Highlights
-
•
The authors report a patient with biopsy-proven adult endocardial fibroelastosis.
-
•
Transthoracic echocardiography revealed diffuse coarse endocardial calcifications.
-
•
Native CT of the chest revealed LV endocardial calcifications.
Keywords: Adult endocardial fibroelastosis, Adult cardiomyopathy, Multimodality imaging
Graphical abstract
Introduction
Endocardial fibroelastosis (EFE) is a type of cardiomyopathy affecting mainly infants, rarely reported in adolescents and adults. It is characterized by massive thickening of the endocardium with subsequent myocardial dysfunction. We report an unusual case in an adult who was being evaluated for heart and lung transplantation. We present the imaging findings on various imaging techniques and also review the pathologic findings.
Case Presentation
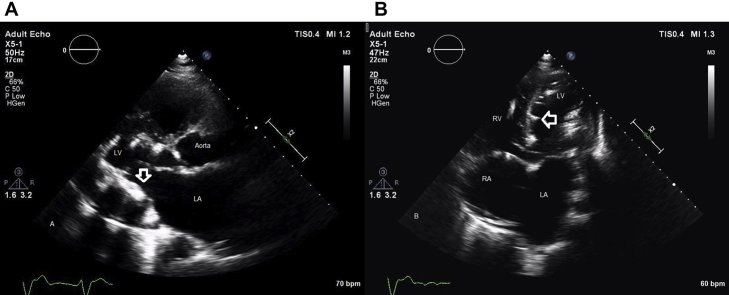
A 48-year-old man with restrictive cardiomyopathy was admitted for progressive shortness of breath. The patient had a long-standing history of hypertension, diabetes, severe secondary pulmonary hypertension, and chronic renal disease. The patient had also previously undergone remote surgical repair of aortic coarctation. On physical examination, cardiac auscultation revealed a pronounced P2 and systolic murmur consistent with tricuspid and mitral regurgitation. Electrocardiography revealed a ventricular paced rhythm. On the 6-min walk test, the patient walked 1,290 feet, which was 200 feet less than on a previous test performed 2 months earlier. Chest radiography was notable for enlargement of the cardiomediastinal silhouette and coarse calcifications overlying the left heart (Figure 1). Transthoracic echocardiography showed reduced left ventricular (LV) systolic function with an ejection fraction of 45%. There was also evidence of concentric LV hypertrophy with diffuse coarse endocardial calcifications (Figure 2, Videos 1 and 2). LV diastolic function showed a stage 3 restrictive pattern in the form of increased filling pressures (E/A ratio > 1.5, pulmonary vein a-wave flow reversal > 25 cm/sec). There was moderate mitral and severe tricuspid regurgitation and severe pulmonary hypertension, with a right ventricular systolic pressure of 129 mm Hg (Figure 3). The right ventricle was dilated, with reduced systolic function (Video 3). Echocardiographic parameters are listed in Table 1.
Figure 1.
Chest radiograph in the frontal projection showing coarse calcifications over the left heart (black arrow).
Figure 2.
(A,B) Transthoracic echocardiography in the parasternal long-axis and apical four-chamber images showing hyperechoic foci with associated acoustic shadowing of myocardial calcifications (white arrows). LA, Left atrial; LV, left ventricular; RA, right atrial; RV, right ventricular.
Figure 3.
Transthoracic echocardiography in the parasternal apical four-chamber images showing central jet of tricuspid regurgitation (white arrow).
Table 1.
Echocardiographic parameters
| Measurement | Value | Indexed | Normal |
|---|---|---|---|
| Left atrial volume | 157 mL (four-chamber A-L) | 87 mL/m2 | LAVi ≤ 34 mL/m2 |
| Left atrial diameter | 4.5 cm (M-mode) | ||
| IVS, leaflet tips | 1.3 cm (2D) | ||
| Posterior wall thickness | 1.8 cm (2D) | ||
| LV mass | 229 g (2D) | 126 g/m2 | |
| LV stroke volume | 37 mL (2D biplane) | ||
| LV outflow tract stroke volume | 60 mL | 33 mL/m2 | |
| LV end-diastolic volume | 73 mL (2D biplane) | 40.3 mL/m2 | 34 mL/m2 ≤ EDVi < 75 mL/m2 |
| LV end-systolic volume | 36 mL (2D biplane) | 19.7 mL/m2 | |
| Ejection fraction | 45% (2D biplane) | >52% |
A-L, Area-length technique; EDVi, end-diastolic volume index; IVS, interventricular septum; LAVi, left atrial volume index; 2D, two-dimensional.
A computed tomographic scan of the chest without contrast demonstrated diffuse coarse LV calcifications, predominantly along the endocardial aspect (Figure 4). Left atrial calcifications were also noted. For further evaluation of the restrictive cardiomyopathy, catheter-directed percutaneous right ventricular endomyocardial biopsy was performed (Figure 5). Microscopic examination revealed myocyte hypertrophy. Movat stain noted focal areas of thickened endocardium demonstrating focal EFE. No evidence of inflammatory infiltrates, giant cells, or granulomata was noted. Thioflavin-S stain was negative for amyloid, and there was no evidence of glycogen or iron storage. The patient is being considered for heart and lung transplantation.
Figure 4.
(A-C) Four-chamber, short-axis, and two-chamber reformatted images from chest computed tomography without contrast showing coarse endocardial calcification with extensive involvement of the mitral annulus (black arrows). LA, Left atrial; LV, left ventricular; RA, right atrial; RV, right ventricular.
Figure 5.
Endomyocardial biopsy. (A) Light micrograph of the subendocardium of a biopsy piece showing wavy eosinophilic material corresponding to fibrous tissue, which thickens the endocardium and is birefringent. This tissue also infiltrates the subjacent myocardium. The myocytes show mild hypertrophy with diameters ranging from 30 to 40 μm. There are no inflammatory infiltrates. Hematoxylin and Eosin stain, 400×. (B) The same field of biopsy shown in (A) distinctly demonstrates elastic fibers (black) interspersed between the dense fibrous tissue (yellow) in the areas of endocardial thickening, thus demonstrating endocardial fibroelastosis. Movat pentachrome stain, 400×.
Discussion
EFE is a cardiac condition classically seen in infants and children that presents as unexplained heart failure. In one review of 52 pediatric heart transplantations, evidence of EFE was found in 25% of patients, suggesting that this condition may not be as uncommon as previously thought. EFE is classified into primary and secondary forms; the primary form is not associated with other structural heart diseases, whereas secondary forms are. Pathologically, two forms are recognized: contracted (less common) and dilated (more common).1 Several pathophysiologic mechanisms have been postulated for this condition, including familial associations and viral etiologies. EFE presenting in adults is very rare, with only a few isolated reports in the literature. To the best of our knowledge, a case with histopathological confirmation has not been reported previously. EFE is characterized by widespread white fibroelastic tissue that lines the endocardium, resulting in incomplete ventricular obliteration with reduced distensibility and impaired diastolic filling. Involvement of chordae tendineae may lead to mitral or tricuspid regurgitation.2 The imaging appearance of EFE is fairly classic and includes very few differential diagnoses. Granulomatous infections such as Mycobacterium tuberculosis infection may be considered, but these infections usually cause myocardial and annular calcifications; the pericardium is usually affected as well.3
Conclusion
EFE causes restrictive cardiomyopathy and can present in adults, causing congestive heart failure. Cardiac transplantation is indicated in patients with end-stage disease.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2017.05.005.
Supplementary Data
Transthoracic echocardiography in the short-axis video view showing coarse endocardial calcifications with posterior acoustic shadowing.
Transthoracic echocardiography in the two-chamber video view showing coarse endocardial calcifications with posterior acoustic shadowing.
Transthoracic echocardiography in the two-chamber video view showing reduced systolic function of the right ventricle.
References
- 1.Seki A., Patel S., Ashraf S., Perens G., Fishbein M.C. Primary endocardial fibroelastosis: an underappreciated cause of cardiomyopathy in children. Cardiovasc Pathol. 2013;22:345–350. doi: 10.1016/j.carpath.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Ni J., Bowles N.E., Kim Y.H., Demmler G., Kearney D., Bricker J.T. Viral infection of the myocardium in endocardial fibroelastosis. Molecular evidence for the role of mumps virus as an etiologic agent. Circulation. 1997;95:133–139. doi: 10.1161/01.cir.95.1.133. [DOI] [PubMed] [Google Scholar]
- 3.Hitsumoto T., Ikeda S., Matsukage S., Hamada M. Extensive myocardial calcinosis due to Mycobacterium tuberculosis. Eur Heart J. 2016;37:1195. doi: 10.1093/eurheartj/ehv604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography in the short-axis video view showing coarse endocardial calcifications with posterior acoustic shadowing.
Transthoracic echocardiography in the two-chamber video view showing coarse endocardial calcifications with posterior acoustic shadowing.
Transthoracic echocardiography in the two-chamber video view showing reduced systolic function of the right ventricle.