Value Health. 2014;17(6):744-748. [DOI] [PubMed] [Google Scholar]
- 30.Xie F, Lo N-N, Tarride J-E, O’Reilly D, Goeree R, Lee H-P. Total or partial knee replacement? Cost-utility analysis in patients with knee osteoarthritis based on a 2-year observational study. Eur J Health Econ. 2010;11(1):27-34. [DOI] [PubMed] [Google Scholar]
- 31.Fryar CD, Gu Q, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2011-2014. Vital Health Stat. 2016;(39):1-46. [PubMed] [Google Scholar]
- 32.Pfuntner A, Wier LM, Steiner C. Costs for hospital stays in the United States, 2010. Available at: https://www.ncbi.nlm.nih.gov/books/NBK121966/. Accessed 12 April 2018.
- 33.Knight C, Paisley S, Wight J, Jones ML. Economic modelling of different treatment strategies for haemophilia A with high-responding inhibitors. Haemophilia. 2003;9(4):521-540. [DOI] [PubMed] [Google Scholar]
- 34.Tencer T, Friedman HS, Li-McLeod J, Johnson K. Medical costs and resource utilization for hemophilia patients with and without HIV or HCV infection. J Manag Care Pharm. 2007;13(9):790-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon JL, Zhou ZY, Doctor JN, et al. Quality of life in haemophilia A: Hemophilia Utilization Group Study Va (HUGS-Va). Haemophilia. 2012;18(5):699-707. [DOI] [PubMed] [Google Scholar]
- 36.Wong TE, Majumdar S, Adams E, et al. ; Healthy Weight Working Group. Overweight and obesity in hemophilia: a systematic review of the literature. Am J Prev Med. 2011;41(6 suppl 4):S369-S375. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann J, Croteau SE. 2017 clinical trials update: innovations in hemophilia therapy. Am J Hematol. 2016;91(12):1252-1260. [DOI] [PubMed] [Google Scholar]