Abstract
Essentials.
Nonacog beta pegol (N9‐GP) activity can be underestimated in clot method using diverse reagents.
Mimicking clotting phase with representative reagents reveals impaired FIX and N9‐GP activation.
Polyethylene glycol conjugation amplifies contact activator‐induced decrease in FIX activation.
The reagent is a pivotal factor for accurate measurement of N9‐GP activity in clinical samples.
Background
In clinical practice, factor IX (FIX) activity is routinely quantified by measurement of the activated partial thromboplastin time (APTT) in a one‐stage (OS) FIX clotting assay. APTT reagents provide a contact activator and phospholipid surfaces required for triggering and sustaining the plasma clotting process. The large diversity in reagent components is reflected in the variable recovery of nonacog beta pegol (N9‐GP; N‐glycoPEGylated recombinant FIX) activity when assayed against a FIX standard. This variation warrants mechanistic studies and is plausibly attributable to the nature and amount of contact activator.
Objective
To identify the cause of the N9‐GP activity underestimation observed with a heterogeneous group of APTT reagents.
Methods
Experiments mimicking the clotting phase (omitting the contact activation phase) of the OS assay, complemented by measurements of activated factor XI (FXIa) activity, were performed to characterize and explain the influence of APTT reagents/contact activators on the conversion of N9‐GP and regular FIX (N9) to activated FIX (FIXa).
Results
In the presence of an intact underestimating APTT reagent or the isolated contact activator, clotting phase activation of N9‐GP proceeded at a reduced rate compared with that of N9. APTT reagent and contact activator negatively affected the activity of FXIa, conceivably as a consequence of FXIa adsorption. Thus, activation of FIX apparently poses a greater steric challenge after polyethylene glycol (PEG) conjugation.
Conclusions
Some OS clotting assay contact activators reduce FXIa‐mediated activation of N9‐GP to a larger degree than that of N9, causing underestimation of N9‐GP activity of potential clinical significance.
Keywords: blood coagulation tests, coagulation factor IX, contact activator, hemophilia B, nonacog beta pegol
1. INTRODUCTION
Ideally, treatment of haemophilia should be prophylactic to minimize the emergence of bleeding events. This has spurred the development of modified coagulation factors with prolonged duration of action, conveniently reducing the required frequency of administration.1 One example is nonacog beta pegol (N9‐GP), a recombinant factor IX (FIX) with a 40‐kDa polyethylene glycol (PEG40) moiety attached to an N‐linked glycan in the activation peptide,2 which exhibits doubled recovery and a five‐fold extension of the mean terminal half‐life in humans arriving at a t½ value of 93 hours.3, 4 Unfortunately, modification of a coagulation factor is often accompanied by altered properties, potentially in a way that poses a challenge for the assay used to measure the biological activity against an unmodified counterpart.5, 6
N9‐GP activity can be accurately measured by a one‐stage (OS) FIX clotting assay when using select commercially available activated partial thromboplastin time (APTT) reagents, albeit a majority of APTT reagents under‐ or overestimate N9‐GP activity when analyzed against a plasma‐derived FIX standard.7, 8 This awareness is of utmost importance in the clinical setting because erroneous estimates of N9‐GP activity could result in suboptimal treatment of haemophilia B patients. Most, but not all, silica‐based APTT reagents overestimate the N9‐GP activity owing to silica‐mediated precocious conversion to activated FIX (FIXa), a process catalyzed by activated factor XI (FXIa) and plasma kallikrein occurring during the contact activation phase preceding recalcification.9 Here, we aimed at elucidating the mechanism underlying the underestimation of N9‐GP activity observed with a heterogeneous group of APTT reagents. This intra‐assay phenomenon is puzzling because the responsible reagents contain either ellagic acid, silica, or kaolin as the contact activator. Our findings allow us to propose a unifying mode by which a diverse set of APTT reagents exert similar effects.
2. MATERIALS AND METHODS
2.1. Materials and standard methods
N9‐GP and non‐PEGylated recombinant FIX (N9) were produced by Novo Nordisk A/S (Måløv, Denmark) as described.2 HemosIL SynthAFax and HemosIL SynthASil were from Instrumentation Laboratory (Bedford, MA, USA), STA‐C.K. Prest from Diagnostica Stago (Asnières sur Seine, France), and Actin FS from Siemens (Marburg, Germany). APTT reagent filtrates (with contact activators and phospholipids removed) were prepared by passage through centrifugal filters (Amicon Ultra, Ultracel) from Merck Millipore (Carrigtwohill, Ireland) with a molecular mass cut‐off of 10 or 100 kDa at 14 000 × g for 15 minutes. The SCT Screen reagent from the HemosIL Silica Clotting Time kit (Instrumentation Laboratory) was the source of colloidal silica, kaolin was from the STA‐C.K. Prest kit, and ellagic acid (≥95%; from tree bark) was obtained from Sigma‐Aldrich (Munich, Germany). Functionally active ellagic acid (as judged from the ability to decrease FXIa amidolytic activity and/or promote contact activation of FXI9) was prepared essentially according to Bock et al.10 The final dilution of ellagic acid to 0.1 mmol L−1 was made in Actin FS filtrate collected after passage through a 10‐kDa cut‐off filter. Optionally, a syringe‐driven 0.22‐μm filter unit (Millex‐GV, Merck Millipore) was used to filter the ellagic acid solutions twice.10 FIX‐depleted plasma was from Precision BioLogic (Dartmouth, Canada), FIXa from Haematologic Technologies (Essex Junction, VT, USA), FXIa from Enzyme Research Laboratories (South Bend, IN, USA), the chromogenic substrate S‐2366 from Chromogenix (Milan, Italy), and the fibrin polymerization inhibitor I‐2882 from Chiralix (Nijmegen, The Netherlands). PEG hydroxylamine (average molecular mass 40 kDa [PEG40, Sunbright GL2‐400CA]) was obtained from NOF America (White Plains, NY, USA), and Rox FIX‐A kit for measurement of FIXa activity was from Rossix AB (Mölndal, Sweden).
Electrophoretic analyses were performed using 4‐12% NuPAGE Novex Bis‐Tris gels and MES buffer (Invitrogen, Carlsbad, CA, USA), and proteins visualized by Coomassie staining. Samples for electrophoresis were withdrawn from incubations of 5 μmol L−1 N9‐GP or N9 with 5 nmol L−1 FXIa in 50 mmol L−1 imidazole, pH 7.3, containing 0.1 mol L−1 NaCl and 5 mmol L−1 CaCl2, containing either 25% (v/v) SynthASil or the same fractional volume of buffer.
2.2. Sampling under conditions mimicking the clotting phase and measurement of generated FIXa
N9‐GP and N9 were reconstituted, prepared, and used as described.9 Measurements of FIXa activity were performed using the Rox FIX‐A kit as described9 on samples taken 60 seconds after Ca2+ initiation of the clotting phase (FIXa generation was linear during the first minute). Except in an initial comparative experiment, the preceding 5‐min contact activation phase was omitted and instead 5 nmol L−1 FXIa was added directly to the APTT reagent. Optionally, 2 μmol L−1 PEG40 was also added to the APTT reagent. In some experiments, APTT reagent filtrate, isolated contact activator or buffer replaced the complete APTT reagent.
2.3. FXIa amidolytic activity assay
One hundred μL of 1 nmol L−1 FXIa (in 50 mmol L−1 imidazole, pH 7.3, containing 0.1 mol L−1 NaCl, 5 mmol L−1 CaCl2, 1% [w/v] bovine serum albumin) was mixed with 50 μL APTT reagent (optionally supplemented with 2 μmol L−1 PEG40 or replaced by APTT reagent filtrate, isolated contact activator or imidazole buffer), and the activity measurement started by adding 50 μL of chromogenic substrate S‐2366 (4 mmol L−1 in buffer). The A405 nm development was monitored for 30 min using a SpectraMax 190 kinetic microplate reader (Molecular Devices, Sunnyvale, CA, USA).
3. RESULTS AND DISCUSSION
The present study focused on APTT reagents previously shown to underestimate N9‐GP activity in a OS FIX clotting assay and employed Actin FS, SynthASil and STA‐C.K. Prest.7, 11 They were selected to represent the broad spectrum of reagent compositions. Of particular interest is the fact that they are based on various contact activator surfaces, ie, ellagic acid, colloidal silica, and kaolin, respectively. Initially, we established that the presence of any of these APTT reagents significantly reduced the activation rates for both N9‐GP and N9 (non‐PEGylated). Most important in this context, the activation of N9‐GP progressed slower than that of N9, a difference which was not seen in the absence of APTT reagent (Figure 1).2 These observations were valid regardless of whether the contact activation phase was allowed to precede recalcification or was omitted by supplementing the APTT reagent with added FXIa. Thus, it appeared to be justified and sufficient to mimic the clotting phase, and we applied an experimental design where FXIa was added together with the APTT reagent (or the replacement for the complete reagent) immediately before recalcification. In this setup (Figure 1), the ratios between the rates of N9‐GP and N9 activation in the presence of Actin FS or SynthASil were in good agreement with the degrees of underestimation of N9‐GP activity seen in a recent two‐center study,7 and the results with STA‐C.K. Prest were in line with those of an earlier study.11 The presence of free PEG40 had no influence on the activation of either N9‐GP or N9 using any of the underestimating reagents. Using a reagent (SynthAFax) whose presence gives the expected N9‐GP activity recovery,7 we noticed indistinguishable, marginally inhibited activations of N9‐GP and N9.
Figure 1.
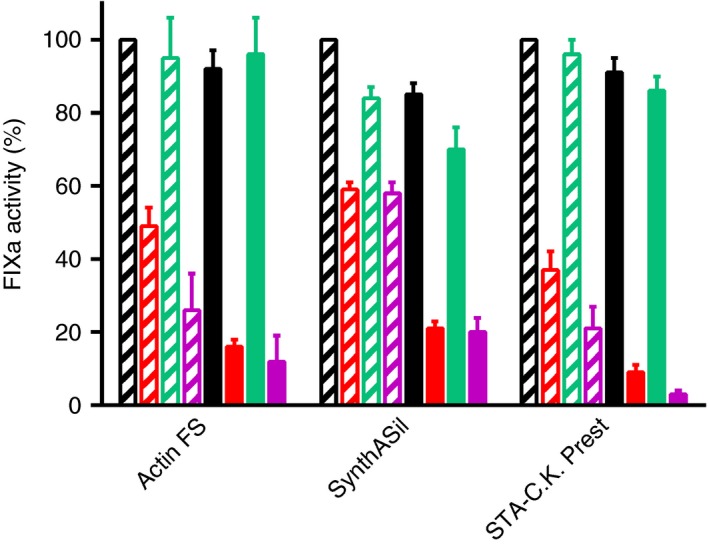
Effects of activated partial thromboplastin time (APTT) reagents, APTT reagent filtrates and contact activators on the activation of nonacog beta pegol (N9‐GP) and factor IX (FIX) (N9) during the clotting phase of a OS FIX clotting assay. The bars represent relative generation of activated factor IX (FIXa) activity in the presence of mere buffer (black), APTT reagent (red), APTT reagent filtrate (green), or isolated contact activator (purple). Striped and solid bars show the results obtained with N9 and N9‐GP, respectively. For each APTT reagent, the generation of FIXa activity was compared to that obtained using N9 in the presence of mere buffer (arbitrarily assigned the value 100%). The APTT reagents are indicated below the graph
A pronounced impairment of N9‐GP activation in the presence of an underestimating APTT reagent was shown by gel electrophoretic visualization of formed FIXa in incubations of N9‐GP and N9 with FXIa in the presence of SynthASil (Figure 2). An inevitable consequence of these findings is that N9‐GP activity, when measured against a FIX standard in an assay influenced by the activation kinetics, such as a clotting assay, would be underestimated due to relatively slower conversion to FIXa. Moreover, these data support that the reason for N9‐GP underestimation resides in the clotting phase and is independent of PEG‐mediated adsorption to the contact activator.
Figure 2.
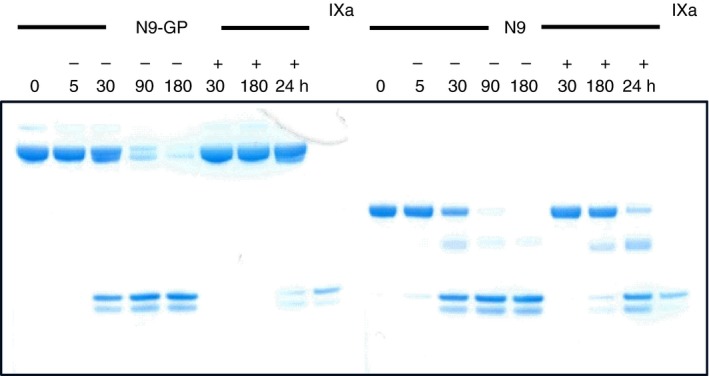
SynthASil‐induced reduction of nonacog beta pegol (N9‐GP) and FIX (N9) conversion to activated factor IX (FIXa) by activated factor XI (FXIa). N9‐GP (left) and N9 (right) were incubated with FXIa in the absence (−) or presence (+) of SynthASil for the indicated times (min, plus 24 hours). A FIXa reference was run in the center and outer right lanes
Filtrates of Actin FS, SynthASil, and STA‐C.K. Prest obtained after passage through a filter with a 10‐ or 100‐kDa cut‐off limit were largely devoid of inhibitory activity affecting N9‐GP and N9 activation and produced very similar rates. In contrast, different degrees of inhibition of N9‐GP and N9 activation, resembling those seen with the parent APTT reagents, could be reproduced if the isolated contact activators were present during the clotting phase, ie, for Actin FS in the form of ellagic acid in Actin FS filtrate, and for SynthASil and STA‐C.K. Prest in the form of a solution of colloidal silica and kaolin alone, respectively (Figure 1). In two out of three cases, the isolated contact activator tended to inhibit more than the corresponding intact APTT reagent. This might be explained by the lack of the phospholipid from the APTT reagent during the mimicked clotting phase (eg, kaolin vs. STA‐C.K. Prest) and by qualitative contact activator differences (Actin FS filtrate plus in‐house ellagic acid vs. intact Actin FS).
Direct effects of contact activators on the enzymatic activity of FXIa were assessed by monitoring the cleavage of a small peptide substrate. All three APTT reagents and the isolated contact activators, but not the reagent filtrates, inhibited FXIa amidolytic activity (Figure 3). Inhibition was unaffected by free PEG40. With all three APTT reagents, the degree of FXIa inhibition measured with the small substrate was very similar to the inhibition of FXIa‐mediated activation of native FIX (N9) in plasma, and hence less pronounced than the inhibition of N9‐GP activation (Figure 1).
Figure 3.
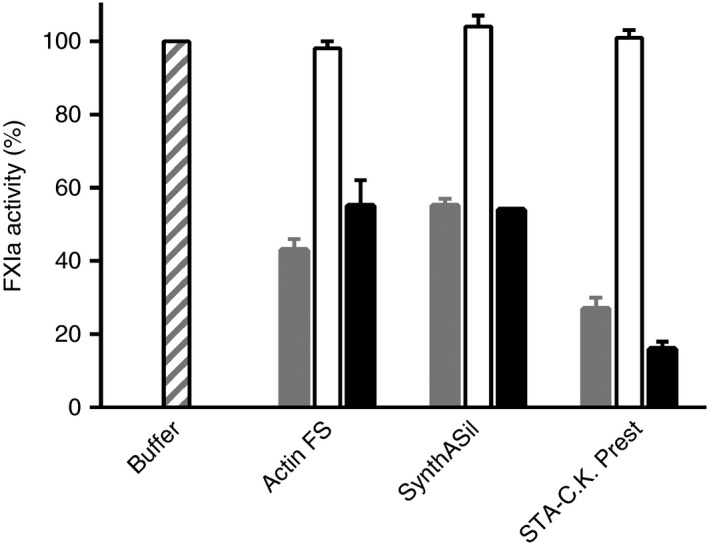
Effects of activated partial thromboplastin time (APTT) reagents, APTT reagent filtrates and contact activators on activated factor XI (FXIa) amidolytic activity. Each bar triplet represents relative FXIa activity in the presence of APTT reagent (gray), APTT reagent filtrate (white) or isolated contact activator (black), with the FXIa activity when instead using assay buffer arbitrarily set to 100% (outer left bar). The APTT reagents are indicated below the graph
Present and previous9 studies have investigated the under‐ and overestimation, respectively, of N9‐GP activity in OS FIX clotting assays. Together, the findings explain why, and illustrate how profoundly, the functional activity assessment depends on the APTT reagent used, or more precisely on the type, quality and amount of contact activator present. The complexity is further illustrated by the observations that silica‐based contact activators can evoke under‐ (SynthASil) as well as overestimation (several reagents) of N9‐GP activity,7, 9 and that ellagic acid either leads to underestimation (with Actin FS) or gives the expected recovery (with SynthAFax or DG Synth).7 Because the measured N9‐GP activity relies heavily on the contact activator, an effort was made to ensure that the isolated contact activators used in the present study mimic as closely as possible those in the corresponding intact APTT reagents. The best available options, considering the uncertainties regarding the detailed composition of APTT reagents, were to add 0.1 mmol L−1 (concentration according to Actin FS insert) of functional ellagic acid prepared in‐house to an Actin FS filtrate, to use a colloidal silica dispersion (SCT Screen) from the manufacturer of SynthASil, and to use the kaolin solution intended for reconstitution of STA‐C.K. Prest.
Deviating recoveries of N9‐GP activity in OS FIX clotting assays appear to be attributable to APTT reagent‐specific adsorption of either the enzymatic activator (primarily FXIa) or N9‐GP itself to the contact activator surface. In the case of FXIa, adsorption results in greater reduction of the rate of N9‐GP activation than of FIX activation during the clotting phase, possibly by inducing a conformational change in the molecule and/or by restricting substrate access. Conceivably, PEG conjugation to FIX poses more of a steric challenge leading to underestimation of N9‐GP activity. If slower activation (of N9‐GP) actually is related to steric hindrance, it might also occur when using certain APTT reagents to measure the activity of other long‐acting FIX molecules, albeit their bulky half‐life‐extending moiety has both a different chemical nature and intramolecular location. In the case of N9‐GP, adsorption facilitates its own premature activation already during the contact activation phase by colocalization with the activator.9 This leads to overestimation of N9‐GP activity. SynthAFax, which returns the expected recovery of N9‐GP activity,7 reveals no or only marginal signs of either adsorption event9 (and present report). In support of unique mechanisms being responsible for the oppositely erroneous estimations of N9‐GP activity, free PEG40 cannot alleviate underestimation (ie, FXIa adsorption) but prevents overestimation (ie, N9‐GP adsorption).9
In conclusion, in order to avoid assay artefacts and obtain the anticipated recovery of N9‐GP activity in the OS FIX clotting assay when using a FIX standard, one must select an APTT reagent which does not elicit any intra‐assay adsorption event.7 Albeit limited overdosage of N9‐GP due to underestimation of its activity may not in itself pose a safety risk for the haemophilia B patients, the importance of accurate measurement is self‐evident.
AUTHOR CONTRIBUTIONS
E. Persson designed the research, analyzed data, and wrote the manuscript. C. La Cour Christoffersen contributed to the research design, performed experiments, and analyzed data.
RELATIONSHIP DISCLOSURE
The authors are employees of Novo Nordisk A/S, the manufacturer of N9‐GP.
ACKNOWLEDGMENTS
The authors thank Drs. N. Lorenzen and P. F. Nielsen, Novo Nordisk A/S, for demonstrating, by size‐exclusion chromatography and mass spectrometry, respectively, the apparent absence of proteinaceous material in any of the APTT reagents. We thank Dr. M. Hansen for valuable discussions.
Persson E, La Cour Christoffersen C. Underestimation of N‐glycoPEGylated factor IX one‐stage clotting activity owing to contact activator‐impaired activation. Res Pract Thromb Haemost. 2017;1:259–263. 10.1002/rth2.12046
REFERENCES
- 1. Peyvandi F, Garagiola I, Biguzzi E. Advances in the treatment of bleeding disorders. J Thromb Haemost. 2016;14:2095–106. [DOI] [PubMed] [Google Scholar]
- 2. Østergaard H, Bjelke JR, Hansen L, et al. Prolonged half‐life and preserved enzymatic properties of factor IX selectively PEGylated on native N‐glycans in the activation peptide. Blood. 2011;118:2333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Negrier C, Knobe K, Tiede A, Giangrande P, Møss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118:2695–701. [DOI] [PubMed] [Google Scholar]
- 4. Collins PW, Young G, Knobe K, et al.; for the paradigm 2 Investigators . Recombinant long‐acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124:3880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitchen S, Gray E, Mertens K. Monitoring of modified factor VIII and IX products. Haemophilia. 2014;20(suppl 4):36–42. [DOI] [PubMed] [Google Scholar]
- 6. Dodt J, Hubbard AR, Wicks SJ, et al. Potency determination of factor VIII and factor IX for new product labelling and postinfusion testing; challenges for caregivers and regulators. Haemophilia. 2015;21:543–9. [DOI] [PubMed] [Google Scholar]
- 7. Bowyer AE, Hillarp A, Ezban M, Persson P, Kitchen S. Measuring factor IX activity of nonacog beta pegol with commercially available one‐stage clotting and chromogenic assay kits: a two‐center study. J Thromb Haemost. 2016;14:1428–35. [DOI] [PubMed] [Google Scholar]
- 8. Sørensen MH, Andersen S, Ezban M. Factor IX‐deficient plasma spiked with N9‐GP behaves similarly to N9‐GP post‐administration clinical samples in N9‐GP ELISA and FIX activity assays. Haemophilia. 2015;21:832–6. [DOI] [PubMed] [Google Scholar]
- 9. Rosén P, Rosén S, Ezban M, Persson E. Overestimation of N‐glycoPEGylated factor IX activity in a one‐stage factor IX clotting assay owing to silica‐mediated premature conversion to activated factor IX. J Thromb Haemost. 2016;14:1420–7. [DOI] [PubMed] [Google Scholar]
- 10. Bock PE, Srinivasan KR, Shore JD. Activation of intrinsic blood coagulation by ellagic acid: insoluble ellagic acid‐metal ion complexes are the activating species. Biochemistry. 1981;20:7258–566. [DOI] [PubMed] [Google Scholar]
- 11. Holm PK, Sørensen MH, Hermit MB, Ezban M. The biological activity of glycoPEGylated recombinant FIX (N9‐GP) is similar in two‐stage chromogenic assays, in SynthAFax‐based one‐stage clot assay and in TEG assay using blood from haemophilia B patients. Haemophilia. 2014;20(suppl 3):24 (abstract). [Google Scholar]


