Graphical abstract
Keywords: TAVR, endocarditis, 3D echocardiography, TEE
Highlights
-
•
Three-dimensional TEE adds value in diagnosing IE in bioprosthetic valves.
-
•
Bioprosthetic valve IE resolves with appropriate antibiotic therapy.
-
•
Misdiagnosis of IE can lead to inappropriate treatment and lethal complications.
Introduction
Prosthetic infective endocarditis (IE) in post–transcatheter aortic valve replacement (TAVR) patients has been reported to occur in 0.5%–3.1% of the patients within 1 year.1 IE is associated with mortality as high as 37%.2 Most patients present with fever or acute congestive heart failure. Definitive diagnosis of IE can be made by major Duke criteria with positive blood cultures and evidence of endocardial involvement.3 Echocardiography is a key part of the evaluation of IE. In the case of TAVR valves, two-dimensional (2D) transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) for visualization of vegetations can be challenging, with reduced sensitivity due to shadowing created by the prosthesis. Advances in three-dimensional (3D) echocardiography have enabled better spatial resolution and visualization of cardiac structures, allowing the identification of any valvular vegetations, abscesses, or nodules. We present the case of a patient, 3 years after TAVR, who presented with positive blood cultures and transient neurologic changes. Neither 2D TTE nor 2D TEE achieved diagnostic visualization of the TAVR valve, because of acoustic shadowing created by the valve stent. However, 3D TEE did demonstrate a mobile vegetation on one of the leaflets, confirming the diagnosis of IE.
Case Presentation
A 97-year-old woman had undergone TAVR (25-mm SAPIEN valve; Edwards Lifesciences, Irvine, CA) 3 years before admission. She had a medical history of hypertension, atrial fibrillation, peripheral vascular disease, and diastolic heart failure. In October 2016 she presented to the hospital with chills, fever, and acute kidney injury. Although two blood cultures returned positive results for Streptococcus mitis, no source of infection was identified, and echocardiography, which had been done 2 weeks previously, was not repeated. Antibiotics were discontinued after a 10-day course of treatment. One week later, the patient presented to the hospital with transient right-sided face, arm, and leg weakness. Upon arrival to the hospital, her symptoms had improved. On examination, heart rate was 88 beats/min, blood pressure was 147/88 mm Hg, and body temperature was 36.9°C. She was alert and oriented, with mild receptive aphasia and generalized weakness. On cardiac examination, she had a soft systolic murmur at the right upper sternal border and no diastolic murmur. Lungs were clear to auscultation. No lower extremity edema or jugular venous dilatation was noted.
Blood cultures were again positive for the same organism. Electrocardiographic findings were unchanged from prior examinations, with sinus rhythm and right bundle branch block (Figure 1). Magnetic resonance imaging of the brain and magnetic resonance angiography of the head were negative for an acute ischemic event. Two-dimensional TTE showed normal left ventricular function, with an ejection fraction of 64%, mild to moderate mitral regurgitation, trivial aortic regurgitation, and normal aortic bioprosthesis function with mean gradient of 12 mm Hg (Figure 2, Videos 1–3). These echocardiographic findings were similar to the findings on echocardiography 1 month previously. There was no increase in aortic regurgitation, and no new mass lesions or vegetations were detected. Precise visualization of the TAVR valve leaflets was limited because of acoustic shadowing. Two-dimensional TEE did not show any mobile vegetations (Figure 3, Videos 4–8), but 3D TEE did show abnormal thickening and a mobile vegetation on one of the leaflets of the TAVR valve (Figure 4, Videos 9–11). The organism was sensitive to penicillin and ceftriaxone, and the patient was treated with ceftriaxone 2 g/d for 8 weeks. Blood cultures drawn 1 month after cessation of antibiotic therapy were negative. Two-dimensional TTE was performed 6 months after completion of therapy, and nonspecific thickening of the bioprosthetic aortic valve leaflets was noted, with mild central and paravalvular regurgitation (Videos 12–15).
Figure 1.
Admission electrocardiogram shows normal sinus rhythm with right bundle branch block.
Figure 2.
(A) Aortic valve continuous-wave Doppler. (B) Parasternal short-axis view of bioprosthetic aortic valve. AV, Aortic valve; LA, left atrium; RA, right atrium; RV, right ventricle.
Figure 3.
Two-dimensional TEE showing long axis of the aortic valve on the left and short axis on the right. Ao, Aorta; LA, left atrium.
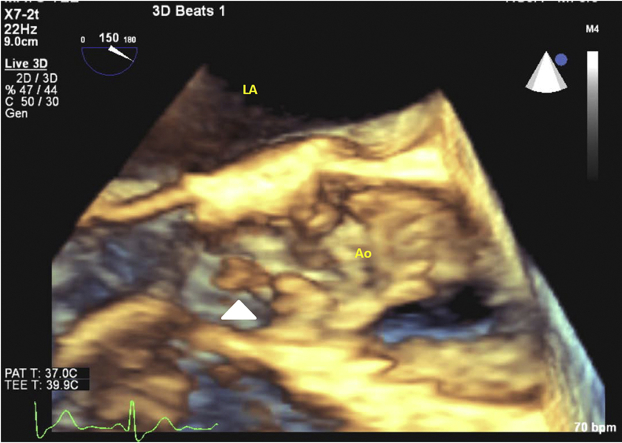
Figure 4.
Three-dimensional TEE showing long axis of the aortic valve, with the white arrow pointing to elongated mobile vegetation originating from the aortic valve leaflets. Ao, Aorta; LA, left atrium.
Discussion
The diagnosis of IE hinges on the findings of blood culture positivity with an organism known to be associated with endocardial infection and visualization of endocardial infection by echocardiography or other imaging modalities. Visualization of endocardial infection is especially challenging in prosthetic valve endocarditis and is made more difficult in TAVR valves, which have greater amounts of prosthetic material in the supra- and infravalvular regions, worsening acoustic shadowing during echocardiographic examination. In the two largest collections of cases, positive echocardiographic findings on TEE were identified in only 58.3%4 or 55%5 of cases. Our report suggests that the use of 3D TEE may improve the yield in suspected cases with typical bacteriologic findings. Importantly, the use of 3D imaging was documented in non-TAVR prosthetic endocarditis by Liu et al.,6 who compared 2D TTE with 3D TTE for the diagnosis of IE on the basis of three parameters: presence of mobile nodules, focal thickening of valve leaflets, and uneven surfaces on valve leaflets. Mobile nodules were detected by 3D TEE with sensitivity of 83%, compared with 0% by 2D TEE. Elsewhere, Berdejo et al.7 evaluated 60 patients with the diagnosis of IE by 2D TEE and 3D TEE for the size of vegetations and occurrence of embolic events. The positive predictive value increased from 59.1% to 65.2% with the use of 3D TEE for a determination of maximum length of vegetation ≥20 mm.
The estimated incidence of bioprosthetic valve endocarditis is about 0.45% per year, based on a 10-year retrospective study.8 The overall incidence of IE has been reported as high as 2.1% per year and 3.1% in the first year after TAVR in a series by Olsen et al.5 The most common presenting symptoms are fever in 80.4% of patients and acute heart failure in 40%.1 The modified Duke criteria used for the diagnosis of endocarditis are divided into major and minor criteria, as shown in Table 1.3, 9 Two major criteria are required for a definitive diagnosis of endocarditis: two positive blood cultures with microorganisms known to cause IE and demonstration of IE by an imaging modality (echocardiography, 18F-fluorodeoxyglucose positron emission tomography–computed tomography [CT], or cardiac CT).9 Definitive diagnosis of IE can also be made with one major and three minor criteria or five minor criteria. Echocardiography is recommended as the first-line imaging modality in the diagnosis of IE. Echocardiographic diagnostic criteria include presence of vegetation, abscess, pseudoaneurysm, or new dehiscence of a prosthetic valve. Multislice cardiac CT allows the detection of abscesses, pseudoaneurysms, fistula formation, and prosthetic valve dehiscence. Fluorine-18-fluorodeoxyglucose positron emission tomography can be used to detect abnormal activity around the site of prosthetic valve implantation for prostheses implanted >3 months earlier. Both multislice cardiac CT and 18F-fluorodeoxyglucose positron emission tomography can be used when the diagnosis of IE remains unclear.9, 10 The most common organisms identified are coagulase-negative staphylococci (24.5%), Staphylococcus aureus (20.8%), and enterococci (20.8%).4 Diagnosis of IE by 2D TTE can be challenging because of acoustic shadowing of the prosthetic valve. Our case illustrates the value of 3D TEE to detect mobile vegetations and allow a definitive diagnosis of prosthetic endocarditis in TAVR patients. As shown in this case report, the use of 3D TEE resulted in definitive diagnosis of IE and proper antibiotic therapy.
Table 1.
Major and minor criteria used in the proposed modified Duke criteria for the diagnosis of IE
| Major criteria | Minor criteria |
|---|---|
Positive blood cultures (two separate cultures) of typical microorganisms
|
Predisposing heart condition |
| History of injection drug use | |
Immunologic phenomena
| |
Evidence of endocardial involvement by one of these modalities
|
Vascular phenomena
|
Microbiologic evidence not meeting major criteria
| |
| Fever >100.4°F/38°C |
CT, Computed tomography; FDG, fluorodeoxyglucose; HACEK, Haemophilus spp, Actinobacillus, Cardiobacterium hominis, Eikenella corrodens, and Kingella spp; IgG, immunoglobulin G; PET, positron emission tomography.
Conclusion
In cases of suspected IE, especially in patients with prosthetic valves, it is imperative to evaluate using different imaging modalities, including 3D echocardiography, as this will allow superior spatial-temporal perspective, improving visualization of the valve leaflets, struts, and associated vegetations. Inadequate evaluation of the valves can result in misdiagnosis of IE and lead to complications and potentially death.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2017.05.002.
Supplementary Data
Two-dimensional TTE, parasternal long-axis view, with no clear vegetation noted on the bioprosthetic aortic valve.
Two-dimensional TTE, parasternal short-axis view of the bioprosthetic aortic valve with difficult-to-visualize leaflets.
Two-dimensional TTE, parasternal short-axis view of the bioprosthetic aortic valve with color Doppler.
Two-dimensional TEE, long-axis view of the bioprosthetic aortic valve and aorta with difficult-to-visualize leaflets.
Two-dimensional TEE, long-axis view of the bioprosthetic aortic valve leaflets.
Two-dimensional TEE, long-axis view of bioprosthetic aortic valve with color Doppler with trivial aortic regurgitation.
Two-dimensional TEE, biplane view of the bioprosthetic aortic valve with long axis to the left and short axis to the right.
Two-dimensional TEE, short-axis view of the bioprosthetic aortic valve with color Doppler.
Three-dimensional TEE, long-axis view of the bioprosthetic aortic valve with mobile vegetation in one of the leaflets.
Three-dimensional TEE, long-axis view of the bioprosthetic aortic valve with mobile vegetation in one of the leaflets into the left ventricular outflow tract.
Three-dimensional TEE, short-axis view of the bioprosthetic aortic valve with thickened aortic bioprosthetic valve leaflets.
Six-month follow-up. Parasternal long-axis view with thickened bioprosthetic aortic valve leaflets.
Six-month follow-up. Zoom in on bioprosthetic aortic valve in the parasternal long-axis view with an echolucent density on the right coronary cusp.
Six-month follow-up. Parasternal short-axis view of the bioprosthetic aortic valve with thickening of the aortic valve leaflets.
Six-month follow-up. Parasternal short-axis view of the bioprosthetic aortic valve with color Doppler showing trivial aortic regugitation.
References
- 1.Regueiro A., Linke A., Latib A., Ihlemann N., Urena M., Walther T. Association between transcatheter aortic valve replacement and subsequent infective endocarditis and in-hospital death. JAMA. 2016;316:1083–1092. doi: 10.1001/jama.2016.12347. [DOI] [PubMed] [Google Scholar]
- 2.Callen D.J., Shroff M.M., Branson H.M., Lotze T., Li D.K., Stephens D. MRI in the diagnosis of pediatric multiple sclerosis. Neurology. 2009;72:961–967. doi: 10.1212/01.wnl.0000338629.01627.54. [DOI] [PubMed] [Google Scholar]
- 3.Li J.S., Sexton D.J., Mick N., Nettles R., Fowler V.G., Jr., Ryan T. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 4.Amat-Santos I.J., Messika-Zeitoun D., Eltchaninoff H., Kapadia S., Lerakis S., Cheema A.N. Infective endocarditis after transcatheter aortic valve implantation: results from a large multicenter registry. Circulation. 2015;131:1566–1574. doi: 10.1161/CIRCULATIONAHA.114.014089. [DOI] [PubMed] [Google Scholar]
- 5.Olsen N.T., De Backer O., Thyregod H.G., Vejlstrup N., Bundgaard H., Sondergaard L. Prosthetic valve endocarditis after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.001939. e001939. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y.W., Tsai W.C., Lin C.C., Hsu C.H., Li W.T., Lin L.J. Usefulness of real-time three-dimensional echocardiography for diagnosis of infective endocarditis. Scand Cardiovasc J. 2009;43:318–323. doi: 10.1080/14017430902737940. [DOI] [PubMed] [Google Scholar]
- 7.Berdejo J., Shibayama K., Harada K., Tanaka J., Mihara H., Gurudevan S.V. Evaluation of vegetation size and its relationship with embolism in infective endocarditis: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2014;7:149–154. doi: 10.1161/CIRCIMAGING.113.000938. [DOI] [PubMed] [Google Scholar]
- 8.Akowuah E.F., Davies W., Oliver S., Stephens J., Riaz I., Zadik P. Prosthetic valve endocarditis: early and late outcome following medical or surgical treatment. Heart. 2003;89:269–272. doi: 10.1136/heart.89.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.P., Del Zotti F. [2015 ESC guidelines for the management of infective endocarditis. The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)] G Ital Cardiol (Rome) 2016;17:277–319. doi: 10.1714/2214.23904. [DOI] [PubMed] [Google Scholar]
- 10.Saby L., Laas O., Habib G., Cammilleri S., Mancini J., Tessonnier L. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374–2382. doi: 10.1016/j.jacc.2013.01.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, parasternal long-axis view, with no clear vegetation noted on the bioprosthetic aortic valve.
Two-dimensional TTE, parasternal short-axis view of the bioprosthetic aortic valve with difficult-to-visualize leaflets.
Two-dimensional TTE, parasternal short-axis view of the bioprosthetic aortic valve with color Doppler.
Two-dimensional TEE, long-axis view of the bioprosthetic aortic valve and aorta with difficult-to-visualize leaflets.
Two-dimensional TEE, long-axis view of the bioprosthetic aortic valve leaflets.
Two-dimensional TEE, long-axis view of bioprosthetic aortic valve with color Doppler with trivial aortic regurgitation.
Two-dimensional TEE, biplane view of the bioprosthetic aortic valve with long axis to the left and short axis to the right.
Two-dimensional TEE, short-axis view of the bioprosthetic aortic valve with color Doppler.
Three-dimensional TEE, long-axis view of the bioprosthetic aortic valve with mobile vegetation in one of the leaflets.
Three-dimensional TEE, long-axis view of the bioprosthetic aortic valve with mobile vegetation in one of the leaflets into the left ventricular outflow tract.
Three-dimensional TEE, short-axis view of the bioprosthetic aortic valve with thickened aortic bioprosthetic valve leaflets.
Six-month follow-up. Parasternal long-axis view with thickened bioprosthetic aortic valve leaflets.
Six-month follow-up. Zoom in on bioprosthetic aortic valve in the parasternal long-axis view with an echolucent density on the right coronary cusp.
Six-month follow-up. Parasternal short-axis view of the bioprosthetic aortic valve with thickening of the aortic valve leaflets.
Six-month follow-up. Parasternal short-axis view of the bioprosthetic aortic valve with color Doppler showing trivial aortic regugitation.