Abstract
Essentials.
Global hemostasis tests generally indicate preserved hemostatic function in cirrhotic patients.
Here we present thromboelastography test results of 270 patients of mixed etiology.
Normal results were seen in the majority of patients, although some were hypo‐ or hypercoagulable.
Thromboelastography did not predict future bleeding, thrombosis, or risk of transplant or death.
Background
New laboratory tests that measure global hemostasis indicate generally preserved hemostatic function in patients with cirrhosis. It is not known whether normal hemostatic function is maintained across various subsets of patients.
Objectives
In the present study, we investigated clot generation and clot lysis kinetics in a large group of patients with different etiologies of disease.
Patients/Methods
Blood samples of 270 patients with cirrhosis were studied using thromboelastography (TEG), which measures the dynamic and physical properties of clot formation and lysis in whole blood. TEG parameters of different subsets of the patient population were compared. Correlations with routine laboratory tests as well as clinical outcomes were explored.
Results
Overall, TEG parameters were normal and similar between underlying disease etiologies. A proportion of subjects showed hypocoagulable features, with the exception of patients with cholestatic cirrhosis in whom TEG readings showed hypercoagulable features. In all groups, K‐time, α‐Angle, and MA correlated well with platelet counts and fibrinogen plasma levels. After a mean follow‐up of 2 years and 11 months, 31 patients had experienced a bleeding event, 8 had developed thrombosis, and 173 patients (64%) had undergone liver transplantation and/or had died. TEG baseline parameters were similar between patients subdivided according to outcome.
Conclusions
TEG parameters reflected generally preserved function of the hemostatic system in patients with cirrhosis, with hypo‐ and hypercoagulable features in subsets of patients with specific underlying disease etiologies. Abnormalities in TEG parameters did however not predict bleeding, thrombosis, or risk of liver transplantation and/or death.
Keywords: hemorrhage, hemostasis, liver diseases, thromboelastography, thrombosis
1. INTRODUCTION
Patients with cirrhosis frequently present with complex hemostatic alterations.1 It has been proposed that the net effect of the hemostatic changes in these patients is a rebalanced, yet precarious, hemostatic system. Indeed, depending on the nature of the trigger and exact physiological/clinical conditions, the balance may easily tip toward either bleeding or thrombosis.2 Identification of the hemostatic phenotype at baseline from individual patients with cirrhosis could potentially predict the likelihood of a thrombotic or bleeding event to occur.
Routine diagnostic tests of hemostasis such as the platelet count and prothrombin time are often indicative of a hypocoagulable state in cirrhosis.1 In contrast, more advanced hemostasis tests that measure global hemostasis, including thrombin generation assay (TGA) and whole blood global viscoelastic tests (VETs), generally indicate a preserved hemostatic balance.3, 4, 5 As opposed to routine tests, these global tests assess the combined hemostatic potential defined by the balance between pro‐ and antihemostatic drivers. TGA for example is determined by the concentration of all the known and potentially yet unidentified clotting factors and inhibitors. In a landmark study, Tripodi and colleagues demonstrated that thrombomodulin‐modified TGA results of patients with cirrhosis are generally comparable to those of healthy individuals. These seminal observations have contributed to the shift in paradigm on the net effects of the coagulopathy in cirrhosis, such that patients are considered to maintain a functional hemostatic system at baseline despite complex alterations in hemostatic components.3
A drawback of TGA, however, is that it is performed with plasma, and therefore, the supporting role of blood cells in hemostasis is excluded. In addition, the test is not designed to measure clot formation, which is the functional endpoint of the hemostatic system. VETs on the other hand not only dissect the dynamics of clot formation in whole blood, they also measure ultimate clot strength and stability. Therefore, VETs may reflect the hemostatic phenotype better than TGA. Two VETs are commercially available, thromboelastography (TEG) and rotational thromboelastometry (ROTEM). Both tests have been used to assess the hemostatic function of patients with cirrhosis in the laboratory.4, 5, 6 In addition, studies have been performed that investigated correlations between TEG/ROTEM parameters and the occurrence of bleeding in these patients.7, 8, 9 For example, in a small number of patients with cirrhosis who presented with acute variceal bleeding, specific parameters of TEG (R‐time, K‐time, and α‐Angle) indicated more hypocoagulable features in those who experienced early rebleeding. In contrast, none of the standard laboratory tests of hemostasis (international normalized ratio (INR), activated partial thromboplastin time (aPTT), or platelet count) differed between those who rebled and those who did not.7
Studies so far have been limited by relatively small cohorts or cohorts of mixed etiology. Therefore, it is yet unknown whether VET parameters are similar across different etiologies of cirrhosis and hence reflect a similar baseline hemostatic function across the whole patient group. It is also unknown whether subdividing hemostatic function according to etiology will show different correlations with clinical events (ie, thrombosis or bleeding).
In the present study, we measured the baseline hemostatic phenotype by TEG in a large group of patients with cirrhosis. The aim was to better define the hemostatic status of patients with cirrhosis across a variety of etiologies and severity of disease. In addition, we investigated possible correlations between TEG, bleeding and thrombosis, but also with disease outcome, since it has been proposed that intrahepatic thrombosis contributes to a progressive disease course in patients with cirrhosis.10, 11
2. PATIENTS & METHODS
Two hundred seventy consecutive outpatients with cirrhosis were prospectively studied at the Virginia Commonwealth University (VCU) Medical Center between October 2008 and January 2012. The mean follow‐up period was 2 years and 11 months. Patients were classified according to severity of disease by using the Child‐Pugh classification and model for end‐stage liver disease (MELD). Patients were grouped according to the following etiologies of disease: hepatitis B or C (HBV/HCV); ethanol (ETOH)‐induced cirrhosis; nonalcoholic steatohepatitis (NASH); cholestatic cirrhosis, defined as primary biliary cirrhosis, primary sclerosing cholangitis or complication of cholecystectomy or choledochal cyst disease; and other non‐cholestatic cirrhosis, defined as auto‐immune hepatitis, alpha‐1 antitrypsin deficiency, Wilson's disease, Budd‐Chiari syndrome, sarcoidosis, or a combination of non‐cholestatic etiologies of cirrhosis. Patients with hepatocellular carcinoma (HCC) of diverse underlying liver disease etiologies were also compared to patients without HCC. Exclusion criteria were a documented history of congenital coagulation disorders, recent viral infection (<2 weeks), use of antiviral drugs, alcohol or anticoagulant drugs in the past 10 days, pregnancy, HIV positivity, transfusion with blood products within the past 7 days, and those who received procoagulant treatments other than vitamin K prior to enrollment. Clinical and laboratory data were collected from the nearest time point to the performance of TEG, usually within 24 hour. TEG was performed once at entry of the study. Informed consent was obtained from each patient before inclusion. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the medical ethics committee of VCU Medical Center.
2.1. Definition of complications and final outcomes of cirrhosis
Patients were seen every 6 months (for Child's class A patients) or at least every 3 months (for Child's class B/C patients) as a matter of routine by one investigator (RTS). Electronic medical records were reviewed on each visit, and patients were specifically asked whether they had any “bleeding or clotting” complications during the interim. Medical records from outside institutions and physicians were requested in the event of a positive response from a patient when the complication occurred at a hospital other than VCU Medical Center.
Bleeding complications specifically included spontaneous (non‐procedure‐related) gastrointestinal bleeding from any source, the need for blood transfusion with evidence of blood loss, or other significant blood loss. Epistaxis was not included in the assessment of bleeding complication unless it required intervention. Thrombotic complications specifically included spontaneous (non‐procedure‐related) deep venous thrombosis of the arms legs and/or pulmonary embolism or new occlusive portal venous thrombosis on ultrasound during 6‐month surveillance exams for hepatocellular carcinoma. Final outcomes of cirrhosis included transplant‐free survival, orthotopic liver transplantation (OLT), or death.
2.2. Thromboelastography
Thromboelastography was performed on a single instrument (Thrombelastograph Haemostasis Analyzer 5000; Haemonetics Corp., Haemoscope Division, Niles, IL, USA). Briefly, 5 mL of 3.2% citrated whole blood was subjected to TEG within 2 hour of blood draw. Clotting was initiated at 37°C by the addition of 40 μL of kaolin to 0.34 mL of re‐calcified blood where 20 μL of 0.2 mol L−1 CaCl2 is added for a final concentration of 11.1 mmol L−1. Kinetic changes in clot formation and clot dissolution were measured for 30 minute after reaching maximal clot firmness. Five parameters were recorded (see Figure 1 and Table 1).
Figure 1.
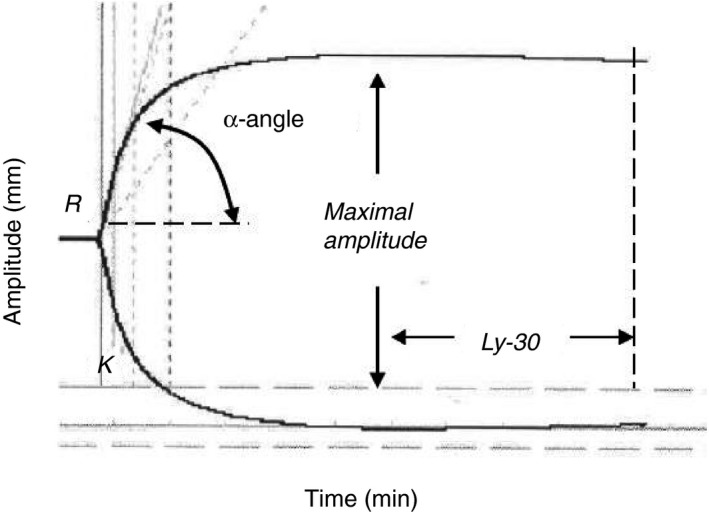
Schematic overview of TEG parameters
Table 1.
Definition and normal range of TEG parameters
| Parameter | Definition | Normal range |
|---|---|---|
| R‐time | Latency of clot formation | 2.5‐7.5 minute |
| K‐time | Kinetics of fibrin formation (time from initial clot formation to an amplitude of 20 mm) | 0.8‐2.8 minute |
| α‐Angle | Kinetics of fibrin formation (rate of fibrin formation and cross‐linking on platelets) | 55.2‐78.4 degrees |
| Maximum amplitude | Maximum clot firmness | 50.6‐69.4 mm |
| Lysis 30 | Fibrinolysis 30 minute from maximum amplitude | 0.0‐7.5% |
An abnormal TEG parameter was defined as an R‐ or K‐time above the upper limit of normal, or an α‐Angle or MA below the lower limit of normal for our laboratory, which are similar to other series.12
2.3. Coagulation tests
PT/INR, platelet counts, and plasma levels of fibrinogen were assayed by the Clinical Coagulation Laboratory at VCU. The PT/INR and fibrinogen plasma concentration were determined using the automated STA‐R Evolution clot detection system. The PT/INR was determined using re‐calcified plasma and recombinant human tissue factor and synthetic thromboplastin (Dade Innovin‐Reagent; Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). Fibrinogen was determined following the von Clauss technique using reagents provided by the manufacturer (Diagnostica Stago, Parsipanny, NJ, USA).
2.4. Statistical analysis
Continuous data were tested for normality and expressed as mean±standard deviation (SD) or median [range], and analyzed by analysis of variance (ANOVA) or Wilcoxon/Kruskal–Wallis Rank Sums test, as appropriate. Categorical variables were analyzed by Chi Square test and correlation of continuous data by Pearson correlation (r‐value). All data were analyzed using Graphpad InStat 3.10 (San Diego, CA, USA) software package. Significance was defined as a P‐value equal or less than .05.
3. RESULTS
3.1. Demographic, laboratory, and clinical characteristics of patients with cirrhosis
Patient demographics, vital signs, and laboratory test results at the time of admission, as well as a selection of clinical outcome data, are presented in Table 2. In brief, the mean age of participants was 57 years and 38% were female. Thirty percent was classified as having Child‐Pugh A cirrhosis, 51% Child‐Pugh B, and 19% Child‐Pugh C. With a median value of 135×109 L−1, platelet counts were highest in cholestatic disease, but this was not significantly higher than in other etiologies of cirrhosis. However, patients who suffered from cholestatic cirrhosis had significantly higher fibrinogen levels (median 396 mg dL−1 compared to 191 mg dL−1 in HBV/HCV cirrhosis, for example). Combined, 26% of the patients developed a bacterial infection during follow‐up, 49% ascites, 21% renal failure, 25% encephalopathy, 3% thrombosis, and 11% suffered from bleeding complications. At the end of the study, 71% of the patients had been hospitalized, 19% underwent liver transplantation, and 46% died. The mean time from performing TEG to bleeding was 347 days and 72 days to thrombosis. It was 328 days to liver transplantation and 484 days to death. Patients in the cholestatic patient group were up to 10 time less likely to develop encephalopathy (3%), but 2‐3 times more likely to undergo liver transplantation (44%) compared to patients suffering from other etiologies of cirrhosis, whilst the distribution among classes of disease severity between the groups was similar (ie, Child‐Pugh and MELD).
Table 2.
Demographic, laboratory and clinical characteristics of the study cohort
| Characteristics | HBV/HCV (n=81) | ETOH (n=33) | NASH (n=34) | Other non‐cholestatic (n=44) | Cholestatic (n=32) | HCC (n=46) | Patients combined (n=270) |
|---|---|---|---|---|---|---|---|
| Value | Value | Value | Value | Value | Value | Value | |
| Age, years | 56 (11) | 57 (11) | 59 (7) | 58 (12) | 55 (14) | 57 (13) | 57 (12) |
| Female sex | 27 {33} | 9 {11} | 21 {62} | 20 {44} | 18 {56} | 8 {17}** | 103 {38} |
| Child‐Pugh score | |||||||
| A | 19 {23} | 6 {18} | 12 {35} | 15 {44} | 9 {28} | 21 {45} | 82 {30} |
| B | 46 {57} | 20 {61} | 18 {53} | 22 {50} | 17 {53} | 15 {33} | 138 {51} |
| C | 16 {20} | 7 {21} | 4 {12} | 7 {16} | 6 {19} | 10 {22} | 50 {19} |
| MELD score | 12 (4) | 13 (5) | 12 (4) | 13 (6) | 14 (5) | 13 (5) | 13 (5) |
| Laboratory blood tests | |||||||
| Hemoglobin, g/dL | 11.8 (2.3) | 11.0 (2.2) | 11.8 (1.9) | 12.2 (2) | 12 (2) | 12.7 (1.9) | 12.3 (7) |
| Platelet count, ×109/L | 84 [58‐323] | 108 [77‐168] | 108 [60‐142] | 81 [60‐160] | 135 [72‐144] | 77 [61‐142] | 92 [62‐142] |
| WBC, ×109/L | 4.7 (1.9) | 5.8 (2.4) | 4.8 (2.0) | 5.0 (2.5) | 6.0 (3.3) | 5.4 (2.3) | 5.2 (2.4) |
| Sodium, mmol/L | 135 (15) | 137 (3) | 138 (1) | 137 (1) | 138 (4) | 137 (4) | 137 (4) |
| Creatinin, mg/dL | 0.84 (0.72) | 1.01 (0.44) | 0.99 (0.31)* | 0.92 (0.54) | 0.9 (0.7) | 1.2 (0.7)* | 1.0 (0.6) |
| Total bilirubin, mg/dL | 3 [2.1 ‐8.9] | 2 [1.1‐3.2] | 1.6 [1.2‐2.5] | 3.9 [3.3‐6.4] | 2.1 [1.3‐7.5] | 1.8 [0.8‐2.9] | 2 [1.2‐3.2] |
| Albumin, g/dL | 3.0 [2.6‐3.4] | 3.2 [3‐3.7] | 3.3 [2.9‐3.9] | 3.4 [2.9‐4] | 3.6 [3.2‐4] | 3.3 [3‐3.8] | 3.2 (2.9‐3.7) |
| Fibrinogen, mg/dL | 191 [167‐236]* | 278 [203‐348]* | 248 [183‐539]* | 229 [179‐339]* | 396 [271‐490] | 247 [205‐318]* | 241 [182‐329] |
| INR | 2.4 (1.5) | 1.3 (0.3) | 1.3 (0.3) | 1.3 (0.3) | 1.3 (0.4) | 1.3 (0.2) | 1.3 (0.3) |
| Complications | |||||||
| Bacterial infection | 11 {14} | 14 {42} | 14 {41} | 11 {25} | 10 {31} | 10 {22} | 70 {26} |
| Ascites | 45 {56} | 22 {67} | 18 {53} | 18 {41} | 12 {38} | 18 {39} | 133 {49} |
| Renal failure | 20 {25} | 11 {33} | 6 {18} | 9 {20} | 4 {13} | 6 {13} | 56 {21} |
| Encephalopathy | 23 {28}** | 9 {27}** | 10 {29}** | 12 {27}** | 1 {3} | 12 {26}** | 67 {25} |
| Thrombosis | 2 {2} | 0 | 1 {3} | 3 {7} | 0 | 2 {4} | 8 {3} |
| Bleeding | 11 {13} | 8 {24} | 3 {9} | 4 {9} | 2 {6} | 3 {7} | 31 {11} |
| Outcome | |||||||
| Hospitalisation | 67 {82} | 25 {76} | 27 {79} | 29 {66} | 24 {75} | 19 {41}** | 191 {71} |
| Liver transplantation | 16 {20}** | 3 {9}** | 5 {15}** | 6 {14}** | 14 {44} | 6 {13}** | 50 {19} |
| Death | 37 {46} | 15 {45} | 15 {44} | 15 {34} | 10 {31} | 31 {67}** | 123 {46} |
ETOH, alcohol; HBV, hepatitis B virus; HCC, Hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalized ratio; MELD, model for end‐stage liver disease; NASH, non‐alchoholic steatohepatitis; WBC, white blood cells. Data are presented as mean (SD), numbers {percentages} or medians [IQR]. Laboratory values normal range: Hemoglobin, 13.5‐18 g/dL (male) or 11.5‐16 g/dL (female); Platelet count, 150‐400 × 109/L; WBC 4.0‐11.0×109/L; Sodium, 135‐145 mmol/L; Creatinin, 0.8‐1.3 mg/dL (male) or 0.6‐1.1 mg/dL (female); Total bilirubin, <1.2 mg/dL; Albumin, 3.4‐5.4 g/dL; Fibrinogen, 150‐400 mg/dL; INR, <1.1. *P<.05 and **P<.01 in comparison with cholestatic patient group.
3.2. Normal R‐times in cirrhosis regardless of the etiology
In Figure 2A, it is shown that the R‐time, indicating the latency of clot formation, was generally within normal range when patients were combined, with a median (range) of 4.3 minute (1‐9.7) (normal range 2.5‐7.5). Six percent of patients had an R‐time outside of the normal range (5% had shortened and 1% prolonged R‐times). When patients were classified according to etiology of disease, median R‐times were normal in all groups: 4.1 minute (1.7‐7.2) in HBV/HCV; 4.4 minute (1.9‐6.8) in ETOH; 4.5 minute (2.6‐7.1) in NASH; 4.3 minute (1‐9.7) in cholestatic; 4.5 (2.5‐7.5) in other non‐cholestatic cirrhosis patients and 4.4 minute (2.2‐7.3) in cirrhotic patients with HCC. Similar results were generated when the cohort was subdivided according to disease severity (ie, median R‐times for Child‐Pugh A, 4.6 minute; B, 4.3 minute and C, 4.2 minute) (Figure 3A).
Figure 2.
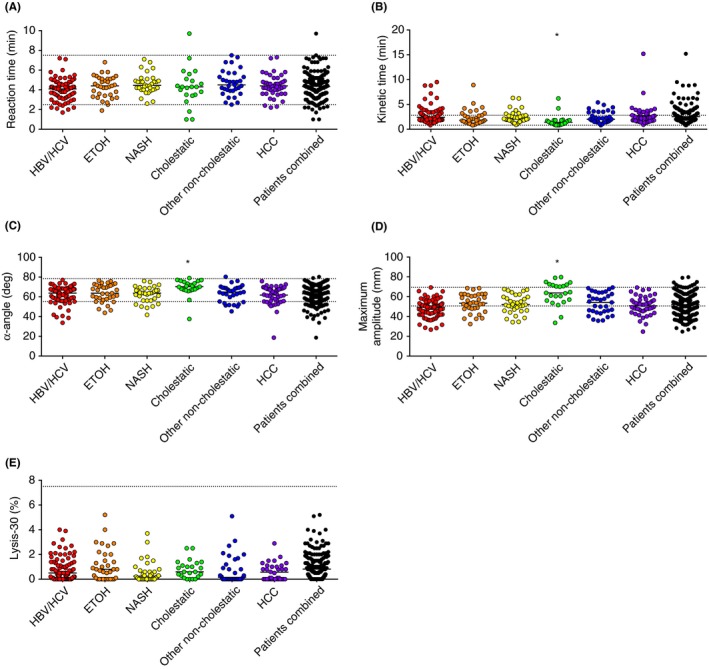
Admission TEG parameters of patients with cirrhosis combined or subdivided by etiology of disease. (A) R‐time, (B) K‐time, (C) α‐Angle, (D) Maximum Amplitude (MA) and (E) Lysis‐30. Reaction and K‐time are expressed in minutes, α‐Angle in degrees, MA in millimeter and Lysis‐30 in percentage of clot lysed 30 minutes after reaching MA. Horizontal bars represent medians. *P<.05 in comparison with each group of non‐cholestatic patients. Grid lines indicate the upper and lower limit of the normal range
Figure 3.
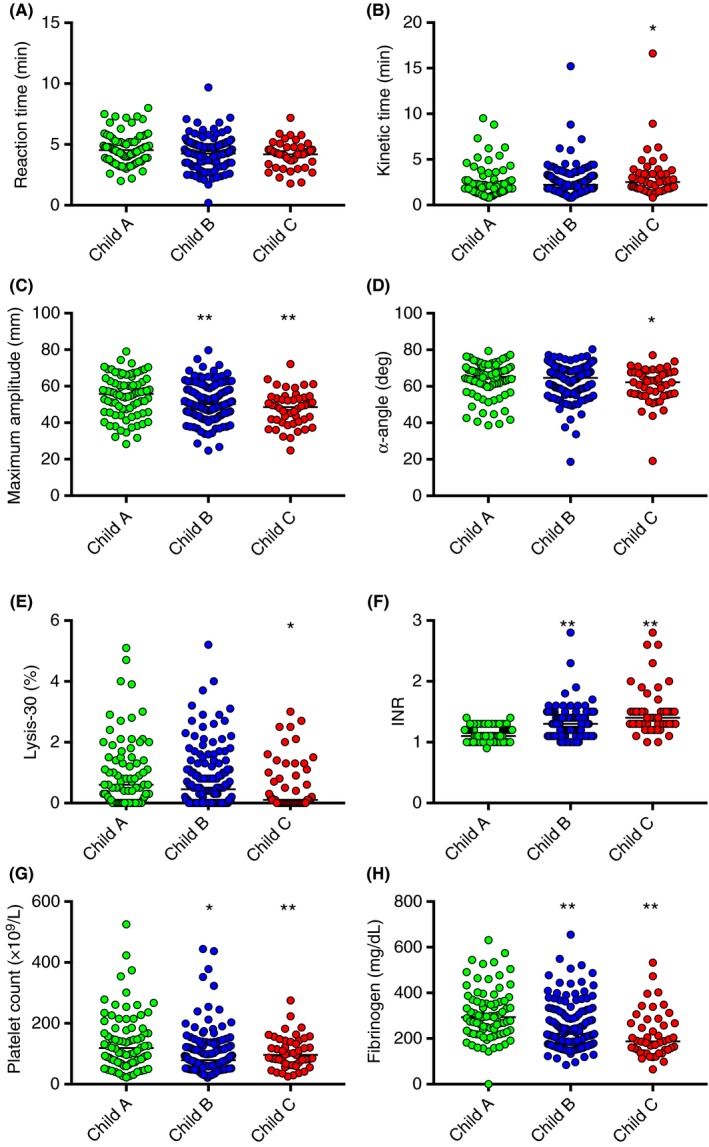
Association between TEG and laboratory parameters measured at admission and disease severity. (A) Reaction time, (B) Kinetic time, (C) α‐Angle, (D) Maximum Amplitude (MA), (E) Lysis‐30, (F) INR, (G) Platelet count, and (H) Fibrinogen levels classified according to Child‐Pugh A, B or C. Horizontal bars represent medians. *P<.05 and **P<.1 in comparison with Child‐Pugh A patients
3.3. Normal‐to‐increased K‐times in cirrhosis regardless of etiology, with lowest values in cholestatic cirrhosis
In Figure 2B, it is shown that the K‐time, the time to initial fibrin formation, was within normal range in 73% of the cases and prolonged in 27%. The median K‐time in all patients combined was 2.1 (0.8‐15.2) (normal range of 0.8‐2.8). The median K‐time increased with disease severity (ie, Child‐Pugh A, 1.9 minute; B, 2.2 minute; and C, 2.5 minute) (Figure 3B). When subdivided by etiology, the K‐time was 2.4 minute (0.8‐9.5) in HBV/HCV; 1.8 minute (0.8‐8.9) in ETOH; 2.1 minute (0.9‐6.3) in NASH; 2.0 minute (0.8‐6.2) in other non‐cholestatic cirrhotic patients and 2.1 minute (0.9‐15.2) in cirrhotic patients with HCC. In contrast, the median K‐time was 1.4 minute (0.8‐6.2) in patients with cholestatic cirrhosis, which was significantly lower than each of the other groups, ETOH cirrhosis excepted. Indeed, only 10% of patients in the cholestatic group presented with K‐times above the normal range.
3.4. Normal‐to‐decreased α‐Angles in cirrhosis regardless of etiology, with highest values in cholestatic cirrhosis
In Figure 2C, it is shown that the α‐Angle, indicating the speed of clot formation, was normal for most, but decreased in 16% of the patients, with a median of 64.9 degrees (18.6‐80.3) (normal range 55.2‐78.4 degrees) in all patients combined. The median α‐Angle decreased with disease severity (ie, Child‐Pugh A, 65 degrees.; B, 65.2 degrees; and C, 62.2 degrees) (Figure 3C). When subdivided by etiology, α‐Angle was 63.8 degrees (33.7‐77.1) in HBV/HCV; 63.7 degrees (43.8‐76.5) in ETOH; 63.7 degrees (41.7‐76.0) in NASH; 64.9 degrees (45.3‐80.3) in other non‐cholestatic cirrhosis and 63.4 degrees (18.6‐75.9) in cirrhotic patients with HCC. In patients with cholestatic cirrhosis, the median α‐Angle was 70.3 degrees (37.5‐79), which is significantly higher than each of the other groups.
3.5. Normal‐to‐decreased MA in non‐cholestatic cirrhosis, but normal‐to‐increased values in cholestatic cirrhosis
In Figure 2D, it is shown that the MA, indicating maximal clot strength, was decreased in 45% of patients combined, normal in 52%, and elevated in 3% with a median of 51.7 mm (24.7‐79.7) against a normal range of 50.6‐69.4. The median MA decreased with disease severity (ie, Child‐Pugh A, 55.4 mm; B, 50.6 mm; and C, 49.4 mm) (Figure 3D). When subdivided by etiology, MA was 49.3 mm (26.7‐69.3 in HBV/HCV; 53.5 mm [32.4‐68.5]) in ETOH; 52.1 mm (34.3‐67.6) in NASH; 54.2 mm (35.7‐69.3) in other non‐cholestatic cirrhosis patients and 50.8 mm (24.7‐69.2) in cirrhotic patients with HCC. Whereas the largest proportion of patients with non‐cholestatic cirrhosis had MA values within or below reference range, 43% of the cholestatic patients had values above it. Indeed, the median MA in this group was 63.8 mm (33.5‐79.7), which is significantly higher than each of the other groups.
3.6. Normal Ly‐30 in cirrhosis regardless of the etiology
In Figure 2E, it is shown that the Ly‐30, indicating the percentage of clot lysed within 30 minute after reaching MA, was normal for all patients, with a median (range) of 0.5% (0‐5.2) of all patients combined (normal range 0‐7.5). Ly‐30 was 0.5% (0‐4) in HBV/HCV; 0.8% (0‐5.2) in ETOH; 0.25% (0‐3.7) in NASH; 0.6% (0‐2.5) in cholestatic; 0.15% (0‐5.1) in other non‐cholestatic cirrhotic patients and 0.1% (0‐2.9) in cirrhotic patients with HCC. The median Ly‐30 decreased with disease severity (ie, Child‐Pugh A, 0.6%; B, 0.5%; and C, 0.1%) (Figure 3E).
3.7. K‐time, α‐Angle and MA correlate with platelets and fibrinogen in cirrhosis
In this large group of patients with cirrhosis, K‐time, α‐Angle and MA correlated with platelet count (r=−.45; r=.52; r=.74, respectively; P<.0001) as well as with fibrinogen levels (r=−.52; r=.55, r=.78, respectively; P<.0001). These correlations were consistent regardless of the etiology, as further demonstrated in Table 3. Platelet count, fibrinogen levels, as well as INR also associated with disease severity as shown in Figure 3F–H.
Table 3.
Correlations between TEG parameters and INR, platelet count or fibrinogen levels according to etiology
| HBV/HCV | ETOH | NASH | Cholestatic | Non‐cholestatic | HCC | Patients combined | |
|---|---|---|---|---|---|---|---|
| INR | |||||||
| K‐time | 0.4024 (P=.002) | 0.8518 (P<.0001) | n.s. | n.s. | 0.373 (P=.0325) | n.s. | 0.4004 (P<.0001) |
| α‐Angle | −0.3982 (P=.002) | −0.48 (P=.047) | n.s. | n.s. | n.s. | n.s. | −0.3141 (P<.0001) |
| MA | −0.4936 (P<.0001) | −0.7028 (P<.0001) | −0.3398 (P=.0493) | n.s. | −0.5765 (P=.0004) | n.s. | −0.3308 (P=.0664) |
| Platelet count | |||||||
| K‐time | −0.5119 (P<.001) | −0.5984 (P=.002) | −0.6305 (P<.0001) | −0.5153 (P=.0141) | −0.5766 (P=0.0004) | −0.3884 (P=.0076) | −0.4525 (P<.0001) |
| α‐Angle | 0.4585 (P<0.001) | 0.5653 (P=.0006) | 0.5964 (P=.0002) | 0.5818 (P=.0045) | 0.5297 (P=.0015) | 0.469 (P=0.001) | 0.5189 (P<.0001) |
| MA | 0.662 (P<.0001) | 0.7542 (P<.0001) | 0.7224 (P<.0001) | 0.8034 (P<.0001) | 0.7073 (P<.0001) | 0.7788 (P<.0001) | 0.7353 (P<.0001) |
| Fibrinogen levels | |||||||
| K‐time | −0.486 (P<.0001) | −0.6338 (P=.0001) | −0.7155 (P<.0001) | −0.6169 (P=.0022) | −0.6384 (P<.0001) | −0.4227 (P=.0038) | −0.517 (P<.0001) |
| α‐Angle | 0.4769 (P<.0001) | 0.3988 (P=0263) | 0.6955 (P<.0001) | 0.617 (P=.0022) | 0.5471 (P=.001) | 0.5015 (P=.0004) | 0.5499 (P<.0001) |
| MA | 0.6637 (P<.0001) | 0.7379 (P<.0001) | 0.7597 (P<.0001) | 0.8063 (P<.0001) | 0.7425 (P<.0001) | 0.7566 (P<.0001) | 0.7766 (P<.0001) |
Coefficients were computed using Pearson r correlation, with P<.05 considered significant and n.s. indicates not significant
3.8. TEG parameters at admission do not predict thrombosis, bleeding events, and transplantation or death in patients with cirrhosis
As shown in Figure 4, TEG parameters at baseline did not significantly differ between patients who experienced thrombosis or bleeding events during follow‐up or between patients that underwent transplantation or died compared to those who survived during follow‐up. Nevertheless, the limited number of patients that experienced a thrombotic event during follow‐up had a lower median R‐ and K‐time and higher median MA and α‐Angle at baseline compared to the median of those who did not suffer from thrombosis. Also, no associations with outcome were found when groups with different etiologies were investigated separately or when patients with Child‐Pugh B and C cirrhosis were combined into a single group (ie, with advanced disease at baseline) (data not shown).
Figure 4.
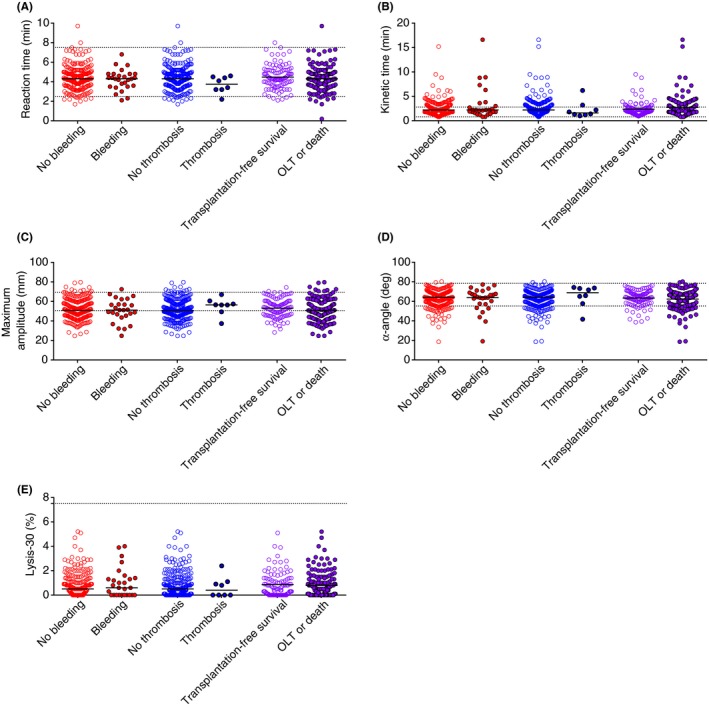
Association between TEG parameters measured at admission and disease outcome. (A) Reaction time, (B) Kinetic time, (C) α‐Angle, (D) Maximum Amplitude (MA), and (E) Lysis‐30 in patients who did or did not experience a bleeding or thrombotic event and, in transplant‐free survivors or patients who received a transplant or died. Horizontal bars represent medians
4. DISCUSSION
The above study of kaolin‐initiated TEG shows a generally preserved function of the hemostatic system in cirrhosis. This is in line with earlier studies using TEG in patients with cirrhosis.5, 13 Nevertheless, this is the largest study of TEG in cirrhosis so far, which allowed comparison between groups with different etiologies of underlying liver disease, but with similar baseline characteristics. In addition, data on outcome were gathered prospectively over an average period of almost 3 years to investigate the predictive potential of the test for bleeding, thrombosis, and liver transplantation and/or death.
We found that regardless of etiology, the majority of patients with cirrhosis had TEG parameters within normal reference range, whereas a significant proportion also had parameters outside the normal range. Hypocoagulable features reflected by increased K‐time and decreased α‐Angle and MA were found in 16‐45% of patients. The latter was not reflective of the cohort as a whole. Indeed, compared to the other etiologies, TEG values in patients with cholestatic liver disease were significantly different. This group demonstrated hypercoagulable tendencies, notably increased MA values above the normal upper limit, probably because of higher fibrinogen concentrations and platelet count compared to the other etiologies in combination with platelet hyperreactivity, which is in accordance with previous studies.6, 14 Ultimately, however, clot dissolution 30 minute after MA was well within normal limits in all groups. In addition, despite some patients having abnormal TEG measurements on admission, this had no implication for their clinical outcome: TEG parameters between patients that did or did not experience bleeding, thrombosis, death and/or liver transplantation were similar, indicating that the test has no value in predicting these events.
We and others have systematically shown that primary and secondary hemostasis are preserved in cirrhosis, with distinct hypercoagulable features, and the present study adds up to the concept of a rebalanced hemostatic system in cirrhosis. We have first demonstrated that elevated levels of the platelet adhesive protein von Willebrand factor (over)compensate for abnormalities in platelet number and function.15 Others have subsequently shown that the thrombin generating capacity in blood samples taken for patients with cirrhosis was preserved3 (even increased in advanced cirrhosis16, 17, 18), as a consequence of a simultaneous decrease in pro‐ and anticoagulant factors. More importantly, these laboratory studies have contributed to a major shift in the understanding of the clinical consequences of cirrhosis‐associated coagulopathy. Expert opinion now suggests that bleeding in many (surgical) cases are/were more likely due to hemodynamic changes in patients with cirrhosis than to an underlying hemostatic disorder.2 It is now also acknowledged that patients with cirrhosis are not “auto‐anticoagulated”; they are not protected from thrombotic events even when PT/INR and aPTT are prolonged, or when platelet counts are low.19 In fact, it is increasingly recognized that thrombosis can be a major complicating factor in cirrhosis20 and may even contribute to disease progression.21
While the results of this study confirm relatively preserved hemostasis in patients with cirrhosis of diverse etiologies, it raises some concerns on how to interpret abnormal TEG results in stable patients with cirrhosis. Notably, the lack of correlation with clinical outcome needs further appreciation, because ultimately this questions the utility of TEG in patient management beyond routine diagnostic tests of hemostasis and the operation theater. So far, there is only evidence that TEG might be useful in detecting an invasive procedure‐related bleeding or thrombotic risk in cirrhotic patients8, 9, 22, 23, as well as early rebleeding following sclerotherapy.7 A recent randomized study looking at patients with cirrhosis undergoing invasive procedures, compared standard hemostasis screening tests (platelet count and PT) with TEG. It showed that the latter was more useful in deciding whether allogenic blood product transfusion was necessary.24 One of the reasons why TEG has not proven useful in stable patients with cirrhosis is that it is still not sophisticated enough detect the full range of potential changes in the hemostatic system. This logically hampers accurate (long‐term) prediction of thrombosis or bleeding, although the lack of association between low K‐time/α‐Angle/MA and bleeding reported here can easily be explained by the current notion that bleeding in cirrhosis is usually secondary to hemodynamic changes (ie, variceal bleeding due to increased pressure in the splanchnic venous system).2
Kaolin‐modified TEG is inherently limited by its initiation of the intrinsic clotting cascade which does not reflect physiological (tissue‐factor driven [TF]) clotting dynamics. However, even in TF‐triggered TEG, the absence of thrombomodulin prohibits protein C activation during clot formation, and therefore TEG does not fully reflect the balance between pro‐ and anticoagulant drivers. Also, while TEG can be useful in recognizing a hyperfibrinolysis‐related bleeding risk, it is not designed to detect a potential thrombotic risk driven by hypofibrinolysis. We have recently shown that clots from patients with cirrhosis are less permeable compared to healthy individuals despite impaired clot formation, and hence these are relatively resistant to lysis.25 The proposed underlying mechanism is that a reduced potential of fibrinolytic proteins to permeate through the clot increases the risk of thrombus development. Failure to detect thrombogenic clot phenotypes in cirrhosis may also partly explain why the TEG has not proven useful in predicting outcome in this study.
A simpler explanation as to why we did not find a correlation between TEG and clinical outcome is that the incidence of thrombosis was either too low to detect one or that the time period between TEG measurements and measures of outcome was too long. In the present study, thrombosis incidence was 3%, and interestingly, there were no cases of thrombosis in cholestatic disease group, which clearly stood apart by demonstrated hypercoagulable features of TEG. Moreover, there were only two cases of thrombosis in the HCC group, while it is well known that malignancies are associated with an increased risk of thrombotic events. The low incidence of thrombotic events in our study was not necessarily lower than previous studies of venous thrombosis in cirrhosis (incidence of 0.5‐6.5%)16, 26, 27, 28, but nevertheless highlights that it will be extremely difficult to predict thrombotic events in the general cirrhotic population. Also, because the clinical phenotype of patients may be altered radically during disease progression, there will be variations in thrombotic or bleeding risk over time, which makes it difficult to identify the ideal time point for TEG measurement in the individual patient. Preferably, future prospective studies would incorporate serial TEG measurements to identify the time window wherein the test has predictive potential. In addition, to validate the test as a predictor of thrombosis and/or disease progression, a critical first move forward will be to identify target groups that have high risk for thrombotic events in the short‐term. One approach would be to adopt the PADUA prediction score that identifies admitted patients at high‐risk for VTE29 and to compare it with TEG in a randomized study of VTE risk detection in hospitalized patients with cirrhosis. Prospectively, TEG could even guide thromboprophylaxis by measuring individual and potentially supra‐/sub‐clinical response to therapy.
In conclusion, a single kaolin‐activated TEG measurement does not predict future bleeding or thrombosis in stable patients with cirrhosis. Future studies designed to determine if the TEG is sensitive enough to detect hemostatic changes in cirrhosis should include either serial TEG measurements from baseline up to the bleeding/thrombotic event (to better evaluate the dynamic nature of the hemostatic abnormalities in patients with cirrhosis), and/or a TEG measurement at the time of or shortly after the bleeding/thrombotic event. Nevertheless, this study of TEG provides evidence of preserved hemostatic function in a large group of patients with cirrhosis across a variety of etiologies. It adds to an increasing number of epidemiological, clinical, and in vitro studies documenting rebalanced hemostatic function in cirrhosis.
AUTHOR CONTRIBUTIONS
G. Hugenholtz analyzed and interpreted the data and wrote the manuscript. T. Lisman interpreted the data, and contributed to writing and reviewing of the manuscript. R. Stravitz designed the study, collected and interpreted the data, and contributed to writing and reviewing. All authors approved the final version of the manuscript.
RELATIONSHIP DISCLOSURE
The authors have no conflict of interest to disclose.
Hugenholtz GCG, Lisman T, Stravitz RT . Thromboelastography does not predict outcome in different etiologies of cirrhosis. Res Pract Thromb Haemost. 2017;1:275–285. 10.1002/rth2.12037
REFERENCES
- 1. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–56. [DOI] [PubMed] [Google Scholar]
- 2. Lisman T, Caldwell SH, Burroughs AK, et al. Coagulation in Liver Disease Study Group . Hemostasis and thrombosis in patients with liver disease: the ups and downs. J Hepatol. 2010;53:362–71. [DOI] [PubMed] [Google Scholar]
- 3. Tripodi A, Salerno F, Chantarangkul V, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553–8. [DOI] [PubMed] [Google Scholar]
- 4. Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol. 2012;8:513–20. [PMC free article] [PubMed] [Google Scholar]
- 5. Tripodi A, Primignani M, Chantarangkul V, et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009;124:132–6. [DOI] [PubMed] [Google Scholar]
- 6. Ben‐Ari Z, Panagou M, Patch D, et al. Hypercoagulability in patients with primary biliary cirrhosis and primary sclerosing cholangitis evaluated by thrombelastography. J Hepatol. 1997;26:554–9. [DOI] [PubMed] [Google Scholar]
- 7. Chau TN, Chan YW, Patch D, Tokunaga S, Greenslade L, Burroughs AK. Thrombelastographic changes and early rebleeding in cirrhotic patients with variceal bleeding. Gut. 1998;43:267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Debernardi Venon W, Ponzo P, Sacco M, et al. Usefulness of thromboelastometry in predicting the risk of bleeding in cirrhotics who undergo invasive procedures. Eur J Gastroenterol Hepatol. 2015;27:1313–9. [DOI] [PubMed] [Google Scholar]
- 9. Bedreli S, Sowa JP, Malek S, et al. Rotational thromboelastometry can detect factor XIII deficiency and bleeding diathesis in patients with cirrhosis. Liver Int. 2017;3:562–8. [DOI] [PubMed] [Google Scholar]
- 10. Poujol‐Robert A, Boelle PY, Poupon R, Robert A. Factor V Leiden as a risk factor for cirrhosis in chronic hepatitis C. Hepatology. 2004;39:1174–5. [DOI] [PubMed] [Google Scholar]
- 11. Anstee QM, Wright M, Goldin R, Thursz MR. Parenchymal extinction: coagulation and hepatic fibrogenesis. Clin Liver Dis. 2009;13:117–26. [DOI] [PubMed] [Google Scholar]
- 12. Scarpelini S, Rhind SG, Nascimento B, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–7. [DOI] [PubMed] [Google Scholar]
- 13. Kleinegris MC, Bos MH, Roest M, et al. Cirrhosis patients have a coagulopathy that is associated with decreased clot formation capacity. J Thromb Haemost. 2014;12:1647–57. [DOI] [PubMed] [Google Scholar]
- 14. Pihusch R, Rank A, Göhring P, Pihusch M, Hiller E, Beuers U. Platelet function rather than plasmatic coagulation explains hypercoagulable state in cholestatic liver disease. J Hepatol. 2002;37:548–55. [DOI] [PubMed] [Google Scholar]
- 15. Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53–61. [DOI] [PubMed] [Google Scholar]
- 16. Gatt A, Riddell A, Calvaruso V, Tuddenham EG, Makris M, Burroughs AK. Enhanced thrombin generation in patients with cirrhosis‐induced coagulopathy. J Thromb Haemost. 2010;8:1994–2000. [DOI] [PubMed] [Google Scholar]
- 17. Tripodi A, Primignani M, Lemma L, et al. Detection of the imbalance of procoagulant versus anticoagulant factors in cirrhosis by a simple laboratory method. Hepatology. 2010;52:249–55. [DOI] [PubMed] [Google Scholar]
- 18. Tripodi A, Primignani M, Chantarangkul V, et al. An imbalance of pro‐ vs anti‐coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105–11. [DOI] [PubMed] [Google Scholar]
- 19. Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101:1524–8. [DOI] [PubMed] [Google Scholar]
- 20. Ambrosino P, Tarantino L, Di Minno G, et al. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta‐analysis. Thromb Haemost. 2016;117:139–48. [DOI] [PubMed] [Google Scholar]
- 21. Lisman T, Violi F. Cirrhosis as a risk factor for venous thrombosis. Thromb Haemost. 2017;117:3–5. [DOI] [PubMed] [Google Scholar]
- 22. Tafur LA, Taura P, Blasi A, et al. Rotation thromboelastometry velocity curve predicts blood loss during liver transplantation. Br J Anaesth. 2016;117:741–8. [DOI] [PubMed] [Google Scholar]
- 23. Zahr Eldeen F, Roll GR, Derosas C, et al. Preoperative thromboelastography as a sensitive tool predicting those at risk of developing early hepatic artery thrombosis after adult liver transplantation. Transplantation. 2016;100:2382–90. [DOI] [PubMed] [Google Scholar]
- 24. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography‐guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63:566–73. [DOI] [PubMed] [Google Scholar]
- 25. Hugenholtz GC, Macrae F, Adelmeijer J, et al. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J Thromb Haemost. 2016;14:1054–66. [DOI] [PubMed] [Google Scholar]
- 26. Aggarwal A, Puri K, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: systematic review. World J Gastroenterol. 2014;20:5737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aldawood A, Arabi Y, Aljumah A, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137:1145–9. [DOI] [PubMed] [Google Scholar]
- 29. Moorehead KJ, Jeffres MN, Mueller SW. A retrospective cohort analysis of pharmacologic VTE prophylaxis and Padua prediction score in hospitalized patients with chronic liver disease. J Pharm Pract. 2017;30:58–63. [DOI] [PubMed] [Google Scholar]


