Abstract
Essentials.
The value of thrombin generation assay (TGA) in monitoring direct oral anticoagulants (DOAC) is not well defined.
TGA parameters were measured and correlated to DOAC levels in 10 healthy volunteers after oral intake of DOACs.
Lag time is the only sensitive TGA parameter across different DOACs, dabigatran, rivaroxaban and apixaban.
Endogenous thrombin potential had weak correlation with DOAC levels and not suitable as stand‐alone parameter.
Background
There are clinical situations where monitoring direct oral anticoagulants (DOACs) may be useful. The clinical application of thrombin generation assay (TGA) in monitoring the effect of DOACs has not been well established. An ex vivo study was performed to systematically evaluate the anticoagulant effect of dabigatran, rivaroxaban and apixaban on each individual TGA parameter through serial measurements over time to assess suitability of these parameters for monitoring the anticoagulant effect of DOACs.
Methods
Ten healthy volunteers were given oral dabigatran 150 mg, rivaroxaban 20 mg, or apixaban 10 mg once. TGA parameters lag time, endogenous Thrombin potential (ETP), and thrombin peak height, time to peak, and velocity index were measured at times 0, 2, 4, and 24 hours after intake of DOAC. TGA parameters and DOAC concentrations were correlated.
Results
The lag time was significantly correlated with all DOAC concentrations (r ≥ .81, P < .0001 for all). Thrombin peak height best correlated with direct Factor Xa inhibitor (FXa) concentrations in nonlinear fashion (R² ≥ .87). ETP was weakly correlated with DOAC levels (r ≤ .68). Besides lag time, the other TGA parameters were not significantly altered over time by dabigatran.
Conclusion
Lag time was the only sensitive TGA parameter across the different classes of DOACs evaluated. Thrombin peak height was strongly correlated to FXa inhibitor concentrations and potentially a useful parameter to monitor FXa inhibitors at low concentrations. ETP had a weak correlation with achieved DOAC concentrations and is likely less suitable for assessment of DOAC effect as a stand‐alone parameter.
Keywords: apixaban, dabigatran, direct oral anticoagulants, rivaroxaban, thrombin generation assay
1. INTRODUCTION
One of the main advantages of the direct oral anticoagulants (DOACs) is fixed daily doses eliminating routine laboratory monitoring for optimal dosing. However, there are certain clinical situations where monitoring the DOAC effect on hemostasis can improve patient management. These situations include, but are not limited to: bleeding complications with or without trauma, thromboembolic events while on therapy, and reversal of anticoagulation.1 Several different laboratory assays have been evaluated for DOAC monitoring, but none are available for routine clinical use in the US. These methods can be classified into the following: (1) Routine coagulations parameters such as prothrombin time (PT), activated partial thromboplastin time (aPTT); (2) Drug specific tests, such as clotting and chromogenic assays calibrated for each DOAC; and (3) Global tests, such as: thrombelastography, and the thrombin generation assay (TGA).2 Of these, PT and aPTT assays are the most widely available. However, it has been demonstrated that these assays lack sufficient sensitivity and specificity for routine measurement of most DOACs due to variations in the measured effects of the DOAC depending on the assay, the instrument used, and the specific DOAC under evaluation.3, 4, 5 TGA measures the concentration of thrombin as a function of time in clotting plasma, providing a global physiologic function test of the hemostatic system.6 TGA is expressed by reporting the following parameters: (1) lag time, the time elapsed between tissue factor initiation and the moment at which 10 nmol L−1 of thrombin is formed, (2) thrombin peak height, the highest concentration of thrombin detected on the thrombin generation curve, and (3) endogenous thrombin potential (ETP), the area under the thrombin generation curve. There are two additional secondary parameters derived from the aforementioned primary parameters: the time to peak, defined as the time from initiation of the test until the thrombin peak height, and velocity index, the slope of the thrombin formation curve, defined as the thrombin peak height divided by the difference between the time to peak and lag time.
The goal of anticoagulation is to reduce the potential thrombin generation in order to decrease the risk of future thromboembolic events. Thus, an assay measuring thrombin generation may have the potential to monitor the effect of anticoagulants such as DOACs. The application of TGA in monitoring the anticoagulant effect of DOACs has been demonstrated in several in vitro studies causing alteration of TGA parameters with increasing concentrations of DOACs in spiked plasma samples.2, 7 There are limited clinical data on the assessment of antithrombotic effect of DOACs. Extensive review of the literature demonstrates the majority of published data on use of TGA assay for assessment of anticoagulation effect of the DOACs are in vitro with a dose response correlation between increasing drug concentrations, increasing lag time, and reduction of thrombin peak height and ETP in blood spiked with dabigatran, rivaroxaban, and apixaban.7 The limited clinical studies on patients or healthy volunteers after oral intake of DOACs will be discussed in the following paragraph.
Helin et al. demonstrated prolongation of lag time, and increase of ETP and thrombin peak height in 49 patients taking dabigatran for atrial fibrillation 150 mg twice daily and DVT prophylaxis 220 mg once daily.8 The study was a retrospective data analysis and specific clinical situations were not reported. The duration of treatment, along with blood sample collection timing in relation to drug intake was not recorded. Conversely, Hermann et al. demonstrated prolongation of lag time and decrease of ETP and thrombin peak height in 17 patients taking dabigatran 150 mg twice daily for atrial fibrillation, and 15 patients taking rivaroxaban 10 mg once daily for DVT prophylaxis when samples were collected 2 hours after drug intake.9 Siegal et al. examined 24 healthy volunteers receiving apixaban 5 mg orally twice daily for 3.5 days and 27 healthy volunteers receiving rivaroxaban 20 mg orally once daily for 4 days. ETP was significantly decreased when blood samples were collected 3 hours after the last intake of apixaban and 4 hours after the last intake of rivaroxaban on day 4 of the study, but the other TGA parameters were not included in the results.10 Cheung et al. examined 6 healthy volunteers receiving apixaban 10 mg twice daily for 3 days.11 ETP was reduced compared to baseline, but other TGA parameters were not reported.11 Marlu et al. examined 10 healthy male volunteers after intake of a single dose of dabigatran 150 mg or rivaroxaban 20 mg. Among subjects receiving rivaroxaban, lag time was increased and thrombin peak height and ETP were reduced 2 hours after drug intake. Among subjects receiving dabigatran, lag time was increased, ETP was modestly reduced, and thrombin peak height was unchanged 2 hours after drug intake.12 Freyburger et al. examined 80 patients after hip replacement surgery, 40 of which were on dabigatran 150‐220 mg once daily, and the other 40 on rivaroxaban 10 mg daily. Among patients on dabigatran, lag time was increased and ETP decreased by 15%. Among patients on rivaroxaban, lag time was increased and ETP was modestly decreased.13 In a separate study by the same group, 51 patients received 2.5 mg apixaban twice daily for DVT prophylaxis after orthopedic surgery. No significant change in the lag time or ETP was observed at baseline compared to 2 hours, 4, and 7 days later during the treatment.14 Lastly, a similar study by Helin and coworkers on orthopedic patients receiving 2.5 mg twice daily of apixaban postoperatively revealed a modest effect on thrombin peak height and ETP as compared to preoperative values.15 The shortcomings of the abovementioned studies include TGA parameters were predominantly measured at single time point. If the parameters were measured beyond a single time point, not all TGA parameters were displayed in the publications. ETP appears to be the TGA parameter most often displayed when the TGA is utilized. The rationale for the use of ETP as compared to the other TGA parameters have not been justified in the reviewed literature, but one can hypothesize that since ETP represents the total bulk coagulability in a sample and is potentially reflective of the thrombotic or bleeding potential in patients.
The purpose of this ex vivo study was to systematically evaluate the anticoagulant effect of dabigatran, rivaroxaban, and apixaban on each individual TGA parameter through serial measurements over time and to establish correlations with the drug concentrations used for standard treatment of atrial fibrillation or venous thromboembolism in order to better define expected alteration of all of TGA parameters after intake of these classes of agents, as well as to demonstrate suitability of these parameters for monitoring the anticoagulant effect of DOACs.
2. MATERIALS AND METHODS
2.1. Study design
The study was approved by the local institutional review board and was performed in accordance with the Helsinki declaration. Healthy male volunteers were included in the study. Because female subjects during reproductive age may be subject to unpredictable variation in their coagulation status based on the timing of the blood sampling and menstrual cycle, it is common practice for coagulation studies with small sample sizes to use only male subjects. The rationale for using exclusively male volunteers in our study was to avoid interaction between the aforementioned phenomenon and the true effect of the DOAC agents on coagulation status. Subjects were included if they met the following inclusion criteria: male gender, age between 18 years and 70 years, normal laboratory screening test results, no abnormalities on physical examination, normal vital signs, and the ability to provide written informed consent. Laboratory screening included renal and hepatic function, hepatitis B virus, hepatitis C virus, and HIV serology, a complete blood cell count, PT, and aPTT. The use of aspirin and non‐steroidal anti‐inflammatories excluded subjects from participation. The exclusion criteria were as follows: a history of allergic reactions to blood products, a personal or family history of coagulation disorders, participation in any other investigational intervention study within the past 30 days, and any medication use within 7 days before the start of the study. Each subject received a single dose of 150 mg dabigatran, 20 mg rivaroxaban, or 10 mg apixaban at different time points with at least 2 weeks between intakes to allow for a wash out period.
The rationale for the choice of the above‐mentioned doses were based on standard recommendations for drug administration at the therapeutic dose, rivaroxaban administered at 20 mg once a daily, dabigatran administered at 150 mg twice daily, and apixaban administered at 5 or 10 mg twice daily based on indication for use (atrial fibrillation vs acute thromboembolism).
Blood samples were obtained at time 0 before intake of the anticoagulant, and subsequently 2, 4, and 24 hours after the drug intake. Phlebotomy was obtained without the use of tourniquet. Samples for coagulation analysis were collected in a standard blue top citrated tube (buffered sodium citrate 0.109 mol L−1 3.2%, BD Vacutainer, Franklin Lakes, NJ, USA). Samples were centrifuged at 7200 rpm for 3 minutes. Platelet poor plasma was kept at −80°Celsius before being utilized in batch analysis.
2.2. Thrombin generation
Thrombin generation measurements were performed using the Calibrated Automated Thrombogram (CAT) system, which consisted of the Fluoroskan Ascent instrument (Thermo, Vantaa, Finland) paired with the Thrombinoscope software (Diagnostica Stago Inc., Parsippany, NJ, USA). Thrombin generation reagents used were the PPP Reagent HIGH, Thrombin Calibrator, and FluCa Kit (Diagnostica Stago Inc.). The PPP Reagent HIGH, which contains a high concentration of tissue factor (TF) along with a standard concentration of phospholipids, was chosen due to the need for a high TF‐containing reagent to overwhelm the effects of the anticoagulant drugs. Briefly, the manufacturer's recommended protocol was used, which involved mixing of 80 μL of each sample plasma or pooled normal plasma (PNP) into 6 wells of a 96 well plastic plate. For each sample plasma or PNP, 20 μL of the PPP Reagent HIGH was added to 3 wells and 20 μL of the thrombin calibrator was added to a separate set of 3 wells. After dispensation, the plate was inserted into the instrument, incubated for 10 minutes at the 37°C set temperature, and the FluCa reagent (buffer/calcium/substrate mixture) dispensed by the instrument to initiate the thrombin generation process. Special care was taken to avoid the mixture of the sample plasma from the nonzero dabigatran time points with the thrombin calibrator, as the presence of dabigatran inhibits the thrombin activity, making the results unreliable. Thus, the baseline samples from a given patient were used as the calibrator wells for all of the dabigatran time points from the respective subject, which was not a concern for the apixaban and rivaroxaban samples. Following completion of the thrombin generation runs, the fluorescence data were automatically analyzed and fit by the Thrombinoscope software. Frozen PNP (CRYOcheck Pooled Normal Plasma, Precision BioLogic, Dartmouth, NS, Canada) was used not only as a control on each plate, but also allowed for normalization of the endogenous thrombin potential (ETP) and thrombin peak height data to correct for inter‐plate variability.
2.3. Measurement of DOAC concentrations
Plasma was collected from each sample and analyzed using dedicated chromogenic anti‐FXa assays for rivaroxaban and apixaban (BIOPHEN Heparin LRT and BIOPHEN DiXaI, HYPHEN BioMed, Neuville‐sur‐Oise, France) clotting and chromogenic anti‐FIIa assays (HEMOCLOT TI and BIOPHEN DTI, HYPHEN BioMed, Neuville‐sur‐Oise, France) for dabigatran. BIOPHEN Heparin LRT and BIOPHEN DiXaI assays are kinetic methods based on the inhibition by apixaban or rivaroxaban of factor Xa (FXa), which is at a constant concentration and in excess. The remaining FXa is then measured by its amidolytic activity on FXa specific chromogenic substrate which releases paranitroaniline (pNA). The amount of pNA generated is inversely proportional to the concentration of apixaban or rivaroxaban in the tested plasma. HEMOCLOT Thrombin Inhibitors is a clotting method based on the inhibition by dabigatran of a defined concentration of human α‐thrombin. Clotting is initiated by the remaining thrombin and a dabigatran calibration curve is used to calculate the dabigatran concentration in the tested plasma based on the clotting time measured. The BIOPHEN DTI assay is a kinetic method based on the inhibition by dabigatran of thrombin, which is at a constant concentration and in excess. The remaining thrombin is then measured by its amidolytic activity on a thrombin specific chromogenic substrate, which releases pNA. The amount of pNA generated is inversely proportional to the concentration of dabigatran in the tested plasma. The assays are calibrated with dedicated specific plasma calibrators and concentrations verified by use of liquid chromatography‐tandem mass spectrometry by the manufacturer.
2.4. Statistical analysis
TGA parameters (lag time, thrombin peak height, ETP, time to peak, and velocity index) were correlated to individual DOAC concentrations. Spearman's correlations coefficient r and P values for significance were reported for linear regression models. If the pattern did not fit a linear model, the best fit nonlinear model was selected and goodness of fit expressed by R squared.
Alteration of TGA parameters over time was analyzed by repeated measures ANOVA test. Whether the alteration of the TGA parameter over time was statistically significant as compared to baseline was expressed by ANOVA P. All data was presented as mean and standard deviation (SD), unless otherwise indicated. P values less than .05 (two tailed) were considered statistically significant. Statistical analyses were performed using Prism software version 7.0 (GraphPad Software Inc., La Jolla CA, USA).
3. RESULTS
Ten healthy male volunteers (mean age 41 ± 15 SD, median 37, range 24‐65 years old) were included in the study. Dabigatran peak levels were achieved at 4 hours with mean concentration of 73.5 ng mL−1 ± 34 SD (median 80.2 ng mL−1, total range 14.4‐120.4). Rivaroxaban, peak levels were achieved at 4 hours with mean concentration of 162.7 ng mL−1 ± 117 SD (median 120.3 ng mL−1 total range 62‐433). Apixaban peak levels were achieved at 2 hours with mean concentration of 162.8 ng mL−1 ± 57 SD (median 168.2 ng mL−1 total range 57‐285) (Figure 1A). The alteration of TGA parameters lag time, ETP and thrombin peak height over 24 hours after intake of single dose of DOAC is demonstrated in Figure 1. The scatter diagram of DOAC concentrations against the TGA parameters are demonstrated in Figures 2, 3, and 4.
Figure 1.
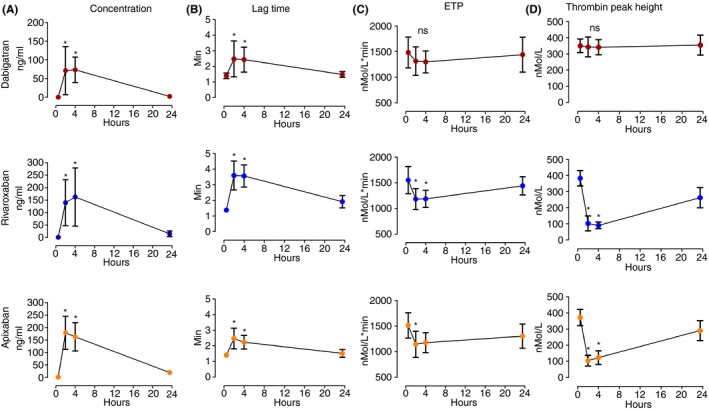
The DOAC concentrations and the effect on TGA parameters, lag time, ETP, and thrombin peak height over time after intake of single dose of DOACs. ETP, Endogenous Thrombin Potential; ns, not significant. *indicates p value of <.05 compared to baseline level at time 0. The bars represent mean ± standard deviation
Figure 2.
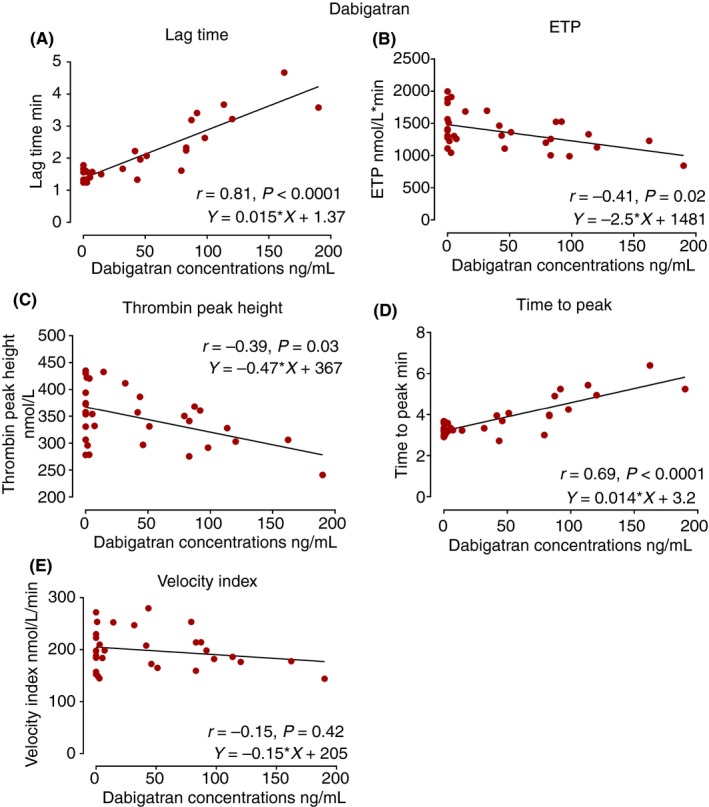
The scatter diagrams of TGA parameters, lag time, ETP, thrombin peak height, time to peak, and velocity index against dabigatran concentrations. The Spearman correlation coefficient r as well as the regression equations are indicated in the diagrams. ETP, endogenous thrombin potential
Figure 3.
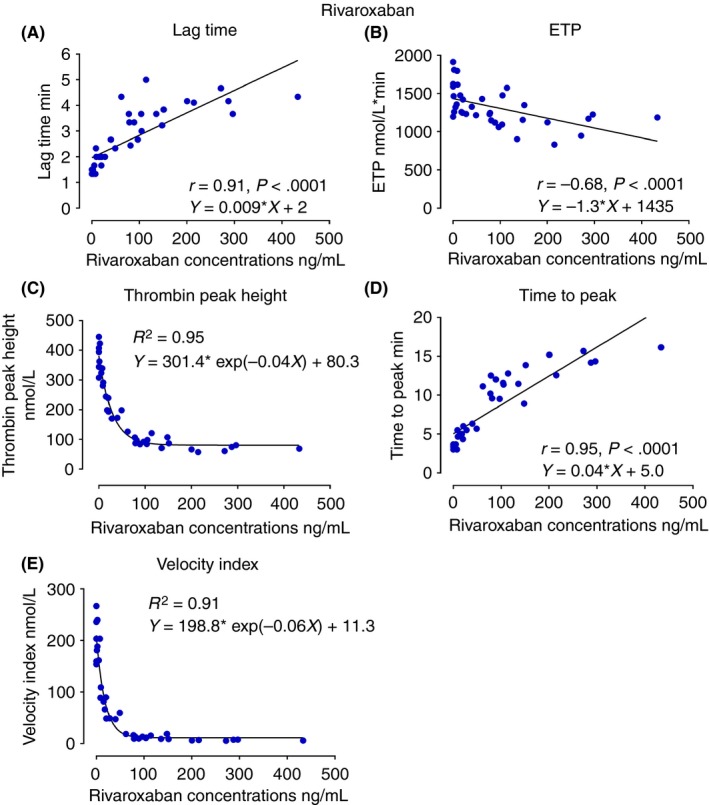
The scatter diagrams of TGA parameters, lag time, ETP, thrombin peak height, time to peak, and velocity index against rivaroxaban concentrations. The Spearman correlation coefficient r as well as the regression equations are indicated in the diagrams. ETP, endogenous thrombin potential
Figure 4.
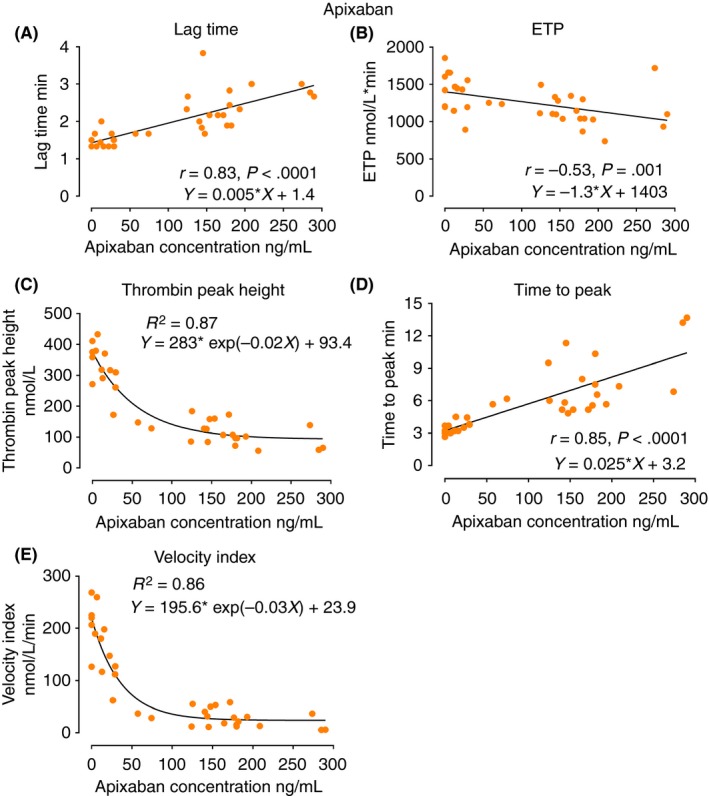
The scatter diagrams of TGA parameters, lag time, ETP, thrombin peak height, time to peak, and velocity index against apixaban concentrations. The Spearman correlation coefficient r as well as the regression equations are indicated in the diagrams. ETP, endogenous thrombin potential
Among all the TGA parameters, lag time was most strongly correlated with DOAC concentrations and significantly increased at two and four hours after administration across the DOAC classes (Figure 1B) (r = .81 for dabigatran, r = .91 for rivaroxaban, r = .83 for apixaban, P < .0001 for all) (Figures 2A, 3A, and 4A). ETP was significantly reduced 2 hours after intake of the FXa inhibitors, rivaroxaban and apixaban (Figure 1C), with moderate correlation with the FXa inhibitor levels (Figures 3B and 4B). ETP levels demonstrated a decreasing trend over time after intake of dabigatran, but the change was not statistically significant (Figure 1C) (ANOVA P = .7). There was a weak but statistically significant correlation, between dabigatran concentration and ETP (r = −.41, P = .02) (Figure 2B). Thrombin peak height was significantly decreased at 2 and 4 hours after intake of FXa inhibitors (Figure 1D), and showed a strong nonlinear correlation with plasma concentrations fitting exponential decay (R² = .95 for rivaroxaban and R² = .87 for apixaban) (Figures 3C and 4C). Thrombin peak height demonstrated no statistically significant trend over time with dabigatran (Figure 1D) (ANOVA P = .96). For the FXa inhibitors, the time to peak was significantly increased and the velocity index was significantly decreased at 2 and 4 hours after intake (ANOVA P < .0001 for both FXa inhibitors using both parameters). Both parameters were strongly correlated with FXa inhibitor levels (Figures 3D, 3E, 4D, and 4E). The pattern of correlation between drug concentrations and velocity index for FXa inhibitors followed exponential decay similar to thrombin peak height. For dabigatran, there were no significant trends over time for time to peak and the velocity index (ANOVA P = .06 for time to peak and P = .96 for velocity index).
4. DISCUSSION
A majority of the previous studies on the use of TGA for evaluation of the effects of DOACs have only displayed certain TGA parameters, or displayed them at a limited number of time points. In the present study, we evaluated all primary and secondary TGA parameters and then correlated them to the DOAC drug concentrations at 4 different time points in healthy volunteers after intake of a single DOAC dose. Lag time was the single parameter relatively equally sensitive to dabigatran, rivaroxaban, and apixaban. In contrast to the general perception, ETP was not strongly correlated to the DOAC concentration, exhibiting only modest variation over time, following drug administration for the FXa inhibitors only, and none for dabigatran. Another original contribution of the present study was to demonstrate that for the FXa inhibitors, the thrombin peak height appears to display superior correlation to drug concentrations as compared to ETP and other TGA parameters. The correlation exhibited nonlinear exponential decay as a function of drug concentration in accordance to previously published literature.16 The decay trend reflects diminishing thrombin generation as more FXa active binding sites being occupied by the FXa inhibitor molecules until saturation point is achieved, and where further increase in FXa inhibitor concentration would no longer impact the thrombin generation. Given this observed decaying pattern, the values of thrombin peak height appear to be most predictive of FXa concentrations at lower concentrations of 100 ng mL−1 or lower for rivaroxaban and 150 ng mL−1 or lower for apixaban (Figures 3C and 4C). As the velocity index is a product of thrombin peak height divided by a time factor, it is clear that this parameter also exhibits an exponential trend similar and parallel to the thrombin peak height. Dabigatran was poorly correlated with the thrombin peak height and therefore similar mechanism was not observed by use of the TGA technique at the concentrations achieved in the present study.
Currently, specific DOAC assays are available capable of measuring exact drug concentrations for each individual anticoagulant.8, 17, 18 However, the use of such assays in patient care requires more clinical and outcome data. The therapeutic DOAC levels are not yet defined by the manufacturers, executive committees of any of the major DOAC trials, guidelines or the FDA and, therefore, the sensitivity or specificity of TGA test for therapeutic drug concentrations cannot be determined. It is plausible that DOAC effect on hemostasis, and its correlation with the clinical events, cannot be determined with a single parameter, such as plasma drug concentration in a similar fashion as the value of international normalized ratio (INR) level in monitoring warfarin. Thus, global assays such as TGA may provide additional useful clinical information beyond simply DOAC concentrations, particularly for FXa inhibitors.
Even though TGA has favorable correlation with DOAC concentrations, and is commonly applied in research studies on the effect of DOACs on hemostasis, it is labor intensive, and requires expertise similar to other manual assays conducted in clinical and research laboratories. Sample preparation and analysis for TGA assays take approximately 2 hours in experienced laboratories. Therefore, in its current format it is likely not suited for emergencies. It is anticipated that the future upgrade of this technology to be more user friendly and better suited for routine clinical use. Another limitation of this technique, as noted in the present study and previously described, is the interaction of samples containing dabigatran with the internal thrombin calibrator, which inhibits the thrombin activity of this reagent, making the results unreliable.13 In order to make accurate measurements, baseline samples from a given subject, not containing dabigatran, were used in the calibrator wells. This issue can be a limiting factor in the case of monitoring subjects on maintenance treatment with no prior baseline samples available to adjust the calibration, which is not a concern for samples containing apixaban and rivaroxaban.
Our study was limited by the fact that it was performed on a limited number of healthy volunteers, rather than patients with atrial fibrillation or thromboembolic disease. Other limitations include the use of a single dose of the anticoagulants, rather than repeated doses to achieve steady state drug concentrations. Peak concentrations achieved (162 ng mL−1 for rivaroxaban and apixaban and 73 ng mL−1 for dabigatran) represented lower ranges of concentrations documented in clinical studies with patients receiving maintenance DOAC treatment for atrial fibrillation or thromboembolism ranging between 60 and 190 ng mL−1 for dabigatran, 40 to 250 ng mL−1 for rivaroxaban, and 70 and 250 ng mL−1 for apixaban.19, 20 Nonetheless, significant correlation was demonstrated between TGA parameters and FXa inhibitor concentrations. A noteworthy limitation of the study was the dose of 150 mg dabigatran administered constituting half of the recommended daily dose for treatment of atrial fibrillation and DVT, as compared to the 10 mg of apixaban and 20 mg rivaroxaban, which constitute the total daily maintenance treatment dose. Thus, there is a potential bias from dabigatran under dosing, which could result in a weaker effect on TGA parameters as compared to the direct FXa inhibitors. Other in vitro and patient studies using TGA have also shown a lack of sensitivity of TGA parameters when dabigatran was used.2, 7, 8, 21 As in the present study, the observation was noted on post‐operative orthopedic patients using prophylactic doses of dabigatran (220 mg once daily) after correcting the thrombin calibrator wells with patients’ baseline plasma samples not containing dabigatran by Freyburger and coworkers.13 Thus, the lack of sensitivity of TGA parameters to dabigatran is unlikely to be related to the interaction between dabigatran and the Thrombin Calibrator. As the subjects in the present study did not achieve adequate dabigatran concentrations, we cannot rule out whether the other TGA parameters beyond lag time would have shown statistically significant alterations had the subjects achieved higher plasma concentrations by receiving higher doses of dabigatran.
In conclusion, TGA lag time was the most sensitive parameter to the effect of the DOACs across the different classes of these anticoagulants evaluated. Thrombin peak height was strongly correlated to drug concentration of the FXa inhibitors in a nonlinear exponential decay pattern, suggesting thrombin peak height may be a useful parameter to monitor the anticoagulant effect of FXa inhibitors particularly at lower concentrations. ETP had a weak to moderate correlation with drug concentrations across the classes evaluated and is likely less suitable for assessment of DOAC effect as a stand‐alone parameter. Larger clinical trials evaluating TGA on patients receiving therapeutic doses of DOACS are needed to correlate the TGA parameters with positive and negative clinical outcomes.
AUTHOR CONTRIBUTIONS
R. Artang was responsible for the design of the study, part of the laboratory analysis, statistical analysis and writing the manuscript. M. Anderson was responsible for the data collection, interpretation of the data and revising the manuscript. P. Riley was responsible for the laboratory analysis and revising the manuscript. J Dalsgaard Nielsen was responsible for design of the study and revising the manuscript. We would like to thank Rebecca Starr for assistance with the language the manuscript.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper.
Artang R, Anderson M, Riley P, Nielsen JD. Assessment of the effect of direct oral anticoagulants dabigatran, rivaroxaban, and apixaban in healthy male volunteers using a thrombin generation assay. Res Pract Thromb Haemost. 2017;1:194–201. 10.1002/rth2.12044
REFERENCES
- 1. Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring Oral Direct Inhibitors (ODIs) of thrombin and factor Xa: A recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2013;11:756–60. [DOI] [PubMed] [Google Scholar]
- 2. Xu Y, Wu W, Wang L, et al. Differential profiles of thrombin inhibitors (heparin, hirudin, bivalirudin, and dabigatran) in the thrombin generation assay and thromboelastography in vitro. Blood Coagul Fibrinolysis. 2013;24:332–8. [DOI] [PubMed] [Google Scholar]
- 3. Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–97. [DOI] [PubMed] [Google Scholar]
- 4. Hillarp A, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133–9. [DOI] [PubMed] [Google Scholar]
- 5. Hillarp A, Gustafsson KM, Faxalv L, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti‐FXa assays. J Thromb Haemost. 2014;12:1545–53. [DOI] [PubMed] [Google Scholar]
- 6. Castoldi E, Rosing J. Thrombin generation tests. Thromb Res. 2011;127(Suppl 3):S21–5. [DOI] [PubMed] [Google Scholar]
- 7. Wong PC, White A, Luettgen J. Inhibitory effect of apixaban compared with rivaroxaban and dabigatran on thrombin generation assay. Hosp Pract. 1995;2013:19–25. [DOI] [PubMed] [Google Scholar]
- 8. Helin TA, Lemponen M, Hjemdahl P, Ronquist‐Nii Y, Lassila R, Joutsi‐Korhonen L. From laboratory to clinical practice: Dabigatran effects on thrombin generation and coagulation in patient samples. Thromb Res. 2015;136:154–60. [DOI] [PubMed] [Google Scholar]
- 9. Herrmann R, Thom J, Wood A, Phillips M, Muhammad S, Baker R. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost. 2014;111:989–95. [DOI] [PubMed] [Google Scholar]
- 10. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet Alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–24. [DOI] [PubMed] [Google Scholar]
- 11. Cheung YW, Barco S, Hutten BA, Meijers JC, Middeldorp S, Coppens M. In vivo increase in thrombin generation by four‐factor prothrombin complex concentrate in apixaban‐treated healthy volunteers. J Thromb Haemost. 2015;10:1799–805. [DOI] [PubMed] [Google Scholar]
- 12. Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non‐specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217–24. [DOI] [PubMed] [Google Scholar]
- 13. Freyburger G, Macouillard G, Labrouche S, Sztark F. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res. 2011;127:457–65. [DOI] [PubMed] [Google Scholar]
- 14. Freyburger G, Macouillard G, Khennoufa K, Labrouche S, Molimard M, Sztark F. Rivaroxaban and apixaban in orthopaedics: is there a difference in their plasma concentrations and anticoagulant effects? Blood Coagul Fibrinolysis. 2015;26:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helin TA, Virtanen L, Manninen M, et al. Effects of thromboprophylactic doses of apixaban and rivaroxaban on coagulation and thrombin generation in association with total hip replacement. J Thromb Thrombolysis. 2017;43:562–9. [DOI] [PubMed] [Google Scholar]
- 16. Burghaus R, Coboeken K, Gaub T, et al. Evaluation of the efficacy and safety of rivaroxaban using a computer model for blood coagulation. PLoS ONE. 2011;6:e17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konigsbrugge O, Quehenberger P, Belik S, et al. Anti‐coagulation assessment with prothrombin time and anti‐Xa assays in real‐world patients on treatment with rivaroxaban. Ann Hematol. 2015;94:1463–71. [DOI] [PubMed] [Google Scholar]
- 18. Skeppholm M, Al‐Aieshy F, Berndtsson M, et al. Clinical evaluation of laboratory methods to monitor apixaban treatment in patients with atrial fibrillation. Thromb Res. 2015;136:148–53. [DOI] [PubMed] [Google Scholar]
- 19. Testa S, Tripodi A, Legnani C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: Results observed in four anticoagulation clinics. Thromb Res. 2016;137:178–83. [DOI] [PubMed] [Google Scholar]
- 20. Reilly PA, Lehr T, Haertter S, et al. RE‐LY Investigators . The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY Trial. J Am Coll Cardiol. 2014;63:321–8. [DOI] [PubMed] [Google Scholar]
- 21. Dale B, Eikelboom JW, Weitz JI, et al. Dabigatran attenuates thrombin generation to a lesser extent than warfarin: could this explain their differential effects on intracranial hemorrhage and myocardial infarction? J Thromb Thrombolysis. 2013;35:295–301. [DOI] [PubMed] [Google Scholar]


