Key Points
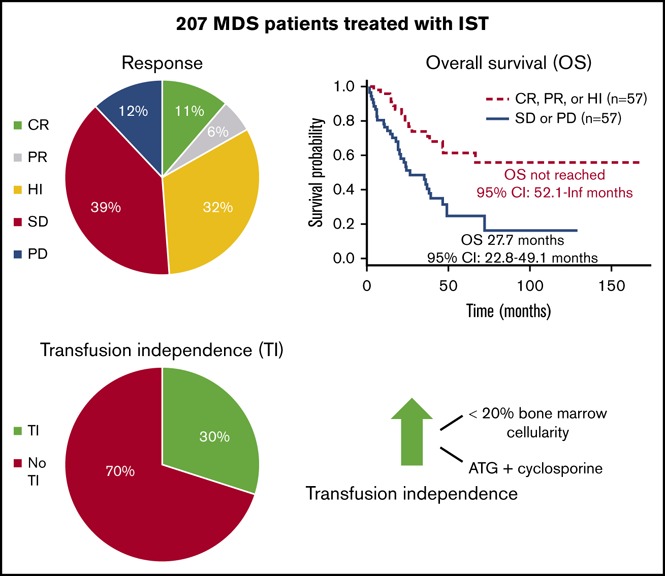
IST leads to a response in nearly half, and to RBC transfusion independence in about a third, of selected lower-risk MDS patients.
Hypocellularity of bone marrow and the use of horse ATG plus cyclosporine are associated with increased rates of transfusion independence.
Abstract
Most studies of immunosuppressive therapy (IST) in myelodysplastic syndromes (MDS) are limited by small numbers and their single-center nature, and report conflicting data regarding predictors for response to IST. We examined outcomes associated with IST and predictors of benefit in a large international cohort of patients with MDS. Data were collected from 15 centers in the United States and Europe. Responses, including red blood cell (RBC) transfusion independence (TI), were assessed based on the 2006 MDS International Working Group criteria, and overall survival (OS) was estimated by Kaplan-Meier methods. Logistic regression models estimated odds for response and TI, and Cox Proportional Hazard models estimated hazards ratios for OS. We identified 207 patients with MDS receiving IST, excluding steroid monotherapy. The most common IST regimen was anti-thymocyte globulin (ATG) plus prednisone (43%). Overall response rate (ORR) was 48.8%, including 11.2% (95% confidence interval [CI], 6.5%-18.4%) who achieved a complete remission and 30% (95% CI, 22.3%-39.5%) who achieved RBC TI. Median OS was 47.4 months (95% CI, 37-72.3 months) and was longer for patients who achieved a response or TI. Achievement of RBC TI was associated with a hypocellular bone marrow (cellularity < 20%); horse ATG plus cyclosporine was more effective than rabbit ATG or ATG without cyclosporine. Age, transfusion dependence, presence of paroxysmal nocturnal hemoglobinuria or large granular lymphocyte clones, and HLA DR15 positivity did not predict response to IST. IST leads to objective responses in nearly half the selected patients with the highest rate of RBC TI achieved in patients with hypocellular bone marrows.
Visual Abstract
Introduction
Patients with lower-risk myelodysplastic syndromes (LR-MDS), traditionally defined as low or intermediate-1 risk groups as stratified by the 1997 International Prognostic Scoring System (IPSS), are most often treated with transfusions, erythropoiesis-stimulating agents (ESAs), lenalidomide, or hypomethylating agents (HMAs), often primarily directed at alleviating transfusion needs.1 However, patients frequently experience either primary or secondary treatment failure to these agents, after which therapeutic options are quite limited.1 Immunosuppressive therapy (IST) has been used in patients with LR-MDS, based on the observation that a subset of these patients develops cytopenias as a consequence of hyperactivated T cells, leading to suppression of hematopoiesis similar to that seen in aplastic anemia.2,3 In addition, concomitant autoimmune diseases are common in MDS; in a recent study, 48% of patients with MDS had serological or clinical evidence of an autoimmune disease, which was found to be an independent marker for a worse prognosis.4 IST with anti-thymocyte globulin (ATG),5-8 cyclosporine,9-11 or alemtuzumab,12 as well as IST combinations of ATG with cyclosporine13-17 or etanercept,18 have all been studied in this context. Although some studies reported clinical activity with IST, others were not able to confirm a benefit of IST in unselected or older patients with MDS.19-21 However, most studies published to date have included a small number of patients and were restricted to single-institution experiences, limiting their ability to reliably identify predictors of response to IST.22 The goal of this study was to use a large, multicenter international cohort to retrospectively examine the clinical outcomes and to identify predictors of response and overall survival (OS) for patients with MDS treated with IST.
Patients and methods
Data source and eligibility
Patients aged 16 years or older with pathologically confirmed MDS, as defined by the 2008 World Health Organization (WHO) criteria23 who were treated with IST at any time during their disease course, were considered eligible for the study. Data from patients who met eligibility criteria were collected retrospectively for the period that spanned from 2006 to 2016. Seven centers were in the United States, and 4 were in Europe. In addition, 4 centers from the European MDS Registry (EUMDS) (Austria, Greece, Poland, and Israel) contributed cases to the study. Types of IST included ATG (rabbit and horse), cyclosporine, tacrolimus, prednisone, and alemtuzumab, and combinations of them. Patients treated with prednisone or other steroids as monotherapy were excluded, as steroid monotherapy is generally ineffective in MDS. Investigators at each center collected data and reported responses in deidentified datasets, which were later centrally combined and analyzed at the coordinating center (Yale University). The study was approved by the institutional review boards at participating institutions.
Variable and patient characteristics
Individual patient characteristics as well as disease characteristics, including 2008 WHO subtype23 and MDS risk category per the IPSS,24 revised IPSS (IPSS-R),25 WHO-based prognostic scoring system,26 and LR-MDS Prognostic Scoring System,27 were collected. Cytogenetic risk was classified according to the IPSS.24 We also collected information about the presence of paroxysmal nocturnal hemoglobinuria (PNH) and large granular lymphocyte (LGL) clones, HLA-DR15 positivity and the presence of mutations in TP53, IDH1/2, ASXL1, and SF3B1 genes, as well as the specific IST used and the treatments that preceded and/or succeeded IST.
Response criteria and survival
Responses were defined using the modified 2006 MDS International Working Group criteria.28 Red blood cell (RBC) transfusion independence (TI) was defined as the patient’s ability to maintain a hemoglobin level ≥8 g/dL for at least 6 weeks without any RBC transfusion support after being transfusion dependent before. OS was measured from time of initiation of IST until death or last follow-up.
Statistical analysis
Descriptive statistics were calculated to characterize the study cohort. We used Student t test and χ2 test to compare continuous and categorical variables, respectively. Kaplan-Meier methods estimated OS from initiation of IST to death or end of follow-up. Univariate and multivariate logistic regression models estimated odds for response and TI, and univariate and multivariate Cox proportional hazard models estimated hazards ratios (HR) for OS. A stepwise procedure was used to select each multivariate model. For each of the three multivariate models, a group of candidate predictors with univariate Wald test P < .25 was selected for consideration in the final model. Within the stepwise procedure for each model, patients with missing data for any of the candidate predictors were removed from the analysis, and no imputation was conducted. All tests were 2-sided, with an α significance level of 0.05. All analyses were performed using R version 3.3.2.29
Results
Study population
A total of 207 patients met study eligibility and were included. Another 160 patients were excluded because they received steroid monotherapy as their only therapy. Disease risk according to the IPSS was low (22%), intermediate-1 (69%), or either intermediate-2 or high risk (9%). Median age at diagnosis was 65 years (range, 15-95 years), and 63% were male (Table 1). Median white blood cell count, hemoglobin level, and platelet count at time of IST initiation were 2.4 × 109/L (range, 0.1-26.4 × 109/L), 8.9 g/dL (5.3-12.8 g/dL), and 44.5 × 109/L (0-1111 × 109/L), respectively.
Table 1.
Patient characteristics
| Characteristic | Median or N | Range or % |
|---|---|---|
| Sex | ||
| Male | 124 | 63.3% |
| Female | 72 | 36.7% |
| Age, y | 61 | 17-88 |
| WHO subtype | ||
| RA | 14 | 8.9% |
| RARS | 8 | 5.1% |
| RCUD | 5 | 3.2% |
| RCMD | 93 | 59.2% |
| RAEB-1 | 15 | 9.6% |
| RAEB-2 | 5 | 3.2% |
| MDS-U | 11 | 7.0% |
| Isolated 5q- | 6 | 3.8% |
| Complete blood count | ||
| White blood cell count | 2.4 | 0.1-26.4 |
| Absolute neutrophil count | 0.85 | 0-5.95 |
| Hemoglobin level | 8.9 | 5.3-12.8 |
| Platelet count | 44.5 | 0-1111 |
| Bone marrow blast % | 1.8 | 0-20 |
| Bone marrow cellularity % | 45 | 0-100 |
| Hypocellular bone marrow (<20%) | 22/82 | 26.8% |
| Peripheral blood blast % | 0 | 0-3.3 |
| IPSS | ||
| Low | 33 | 22% |
| Intermediate-1 | 104 | 69.3% |
| Intermediate-2 | 12 | 8% |
| High | 1 | 0.7% |
| LR-PSS | ||
| Risk category 1 | 38 | 25.3% |
| Risk category 2 | 62 | 41.3% |
| Risk category 3 | 50 | 33.3% |
| Molecular analysis | ||
| PNH clone (present/absent) (n = 62) | 16/46 | 26% |
| LGL clone (present/absent) (n = 44) | 16/28 | 36% |
| HLA-DR15 (positive/negative) (n = 52) | 28/24 | 54% |
| TP53 (mutated/nonmutated) (n = 43) | 2/41 | 5% |
| IDH1 (mutated/nonmutated) (n = 74) | 2/72 | 3% |
| IDH2 (mutated/nonmutated) (n = 39) | 0/39 | 0% |
| ASXL1 (mutated/nonmutated) (n = 41) | 6/35 | 15% |
| SF3B1 (mutated/nonmutated) (n = 73) | 10/63 | 14% |
LR-PSS, LR-MDS Prognostic Scoring System.
Sixty percent of patients had received a median of 1 other therapy (range, 1-7) before IST. Prior treatments included the following agents: ESAs (33%), HMAs (33%), thalidomide or lenalidomide (9%), cytotoxic chemotherapy (11%), androgens (5%), and other therapies including other IST, iron chelation therapy, and experimental therapies (9%), either as mono or combination therapies. Median follow-up time was 25.2 months (range, 0.5-245.1 months).
Patterns of treatment with IST
Of the 207 patients, IST regimens included several different ATG-based combinations (76%), as well as cyclosporine (13%) tacrolimus (4%) and others (7%). Combination regiments with ATG as the backbone included ATG plus prednisone (43%), ATG plus cyclosporine (21%), ATG plus tacrolimus (4%), and ATG plus cyclosporine and etanercept (8%). ATG was given as the rabbit isoform in 62% of patients and as horse isoform in 38% of patients. Among patients who were reported to discontinue IST, 29.4% discontinued IST because of adverse effects, whereas 23.4%, 14.7%, 5.9%, and 17.6% discontinued IST because of lack of response, disease progression, completion of treatment regimen, or other reasons, respectively.
Response to IST therapy and predictors
Of 125 patients whose response data were recorded (Table 2), 11.2% (95% confidence interval [CI], 6.5%-18.4%) had CR, 5.6% (95% CI, 2.5%-11.6%) had PR, and 32% (95% CI, 24.1%-41%) achieved HI, resulting in an ORR of 48.8% (95% CI, 39.8%-57.9%). In contrast, 39.2% (95% CI, 30.7%-48.4%) of patients had stable disease, and 12% (95% CI, 7.1% to 19.3%) had progressive disease. RBC TI was achieved in 30% (95% CI, 22.3%-39.5%) of patients who were dependent on RBC transfusions before IST. For patients who achieved RBC TI, the median time from initiation of IST to TI was 9.4 weeks (95% CI, 6.3-12.6 weeks), and the median duration of TI was 19.9 months (95% CI, 12.8-27 months).
Table 2.
Response to IST
| Response | Percentage | 95% CI |
|---|---|---|
| CR | 11.2 | 6.5-18.4 |
| PR | 5.6 | 2.5-11.6 |
| HI | 32.0 | 24.1-41.0 |
| SD | 39.2 | 30.7-48.4 |
| PD | 12.0 | 7.1-19.3 |
| ORR (CR+PR+HI) | 48.8 | 39.8-57.9 |
| TI | 30 | 22.3-39.5 |
CR, complete response; HI, hematologic improvement; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; TI, RBC transfusion independence.
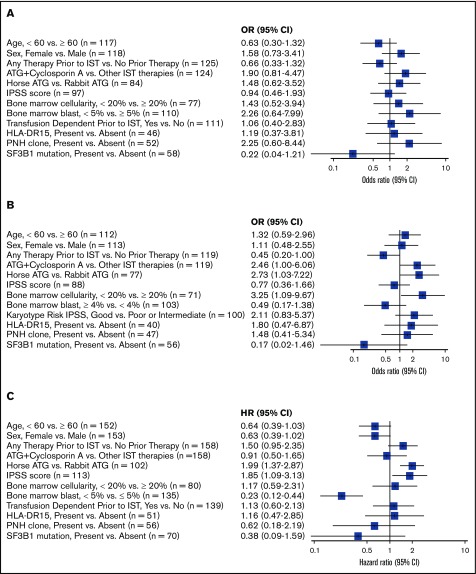
In univariate analysis of predictors of response (CR+PR+HI), the presence of SF3B1 mutation (reported in 10 of 73 patients who had SF3B1 sequenced) was associated with a lower response rate; however, it did not achieve statistical significance (mutated vs nonmutated: OR, 0.2; 95% CI, 0.04-1.2; P = .08; Figure 1A). In univariate analysis of predictors of TI, the receipt of IST as a second or subsequent lines of treatment (any prior therapy vs no prior therapy: OR, 0.5; 95% CI, 0.2-0.9; P = .048) was associated with decreased odds of achieving TI. On the contrary, a hypocellular bone marrow (<20% vs ≥20%: OR, 3.3; 95% CI, 1.1-10; P = .03), horse ATG (horse vs rabbit ATG: OR, 2.7; 95% CI, 1.03-7.2; P = .043), and ATG plus cyclosporine (vs all other treatment regimens: OR, 2.5; 95% CI, 1.1-6.0; P = .048) were all significantly associated with achievement of TI (Figure 1B).
Figure 1.
Univariate analysis (forest plot) of clinical and molecular predictors of (A) response (CR+PR+HI), (B) achievement of TI, and (C) OS.
In multivariate analysis of predictors of response, no predictive factors for response were identified. In multivariate analysis of predictors of TI, only a hypocellular bone marrow remained a significant predictor of achieving RBC TI (<20% vs >20%: OR, 4.0; 95% CI, 1.2-13; P = .03). Age, prior transfusion dependence, MDS risk scores, presence of a PNH or LGL clone, and HLA DR15 positivity were not predictive of response with IST.
Overall survival after IST treatment and predictors
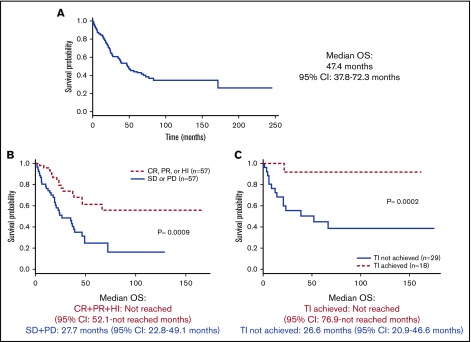
Median OS from time of IST initiation for all patients was 47.4 months (95% CI, 37-72.3 months; Figure 2A). For patients who achieved a response (CR+PR+HI) to IST, the median OS was not reached (95% CI, 52.1 months-not reached), whereas patients without a response had a median OS of 27.7 months (95% CI, 22.8-49.1 months; P = .0009) (Figure 2B). Similarly, for patients who achieved TI with IST, median OS was not reached (95% CI, 76.9 months-not reached), whereas patients who remained transfusion-dependent had a median OS of 26.6 months (95% CI, 20.9-46.6 months; P = .0002; Figure 2C).
Figure 2.
OS from onset of IST. (A) For all patients treated with IST. (B) According to response (CR+PR+HI) achieved vs no response achieved. (C) According to TI achieved vs TI not achieved.
In univariate analysis of predictors of OS, higher-risk IPSS score predicted worse OS (HR, 1.8; 95% CI, 1.1-3.1; P = .02), whereas low bone marrow blast percentage predicted improved OS (<5% vs ≥5%; HR, 0.2; 95% CI, 0.1-0.4; P < .001; Figure 1C). In multivariate analysis, a low bone marrow blast count (<5% vs ≥5%; HR, 0.2; 95% CI, 0.1-0.4; P < .0001) was a predictor of improved OS.
Discussion
To our knowledge, this is the largest reported cohort of patients with MDS treated with IST. We observed that IST led to durable objective responses in about half and to RBC TI in approximately one third of patients, which is in line with prior reports.22 Acknowledging the limitations of selection bias of this retrospective experience and cross-study comparisons, these response rates appear comparable (or even slightly better) than other treatment modalities used for patients with MDS with low and intermediate-1 IPSS risk including ESAs, lenalidomide, and HMAs.30 ESAs result in HI in 30% to 50% and lead to TI in about 20% to 40% of anemic unselected patients with LR-MDS.31-34 In a minority of patients with deletion of the long arm of chromosome 5 (5q-), lenalidomide results in HI in 56% to 76% and TI in in 26% of patients.35-37 However, in all other patients without 5q-, lenalidomide leads to HI in 43% and TI in 26% of patients.35,38,39 HMAs are more frequently used in higher-risk patients with MDS (high and intermediate-2 IPSS risk), but response rates in lower-risk patients with MDS have been reported to be around 50%.40,41 However, several other papers reported lower rates of response with HMAs.42,43
Not surprisingly, in our study, OS was significantly longer for patients who achieved an objective response or TI, which is also consistent with prior studies.13 Our results show, similar to the aplastic anemia experience, that the use of horse ATG is superior than rabbit ATG. Furthermore, although ATG has often be used alone, our data suggest that the combination of horse ATG with CSA seems to be the most effective form of IST. We were able to confirm the predictive value of marrow hypocellularity for clinical benefit of IST, but could not confirm it for any of the previously reported variables.
Despite a clear benefit of IST in a subgroup of patients, IST is infrequently used in clinical practice.1 This is partly because of operational challenges in administering these drugs, which are often given in the inpatient setting and can be associated with severe reactions, and difficulties in predicting whether patients will benefit from IST therapy. Several patient characteristics have been identified as predictors of response to IST in prior studies. These include younger age (<65 years) and limited prior transfusion history (<2 years), as well as use as first-line treatment (or after lenalidomide), low blast percentage with hypocellular marrow, and good prognostic karyotype, in addition to HLA DR15 and PNH clone positivity and a higher CD8+ terminal memory T-cell percentage.5,6,44-46 On the basis of the patient’s age, the duration of transfusion dependence before IST, and the HLA-DR15 genotype, the National Institutes of Health response model for IST in MDS was developed.44 However, reports have varied widely in what characteristics predict response to IST, and the National Institutes of Health response model could not be validated in some studies.45,46
In our patient cohort, we were not able confirm the predictive value of several previously described biomarkers of response. Age, prior transfusion dependence, MDS risk assessment scores, presence of PNH or LGL clones, HLA DR15 positivity, and gene mutations did not appear to predict response to IST. Although we are not able to explain these differences with certainty, this could be related to several factors. These include potential selection bias of which patients received IST and differences in interpretation of variable positivity in the size of the PNH or LGL clone considered positive by the local investigator. Furthermore, other differences between the study cohorts regarding biologic factors that were not controlled for might have played a role as well. In addition, the limited number of data points for bone marrow cellularity as well as HLA DR15 positivity and presence of a PNH clone could have played a role as well. However, apart from the National Institutes of Health cohort of patients,13,44 in most of the other studies examining predictive factors for IST in MDS,5,6,45,47 bone marrow cellularity and HLA DR15 status were assessed in only 10 to 20 patients, whereas PNH clones were only tested for in less than 10 patients if tested for at all. In comparison, in our study, in 70 to 80 patients, data on bone marrow cellularity, and in 40 to 50 patients, data on HLA DR15 and PNH clonal status was available to correlate with response and OS.
In contrast, increased rates of TI were seen in patients with hypocellular bone marrow (bone marrow cellularity, <20%), and patients who were not exposed to any therapies before IST. Furthermore, patients treated with horse ATG (compared with rabbit ATG) had improved rates of TI in univariate analysis, which has been described before in aplastic anemia.48 On the contrary, one prior study in MDS reported no difference in the response rate achieved with horse ATG vs rabbit ATG47; however, this was a much smaller study of just 35 patients. In addition, ATG in combination with cyclosporine was superior to all other IST regimens examined regarding the achievement of TI in univariate analysis, which is similar to studies in MDS45 and in severe aplastic anemia.49
There are limited data regarding the effect of somatically recurrent genetic mutations on response to IST among patients with MDS. Our analyses were limited to only five genes because these genes were the ones most frequently reported by the centers, and therefore these numbers allowed meaningful analyses, which was not the case for the other recurrently mutated genes in MDS.
While providing important insights, such as any retrospective study, our study has important limitations. Selection bias could have inflated the benefit of IST, as the patients in the cohort were selected by their treating physicians to receive IST. Missing response or predictor data might have also affected the results because of a lack of power or selection bias of ascertainment, as we chose not to impute missing data, given the heterogeneity of centers that supplied their data. MDS diagnosis and response to therapy were reported by local investigators, and no centralized review of bone marrow biopsy results and responses was performed. However, all local investigators are MDS experts with extensive experience in hematopathology and in applying response MDS International Working Group criteria, and the centers included in the study were tertiary academic centers with extensive clinical and research history in the management of MDS. In contrast, the fact that patients were treated in specialized tertiary centers may also reduce the broad applicability of these data to the general population. Although the disease pathology was not centrally confirmed, exclusion of patients who received only steroids would likely eliminated cases of MDS associated with autoimmune or rheumatologic diseases; an association that has been previously established.
In summary, in this large retrospective international cohort study examining IST use in selected patients with LR-MDS, we found that IST leads to durable objective responses in nearly half the selected patients. Our results suggest the preferred IST regimen is horse ATG in combination with CSA to be used in patients’ hypocellular bone marrows.
Acknowledgments
The authors acknowledge all our patients whose data were contributed to this database and the Frederick A. DeLuca Foundation for supporting the statistical analyses.
T.d.W. and A.S. submitted the data on behalf of the European Myelodysplastic Syndrome Registry (EUMDS).
Footnotes
Presented as an oral presentation at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017.
Authorship
Contribution: M.S., M.D., and A.M.Z. performed conception and design; all authors provided study materials or patients; all authors performed collection and assembly of data; M.S., M.D., and A.M.Z. performed data analysis and interpretation; all authors performed manuscript writing, manuscript critical revision, and final approval of manuscript; and all authors are accountable for all aspects of the work.
Conflict-of-interest disclosure: M.A.S. has a membership on the board of directors or advisory committees of Celgene, Millenium-Takeda, and Opsona. A.M.B. received research funding to his institution from Celgene and Takeda. G.J.R. performs consultancy for AbbVie, Agios, Amgen, Amphivena, Array Biopharma Inc., Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche Pharmace, Boehringer Ingelheim, GlaxoSmithKline, Shire, Astex Pharmaceuticals, Cellectis, and Sunesis Pharmaceuticals; received research funding to her institution from Abbvie, Agios, Astex Pharmaceuticals, Celgene, CTI, Karyopharm Therapeutics, MedImmune, MEI Pharma, Moffitt, Novartis, Onconova Therapeutics, Pfizer, Sunesis Pharmaceuticals, Tensha Therapeutics, Cellectis; received funding for travels from AstraZeneca, Shire, Astellas Pharma,Celator, Incyte, Roche, Amphivena, MEI Pharma, Astex Pharmaceuticals, Janssen, and Juno Therapeutics; and received research funding from Cellectis. D.P.S. received consultance and research funding from Jansen; had a consultancy with Onconova; had equity ownership from Incyte; had a consultancy with H3 Biosciences, Takeda, and Celgene; and had a consultancy with and membership on the board of directors or advisory committee for Amgen, Pfizer, and Novartis. U.P. had a consultancy with and received honoraria and research funding from Celgene, Janssen, and Acceleron. R.I. received research funding from Janssen and Novartis. P.F. received honoraria and research funding from Amgen, Astex, Celgene, and Janssen. A.T.F. had a consultancy with and membership on the board of directors or advisory committee and received honoraria and research funding from Seattle Genetics; had membership on the board of directors or advisory committee for Juno; received research funding from Takeda; had a consultancy with and membership on the board of directors or advisory committee and received honoraria and research funding from Celgene; had a consultancy with and membership on the board of directors or advisory committee and received honoraria from Agios; had a consultancy with and membership on the board of directors or advisory committee for Medimmune and Amgen; and received honoraria from Pfizer. U.G. received honoraria and research funding from Celgene and Novartis and honoraria from Janssen. E.K.R. had a consultancy with Novartis and received research funding to her institution; had a consultancy or advisory role for and was on the speakers bureau for Incyte; had a consultancy or advisory role for and was on the speakers bureau for and received travel funding from Celgene and Novartis; was on the speakers bureau for ARIAD; had a consultancy or advisory role for and received research funding to her institution from Pfizer; and received research funding to her institution from Astellas Pharma, Bristol-Myers Squibb, and NS Pharma. N.A.P. had a consultancy with CTI biopharma/Baxalta, Alexion, Ariad, and Incyte; and received research funding to his institution from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Celator, Pfizer, and Astex Pharmaceuticals. V.S. received honoraria and research funding from Celgene; had a consultancy with and received honoraria from Janssen; received honoraria from Novartis; had a consultancy with and membership on the board of directors or advisory committee for AbbVie; had membership on the board of directors or advisory committee for Amgen; and had a consultancy with Otsuka. R.S.K. received honoraria from, had a consultancy with, and received travel funding from Celgene; received honoraria from, had a consultancy with, was on the speakers bureau for, and received travel funding from Novartis; was on the speakers bureau for and received travel funding from Alexion Pharmaceuticals; had stock or other ownership in AbbVie; received research funding to his institution and travel funding from Incyte; and received research funding to his institution from Celgene, GlaxoSmithKline, Eleos, and Boehringer Ingelheim. A.M.Z. received research funding to his institution from Celgene, Pfizer, Incyte, ADC Therapeutics, Medimmune, Takeda, AbbVie, and Boehringer Ingelheim; had a consultancy with and received honoraria from AbbVie, Ostuka, Pfizer, Gilead, Celgene, Ariad, Incyte, Agios, Novartis, Takeda, Daiichi Sankyo, and Boehringer Ingelheim; and received honoraria from and was on the speakers bureau for Takeda. The remaining authors declare no competing financial interests.
Correspondence: Amer M. Zeidan, Department of Internal Medicine, Section of Hematology, Yale School of Medicine, 333 Cedar St, New Haven, CT 06510-3222; e-mail: amer.zeidan@yale.edu.
References
- 1.Fenaux P, Adès L. How we treat lower-risk myelodysplastic syndromes. Blood. 2013;121(21):4280-4286. [DOI] [PubMed] [Google Scholar]
- 2.Biesma DH, van den Tweel JG, Verdonck LF. Immunosuppressive therapy for hypoplastic myelodysplastic syndrome. Cancer. 1997;79(8):1548-1551. [DOI] [PubMed] [Google Scholar]
- 3.Teramura M, Kimura A, Iwase S, et al. Treatment of severe aplastic anemia with antithymocyte globulin and cyclosporin A with or without G-CSF in adults: a multicenter randomized study in Japan. Blood. 2007;110(6):1756-1761. [DOI] [PubMed] [Google Scholar]
- 4.Montoro J, Gallur L, Merchán B, et al. Autoimmune disorders are common in myelodysplastic syndrome patients and confer an adverse impact on outcomes. Ann Hematol. 2018;97(8):1349-1356. [DOI] [PubMed] [Google Scholar]
- 5.Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med. 2002;137(3):156-163. [DOI] [PubMed] [Google Scholar]
- 6.Komrokji RS, Mailloux AW, Chen DT, et al. A phase II multicenter rabbit anti-thymocyte globulin trial in patients with myelodysplastic syndromes identifying a novel model for response prediction. Haematologica. 2014;99(7):1176-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, Barrett AJ. Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol. 1997;99(3):699-705. [DOI] [PubMed] [Google Scholar]
- 8.Killick SB, Mufti G, Cavenagh JD, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol. 2003;120(4):679-684. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Jiang B, Da W, Gong M, Guan M. Treatment of myelodysplastic syndrome with cyclosporin A. Int J Hematol. 2007;85(1):11-17. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa T, Tohyama K, Nakao S, et al. A prospective study of cyclosporine A treatment of patients with low-risk myelodysplastic syndrome: presence of CD55(-)CD59(-) blood cells predicts platelet response. Int J Hematol. 2007;86(2):150-157. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Okada M, Yamada S, et al. Good response to cyclosporine therapy in patients with myelodysplastic syndromes having the HLA-DRB1*1501 allele. Leukemia. 2000;14(2):344-346. [DOI] [PubMed] [Google Scholar]
- 12.Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab treatment of intermediate-1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol. 2010;28(35):5166-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26(15):2505-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passweg JR, Giagounidis AA, Simcock M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol. 2011;29(3):303-309. [DOI] [PubMed] [Google Scholar]
- 15.Yazji S, Giles FJ, Tsimberidou AM, et al. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17(11):2101-2106. [DOI] [PubMed] [Google Scholar]
- 16.Jonásova A, Neuwirtová R, Cermák J, et al. Cyclosporin A therapy in hypoplastic MDS patients and certain refractory anaemias without hypoplastic bone marrow. Br J Haematol. 1998;100(2):304-309. [DOI] [PubMed] [Google Scholar]
- 17.Miescher PA, Favre H, Beris P. Autoimmune myelodysplasias. Semin Hematol. 1991;28(4):322-330. [PubMed] [Google Scholar]
- 18.Scott BL, Ramakrishnan A, Fosdal M, et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149(5):706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steensma DP, Dispenzieri A, Moore SB, Schroeder G, Tefferi A. Antithymocyte globulin has limited efficacy and substantial toxicity in unselected anemic patients with myelodysplastic syndrome. Blood. 2003;101(6):2156-2158. [DOI] [PubMed] [Google Scholar]
- 20.Atoyebi W, Bywater L, Rawlings L, Brunskill S, Littlewood TJ. Treatment of myelodysplasia with oral cyclosporin. Clin Lab Haematol. 2002;24(4):211-214. [DOI] [PubMed] [Google Scholar]
- 21.Broliden PA, Dahl IM, Hast R, et al. Antithymocyte globulin and cyclosporine A as combination therapy for low-risk non-sideroblastic myelodysplastic syndromes. Haematologica. 2006;91(5):667-670. [PubMed] [Google Scholar]
- 22.Parikh AR, Olnes MJ, Barrett AJ. Immunomodulatory treatment of myelodysplastic syndromes: antithymocyte globulin, cyclosporine, and alemtuzumab. Semin Hematol. 2012;49(4):304-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-2088. [PubMed] [Google Scholar]
- 25.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503-3510. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22(3):538-543. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419-425. [DOI] [PubMed] [Google Scholar]
- 29.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing. Available at: http://www.R-project.org/. Accessed 20 December 2017.
- 30.Stahl M, Zeidan AM. Management of lower-risk myelodysplastic syndromes without del5q: current approach and future trends. Expert Rev Hematol. 2017;10(4):345-364. [DOI] [PubMed] [Google Scholar]
- 31.Italian Cooperative Study Group for rHuEpo in Myelodysplastic Syndromes, Ferrini PR, Grossi A, et al. A randomized double-blind placebo-controlled study with subcutaneous recombinant human erythropoietin in patients with low-risk myelodysplastic syndromes. Br J Haematol. 1998;103:1070-1074. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JA, Gilliland DG, Prchal JT, et al. ; GM/EPO MDS Study Group. Effect of recombinant human erythropoietin combined with granulocyte/ macrophage colony-stimulating factor in the treatment of patients with myelodysplastic syndrome. Blood. 2000;95(4):1175-1179. [PubMed] [Google Scholar]
- 33.Casadevall N, Durieux P, Dubois S, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood. 2004;104(2):321-327. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg PL, Sun Z, Miller KB, et al. Treatment of myelodysplastic syndrome patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase 3 trial by the Eastern Cooperative Oncology Group (E1996). Blood. 2009;114(12):2393-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stahl M, Zeidan AM. Lenalidomide use in myelodysplastic syndromes: Insights into the biologic mechanisms and clinical applications. Cancer. 2017;123(10):1703-1713. [DOI] [PubMed] [Google Scholar]
- 36.List A, Dewald G, Bennett J, et al. ; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456-1465. [DOI] [PubMed] [Google Scholar]
- 37.Fenaux P, Giagounidis A, Selleslag D, et al. ; MDS-004 Lenalidomide del5q Study Group. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118(14):3765-3776. [DOI] [PubMed] [Google Scholar]
- 38.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86-93. [DOI] [PubMed] [Google Scholar]
- 39.Santini V, Almeida A, Giagounidis A, et al. Randomized phase III study of lenalidomide versus placebo in RBC transfusion-dependent patients with lower-risk non-del(5q) myelodysplastic syndromes and ineligible for or refractory to erythropoiesis-stimulating agents. J Clin Oncol. 2016;34(25):2988-2996. [DOI] [PubMed] [Google Scholar]
- 40.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429-2440. [DOI] [PubMed] [Google Scholar]
- 41.Short N, Garcia-Manero G, Bravo GM, et al. Low-dose hypomethylating agents (HMAs) are effective in patients (Pts) with low- or intermediate-1-risk myelodysplastic syndrome (MDS): a report on behalf of the MDS clinical research consortium. Blood. 2015;126:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobiasson M, Dybedahl I, Holm MS, et al. Limited clinical efficacy of azacitidine in transfusion-dependent, growth factor-resistant, low- and Int-1-risk MDS: Results from the nordic NMDSG08A phase II trial. Blood Cancer J. 2014;4(3):e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thépot S, Ben Abdelali R, Chevret S, et al. ; Groupe Francophone des Myélodysplasies (GFM). A randomized phase II trial of azacitidine +/- epoetin-β in lower-risk myelodysplastic syndromes resistant to erythropoietic stimulating agents. Haematologica. 2016;101(8):918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunthararajah Y, Nakamura R, Wesley R, Wang QJ, Barrett AJ. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003;102(8):3025-3027. [DOI] [PubMed] [Google Scholar]
- 45.Haider M, Al Ali N, Padron E, et al. Immunosuppressive therapy: exploring an underutilized treatment option for myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2016;16(Suppl):S44-S48. [DOI] [PubMed] [Google Scholar]
- 46.Lim ZY, Killick S, Germing U, et al. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia. 2007;21(7):1436-1441. [DOI] [PubMed] [Google Scholar]
- 47.Stadler M, Germing U, Kliche KO, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18(3):460-465. [DOI] [PubMed] [Google Scholar]
- 48.Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gafter-Gvili A, Ram R, Gurion R, et al. ATG plus cyclosporine reduces all-cause mortality in patients with severe aplastic anemia--systematic review and meta-analysis. Acta Haematol. 2008;120(4):237-243. [DOI] [PubMed] [Google Scholar]