INTRODUCTION
Over the past several decades, significant advances have been made in local-regional therapy for breast cancer. The use of breast conserving therapy (BCT) to include lumpectomy with whole breast irradiation is a practice supported by six prospective trials that demonstrated overall survival is equivalent for patients undergoing BCT versus mastectomy. 1–6 Attention to margins was not standard at the time these trials were conducted and only the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 required negative margins. In the report of 20-year follow-up of B-06, the ipsilateral breast tumor recurrence (IBTR) rate was 14.3% in patients undergoing segmental mastectomy with whole breast irradiation.3 In the B-06 trial, only women with positive lymph nodes received adjuvant chemotherapy. Since that time, the indications for adjuvant therapy including endocrine therapy and chemotherapy have been expanded, and the use of systemic therapy has been shown to decrease local recurrence in the breast. Combined with improvements in diagnostic imaging and pathologic evaluation of specimens, IBTR rates have decreased significantly. 7
The number of patients eligible for BCT has increased as the use of neoadjuvant chemotherapy for larger tumors has demonstrated that downsizing of the primary tumor results in the option for breast preservation in some patients who would otherwise require mastectomy if they underwent surgery first. There is no adverse impact on survival by administering chemotherapy in the neoadjuvant versus adjuvant setting 8–17 and mature results of the largest randomized trial showed trends toward improved disease-free and overall survival in premenopausal women who received neoadjuvant chemotherapy. 14 Two meta-analyses confirmed equivalent survival outcomes, and one reported that neoadjuvant chemotherapy reduced mastectomy rates by 16.6% (95% CI:15.1-18.1%). 18, 19 This is likely to be an underestimation of the impact on surgical options, as most trials did not differentiate whether patients were BCT candidates at presentation and most did not record whether the patient chose mastectomy over BCT even if they were BCT candidates after chemotherapy.
One concern regarding the use of BCT after neoadjuvant chemotherapy has been the ability to determine the extent of residual disease that should be targeted for resection. Due to variability in the response of tumors to chemotherapy, some will shrink concentrically while others will have pockets of residual tumor intermixed with fibrosis spread over a larger area. Defining the appropriate preoperative imaging and extent of breast tissue for resection after neoadjuvant chemotherapy therefore remains a clinically relevant question. At the University of Texas MD Anderson Cancer Center we have developed a standard approach whereby all patients undergo imaging evaluation before and after chemotherapy (diagnostic mammography and ultrasound) 20 and a clip marking the tumor site is placed early in the treatment course to facilitate resection of the primary tumor bed in case of complete radiographic response. Following chemotherapy, any residual radiographic abnormality and the clip are targeted for resection along with a goal of at least a 2 mm margin of normal tissue. In most cases, we do not attempt to excise the entire pre-chemotherapy tumor volume. 21, 22 The goal of this study was to evaluate BCT patients undergoing surgery first versus those receiving neoadjuvant chemotherapy using a standardized treatment approach to discern any clinicopathologic factors or treatment related variables that may impact LRR.
METHODS
Patient Population
A prospectively maintained database was used to identify patients undergoing BCT to include segmental mastectomy and whole breast irradiation from January 1987 through December 2005. Demographic data including age and date of diagnosis were noted. Clinicopathologic data included: histology (invasive ductal, invasive lobular, mixed), clinical stage according to the 6th edition of the American Joint Committee on Cancer staging guidelines, nuclear grade, estrogen and progesterone receptor (ER and PR) and HER2/neu (HER2) status, presence of lymphovascular invasion (LVI), margin status, (negative ≥ 2mm; close < 2mm; or positive), presence of multifocal disease on final pathology, and pathologic stage. At presentation, clinical stage was determined using physical examination, mammography, and ultrasound of the breast and regional nodal basins. Suspicious appearing lymph nodes were evaluated by fine needle aspiration biopsy. 20 For hormone receptor status, >10% staining of the cells by immunohistochemistry (IHC) was considered positive. Tumors were considered HER2-positive if they were IHC 3+ or amplified on fluorescence in-situ hybridization.
Treatment
Breast conserving therapy included segmental mastectomy, axillary node evaluation and whole breast irradiation. Segmental mastectomy involved excision of the primary tumor with a margin of normal tissue. In patients who received neoadjuvant chemotherapy, preoperative imaging was performed in the majority of patients and any radiographic abnormality, calcifications and the clip were targeted for resection. In most cases, no attempt was made to resect the pretreatment disease volume. 21, 22 In the early 1990s, we began performing intraoperative margin analysis and this approach was routinely utilized by 1994. 7 Briefly, the excised specimen is oriented, then inked using a multicolor system, followed by sectioning into 3- to 5-mm sections perpendicular to the long axis. Sections are evaluated grossly by a breast pathologist, then subjected to specimen radiography and reviewed by a breast radiologist. If these evaluations suggest a close or involved margin, additional tissue is resected at the initial operation. When final pathologic evaluation demonstrated close or positive margins, patients were counseled regarding the utility of re-excision. For patients presenting with clinically node negative disease, axillary node evaluation generally consisted of sentinel lymph node (SLN) dissection with a completion axillary dissection performed when the SLN revealed metastatic disease. Patients presenting with clinically node positive disease underwent axillary lymph node dissection. Clinically node positive disease was defined as any lymph node identified by palpation or ultrasound and confirmed by fine needle aspiration biopsy to contain metastatic disease.
All patients received external-beam radiation therapy to the breast with tangential fields. The standard treatment involved a median dose of 50Gy to the breast delivered in 25 fractions over 5 weeks followed by an electron boost to the tumor bed (median dose, 10Gy). Regional nodal irradiation (RNI) was delivered at the discretion of the radiation oncologist. For patients undergoing surgery first, RNI was routinely administered in patients with ≥ 4 positive lymph nodes. Use of RNI was considered in patients with 1 to 3 positive lymph nodes with other adverse factors including young age, LVI or extranodal extension. For patients receiving neoadjuvant chemotherapy, RNI was recommended for patients presenting with clinical stage III disease and for those with residual positive lymph nodes (ypN1).
Patients treated with neoadjuvant chemotherapy received a variety of regimens, often dictated by ongoing clinical trials. The specific regimen was known in 97% and included an anthracycline based regimen in 33%, a taxane based regimen in 7%, or a combination anthracycline/taxane based regimen in 60%. This study predated the routine use of trastuzumab in the neoadjuvant setting. Patients undergoing surgery first were offered adjuvant chemotherapy based on institutional or NCCN guidelines. Patients with hormone receptor positive disease generally received adjuvant endocrine therapy. Early in the study, tamoxifen was offered only to postmenopausal patients. In the later years of the study, tamoxifen was offered to premenopausal patients, and postmenopausal women were considered for an aromatase inhibitor.
Endpoints and Statistical Methods
Endpoints included LRR-free survival, distant metastasis-free survival (DMFS), and disease-specific survival (DSS). Events were measured from the date of diagnosis. LRR was defined as disease recurrence in the ipsilateral breast or the axillary, supraclavicular, infraclavicular or internal mammary lymph nodes. Recurrence at any other site was considered distant metastasis. All LRRs were counted as events regardless of whether they were the first site of failure or occurred with or after distant metastasis. LRR and distant metastases were considered to be concurrent if diagnosed within 3 months. Patients who did not experience any of these events were censored at last follow-up or at the time of death.
Distributions of clinical factors between groups were compared using the Mann-Whitney test for continuous variables and chi-squared test for categorical variables. LVI and HER2 were not routinely assessed in the entire study period, therefore patients with missing data and missing cases were grouped with negative cases. Multivariate analyses were performed using a Cox proportional hazards model with backward stepwise analysis. Five- and 10-year rates of LRR-free, DMFS, and DSS were calculated using the Kaplan-Meier method and differences between patients undergoing surgery first and those receiving neoadjuvant chemotherapy were compared using the log-rank test. All calculations were performed with Stata software (Stata/SE 11; Stata Corp., College Station, TX). Two-tailed p values ≤ .05 were considered statistically significant. This study was approved by the MD Anderson Institutional Review Board.
RESULTS
Clinicopathologic characteristics
The study population consisted of 2983 patients who underwent BCT; 2331 (78%) undergoing surgery first and 652 (22%) undergoing surgery after neoadjuvant chemotherapy. Table 1 lists clinicopathologic characteristics for both cohorts. As demonstrated in the table, 93% of patients receiving neoadjuvant chemotherapy had clinical stage II or III disease compared to 21% of those treated with surgery first. Patients receiving neoadjuvant chemotherapy also had a greater percentage of nuclear grade 3 (66% versus 34%, P<0.001) and ER-negative (48% versus 24%, P<0.001) tumors. Neoadjuvant chemotherapy downsized tumors such that 93% (607/652) presented with clinical stage II or III disease, and only 47% (304/652) had pathologic stage II or III disease (P<0.001). A pathologic complete response was noted in 131 (20%) patients.
Table 1.
Clinicopathologic factors for patients undergoing breast conserving therapy
| Factor | Surgery First (n=2331) |
Neoadjuvant Chemotherapy (n=652) |
P value |
|---|---|---|---|
|
| |||
| Age | |||
| Median | 55 | 50 | < 0.001 |
| Range | 22 – 89 | 25 - 84 | |
|
| |||
| Histology | |||
| IDC | 2033 (87%) | 617 (95%) | <0.001 |
| ILC | 169 (7%) | 19 (3%) | |
| Mixed | 129 (6%) | 16 (2%) | |
|
| |||
| Clinical Stage | |||
| 0 | 31* (1%) | 0 (0%) | <0.001 |
| I | 1823 (78%) | 45 (7%) | |
| II | 459 (20%) | 451 (69%) | |
| III | 18 (1%) | 156 (24%) | |
|
| |||
| Nuclear grade | |||
| 1 | 266 (11%) | 16 (2%) | <0.001 |
| 2 | 1244 (53%) | 196 (30%) | |
| 3 | 785 (34%) | 431 (66%) | |
| Not reported | 36 (2%) | 9 (1%) | |
|
| |||
| ER | |||
| Positive | 1665 (71%) | 331 (51%) | <0.001 |
| Negative | 552 (24%) | 311 (48%) | |
| Unknown | 114 (5%) | 10 (1%) | |
|
| |||
| PR | |||
| Positive | 1364 (59%) | 292 (45%) | <0.001 |
| Negative | 802 (34%) | 347 (53%) | |
| Unknown | 165 (7%) | 13 (2%) | |
|
| |||
| HER2 | |||
| Positive | 122 (5%) | 114 (17%) | <0.001 |
| Negative | 1216 (52%) | 402 (62%) | |
| Unknown | 993 (43%) | 136 (21%) | |
|
| |||
| LVI | |||
| Present | 348 (15%) | 103 (16%) | 0.584 |
| Absent/Unknown | 1983 (85%) | 549 (84%) | |
|
| |||
| Multifocal disease | |||
| Yes | 155 (7%) | 64 (10%) | 0.006 |
| No | 2176 (93%) | 588 (90%) | |
|
| |||
| Margin Status | |||
| Negative | 2239 (96%) | 621 (95%) | 0.144 |
| Close (<2mm) | 63 (3%) | 26 (4%) | |
| Positive | 29 (1%) | 5 (1%) | |
|
| |||
| Pathologic Stage | |||
| 0 | 0 (0%) | 131 (20%) | <0.001 |
| I | 1540 (66%) | 217 (33%) | |
| II | 702 (30%) | 251 (38%) | |
| III | 89 (4%) | 53 (9%) | |
|
| |||
| Adjuvant | |||
| Chemotherapy | |||
| Yes | 980 (42%) | NA | NA |
| No | 1348 (58%) | ||
| Unknown | 4 (<1%) | ||
|
| |||
| Endocrine Therapy§ | |||
| Yes | 1293 (78%) | 294 (89%) | <0.001 |
| No | 372 (22%) | 37 (11%) | |
Abbreviations: IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; Mixed, invasive ductal and lobular carcinoma; ER, estrogen receptor; PR, progesterone receptor; HER2, HER2/neu; LVI, lymphovascular invasion
31 patients who underwent surgery first had a biopsy diagnosis of ductal carcinoma in situ but were found to have invasive disease on final pathologic evaluation.
Administration of endocrine therapy was determined only for patients with ER-positive disease
Survival
In the surgery first group, the median follow-up for surviving patients was 7.9 years (range, 0.02 – 20.7) and 5- and 10-year LRR-free survival rates were 97% (95% CI, 96-98) and 94% (95% CI, 93-95), respectively. In the neoadjuvant chemotherapy group, the median follow-up was 7.2 years (range, 0.19 – 19.2) and 5- and 10-year LRR-free survival rates were 93% (95% CI, 91-95) and 90% (95% CI, 87-93). These differences in LRR-free survival comparing the entire cohorts of surgery first patients to neoadjuvant chemotherapy patients were significant (P<0.001). However, when evaluated by presenting clinical stage, there were no differences in LRR-free survival rates for those undergoing surgery first and those receiving neoadjuvant chemotherapy (P=NS; Figure 1). When local-regional recurrence was separated into local (in breast) recurrence versus regional recurrence, in the surgery first group, the crude local recurrence rate was 4% (94/2331) and the regional recurrence rate was 1% (24/2331). In the neoadjuvant chemotherapy group, 6% (38/653) had a local recurrence and 2% (16/652) had a regional recurrence.
Figure 1.
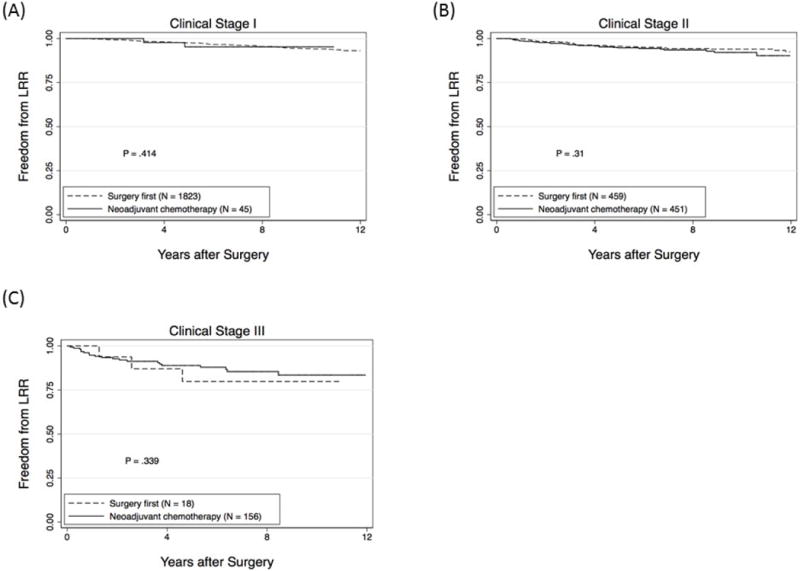
Local-regional recurrence (LRR)-free survival rates according to whether patients underwent surgery first or received neoadjuvant chemotherapy for patients presenting with clinical stage I (A), II (B) or III (C) disease.
With respect to distant metastasis, 7% (165/2331) of the surgery first group experienced a distant metastasis versus 16% (104/652) of those receiving neoadjuvant chemotherapy (P<0.001). Rates of DMFS were significantly worse (P<0.001) in the neoadjuvant chemotherapy group. Five- and 10-year DMFS rates were 95% (95% CI, 94-96) and 92% (95% CI, 91-94), respectively in the surgery first group and 87% (95% CI, 84-89) and 81% (95% CI, 77-84) in the neoadjuvant chemotherapy group (Figure 2A). Patients in the neoadjuvant chemotherapy group were more likely to experience a distant metastasis concurrently with a LRR; 57% of distant metastases occurred concurrently with LRR, whereas 31% occurred after LRR. In the surgery first group, 38% of distant metastases occurred concurrently with LRR and 52% occurred after LRR. DSS was significantly worse (P<0.001) in the neoadjuvant chemotherapy group (Figure 2B), consistent with the greater percentage of patients with clinical stage II or III disease.
Figure 2.
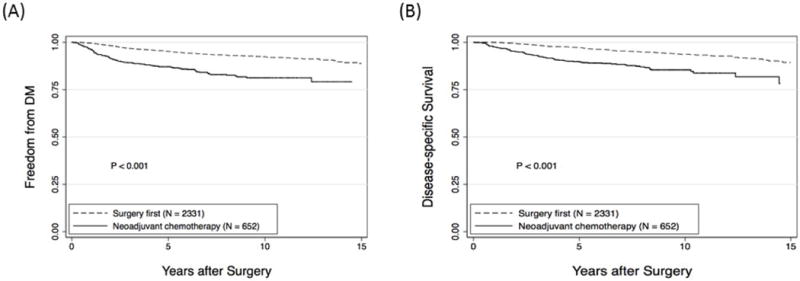
Comparison of (A) distant metastasis-free survival and (B) disease-specific survival for patients undergoing surgery first versus those receiving neoadjuvant chemotherapy.
Risk factors associated with local-regional recurrence
A multivariate analysis of LRR with stepwise inclusion of factors was performed for the entire cohort using all variables from table 1 as candidate factors except for adjuvant therapy in the surgery first group. Factors significant on statistical analysis are shown in table 2. When clinical T stage was considered as a candidate factor, represented as 3 dichotomous variables (T1 versus T2-T4, T1-T2 versus T3-T4 and T1-T3 versus T4), it was not selected as significant. When administration of neoadjuvant chemotherapy was added to the model fit with the 8 adverse factors listed in table 2, it was not significant (P=0.32), suggesting that when controlling for these other factors, there was no difference in LRR for patients undergoing surgery first compared to those receiving neoadjuvant chemotherapy.
Table 2.
Multivariate analysis for factors associated with a local-regional recurrence in breast cancer patients undergoing breast conserving therapy
| Factor | Hazard Ratio | Standard Error | P value | 95% Confidence Interval |
|---|---|---|---|---|
| Age < 50 | 1.91 | .32 | <0.001 | 1.37-2.65 |
| Clinical stage III | 2.52 | .56 | <0.001 | 1.63-3.90 |
| Nuclear grade 3 | 1.90 | .35 | 0.001 | 1.32-2.73 |
| ER negative | 2.35 | .50 | <0.001 | 1.55-3.56 |
| ER positive/no endocrine therapy | 2.82 | .65 | <0.001 | 1.79-4.42 |
| LVI present | 1.49 | .29 | 0.039 | 1.02-2.17 |
| Multifocal disease | 1.90 | .49 | 0.012 | 1.15-3.13 |
| Close/positive margins | 2.53 | .70 | 0.001 | 1.47-4.35 |
Abbreviations: ER, estrogen receptor; LVI, lymphovascular invasion
To further investigate factors associated with LRR, we repeated the multivariate analysis for the individual groups. For patients undergoing surgery first, adjuvant chemotherapy was included as a variable in this analysis. Significant factors for LRR in patients undergoing surgery first versus those receiving neoadjuvant chemotherapy are shown in table 3. For patients receiving neoadjuvant chemotherapy, path stage I disease or greater was a significant factor. We therefore looked at LRR rates for patients who achieved a pCR versus those who did not achieve a pCR. The 5- and 10-yr LRR-free survival rates were 97% (95% CI, 92-99) and 93% (95% CI, 82-97) for patients achieving a pCR versus 93% (95% CI, 90-95) and 90% (95% CI, 86-92) for patients not achieving a pCR (P=0.06). Overall, the results of these analyses revealed that factors associated with LRR were similar between the two groups. There were no adverse factors identified that would preclude an attempt at segmental mastectomy as long as the lesion could be resected with a clear margin.
Table 3.
Multivariate analysis for factors associated with a local-regional recurrence in breast cancer patients undergoing surgery first and in patients receiving neoadjuvant chemotherapy
| Surgery First | Neoadjuvant Chemotherapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | Hazard Ratio | Standard Error | P value | 95% Confidence Interval | Factor | Hazard Ratio | Standard Error | P value | 95% Confidence Interval |
| Clinical stage III | 4.13 | 2.43 | o.016 | 1.3-13.11 | Clinical stage III | 1.97 | .56 | 0.017 | 1.13-3.44 |
| Nuclear grade 3 | 1.69 | .37 | 0.015 | 1.11-2.60 | Nuclear grade 3 | 3.39 | 1.56 | 0.008 | 1.37-8.36 |
| ER negative | 2.18 | .58 | 0.003 | 1.30-3.67 | ER negative | 2.00 | .63 | 0.027 | 1.08-3.69 |
| Close/positive margins | 2.56 | .86 | 0.005 | 1.33-4.94 | Close/positive margins | 3.04 | 1.47 | 0.022 | 1.17-7.86 |
| ER positive/no endocrine therapy | 2.88 | .76 | <0.001 | 1.72-4.84 | LVI present | 2.30 | .71 | 0.007 | 1.26-4.20 |
| Multifocal Disease | 1.89 | .61 | 0.048 | 1.01-3.55 | Path stage ≥ I | 2.73 | 1.21 | 0.024 | 1.14-6.52 |
| Age < 50 | 2.18 | .45 | <.001 | 1.45-3.27 | |||||
Abbreviations: ER, estrogen receptor; LVI, lymphovascular invasion
Taken together, these data suggest that LRR after BCT is driven largely by biologic factors and not the timing of chemotherapy. To further investigate this, we determined LRR-free survival for patients undergoing surgery first versus those who received neoadjuvant chemotherapy based on the number of adverse factors per patient from the eight factors identified on multivariate analysis. As shown in figure 3, in patients with 0, 1, 2, 3 or 4 factors, there was no significant difference in LRR-free survival for surgery first versus neoadjuvant chemotherapy. There were too few patients with 5 (7 surgery first, 18 neoadjuvant chemotherapy), or 6 (1 surgery first, 3 neoadjuvant chemotherapy) factors to perform statistical analyses. No patients had 7 or 8 adverse factors.
Figure 3.
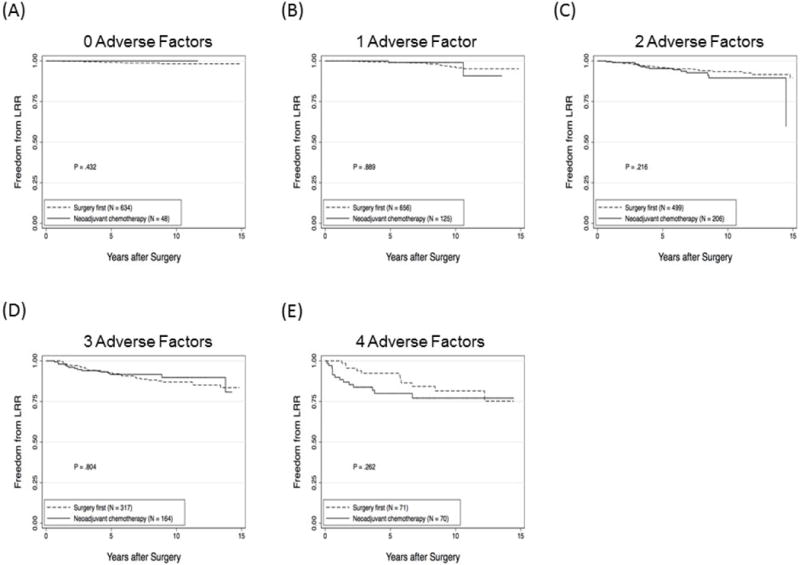
Local-regional recurrence (LRR)-free survival rates for patients undergoing surgery first or receiving neoadjuvant chemotherapy based on the number of adverse factors for each patient. (A) zero factors, (B) one factor, (C) two factors, (D) 3 factors, or (E) 4 factors.
DISCUSSION
Breast conserving therapy is the preferred treatment for patients with early stage breast cancer and is increasingly used in patients with larger tumors and later stage disease whose tumors respond to neoadjuvant chemotherapy. In the current study, we report excellent rates of local-regional control with BCT in appropriately selected patients undergoing upfront surgery or following neoadjuvant chemotherapy. There are no differences in LRR-free survival when evaluated by presenting clinical stage. Importantly, our data demonstrate that LRR is driven largely by biologic factors, including presenting stage, grade, ER status and presence of LVI or multifocal disease after chemotherapy. When controlling for these adverse factors, there is no difference in LRR rates between patients undergoing surgery first versus those treated with neoadjuvant chemotherapy.
With 652 patients undergoing BCT after neoadjuvant chemotherapy, this study represents the largest series to date. This work builds on a previous report from our institution of 340 patients where, after a 60 month median follow-up, we noted a 5-year rate of LRR-free survival of 91%. 22 In the current series, which includes patients from the previous study, we continue to see excellent local-regional control in patients selected for BCT after neoadjuvant chemotherapy with 5- and 10-year LRR-free survival rates of 93% and 90%, respectively. Given that patients receiving neoadjuvant chemotherapy had more advanced stage and significantly worse clinicopathologic characteristics at presentation, these results compare favorably with the 5- and 10-year LRR-free survival rates in patients undergoing upfront surgery (97% and 94%, respectively). The differences in adverse biologic factors between the two groups are reflected in the significantly worse DMFS and DSS rates for the neoadjuvant chemotherapy group.
Data from the NSABP B18 trial represents the second largest series of patients undergoing BCT after neoadjuvant chemotherapy. In that trial, over 1500 patients were randomized to preoperative or postoperative chemotherapy. There were 503 women in the preoperative chemotherapy group that underwent BCT and after a 9.5 year median follow-up, the IBTR rate was 10.7%. This was not statistically significantly different than the 7.6% rate in 448 women treated with BCT followed by adjuvant chemotherapy. Interestingly, in the initial report of this trial, 10 there was a statistically significant increase in the IBTR rate in patients who were converted from mastectomy to BCT after preoperative chemotherapy (P=.04). At the 9.5 year follow-up, this difference persisted (15.9% in downstaged patients versus 9.9% in patients initially planned for BCT). However, when controlling for age and clinical tumor size, the difference did not persist (P=.14). 17 It has been suggested that higher rates of LRR in patients with large tumors that are downsized with neoadjuvant chemotherapy could represent more aggressive biologic behavior in these larger tumors. 23
Data in the current study cannot be directly compared to the NSABP B18 study because this was a retrospective review and we do not know how many patients were BCT candidates at presentation. Likewise, we do not know how many patients were a candidate for BCT after chemotherapy but ultimately chose to undergo mastectomy. As a surrogate marker, we did demonstrate a significant decrease in the percentage of patients that had pathologic stage II or III disease at the time of surgery compared with the patients who had stage II or III disease at presentation. One would hypothesize that downstaging facilitated BCT in many patients. Our favorable 5- and 10-year LRR-free survival rates in patients with clinical stage II or III disease that received neoadjuvant chemotherapy (93% [95% CI, 91-95] and 90% [95% CI, 87-93], respectively) confirms that a selective approach to BCT in these patients can achieve excellent local-regional control. An important aspect of our approach is careful preoperative imaging with an attempt to resect any residual radiographic abnormality, calcifications and the primary tumor site biopsy clip with a negative margin defined as > 2mm. This differs from the NSABP approach which defines a negative margin as “no ink on tumor”. An important caveat of our study is that data regarding rates of re-excision were not evaluated and we do not know how many patients had an attempt at BCT before defaulting to mastectomy due to the inability to obtain a negative margin. Data in the current study only details outcomes in patients who completed BCT, and over 95% of patients from both groups had a negative margin. Inability to secure a negative margin was found to be a significant factor associated with LRR and we would advise mastectomy when a negative margin cannot be achieved. A negative margin has also been shown to be important in patients with earlier stage disease undergoing BCT. 24 It is likely that optimizing surgical management by resecting all radiographic abnormalities with a negative margin reduces tumor burden such that the effectiveness of adjuvant radiation and systemic therapy is enhanced.
Two meta-analyses evaluating trials of neoadjuvant versus adjuvant therapy have reported that neoadjuvant chemotherapy was associated with an increase in the risk of LRR. 18, 19 Importantly, in both analyses, which included many of the same trials, this increased risk was driven by inclusion of studies where patients underwent primary radiation without surgery. In the meta-analysis by Mieog et al, when studies with inadequate local-regional treatment were excluded, there was no difference in the LRR rates between the neoadjuvant and adjuvant groups (HR=1.12; 95% CI: .92-1.37). 19 The trials included in this meta-analysis were not limited to patients undergoing BCT. When analyzed according to the type of surgery, BCT versus mastectomy, there were no differences in LRR rates. In both BCT and mastectomy patients, LRR rates were not impacted by timing of chemotherapy. 19 This is consistent with the findings from the current study where we found that neoadjuvant chemotherapy was not a significant factor when added to a multivariate model of factors associated with LRR. In addition, when we controlled for the presence of adverse factors, there was no difference in LRR rates for patients undergoing surgery first compared with those receiving neoadjuvant chemotherapy.
In conclusion, we have demonstrated that LRR after BCT is driven by biologic factors. In appropriately selected patients receiving neoadjuvant chemotherapy, BCT can be performed with low LRR rates. BCT should be limited to patients in whom a segmental mastectomy can be performed with negative margins and should include whole-breast irradiation in all cases with selective use of RNI.
Acknowledgments
Sources of Support: This study was supported by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA01667233
Footnotes
Presented in part at the ASCO Breast Cancer Symposium, San Francisco, CA, September 8-10, 2011.
References
- 1.Arriagada R, Le MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14(5):1558–64. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 2.Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992;11:19–25. [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 4.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98(4):697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 5.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–50. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 7.Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007;14(4):1458–71. doi: 10.1245/s10434-006-9236-0. [DOI] [PubMed] [Google Scholar]
- 8.Cleator SJ, Makris A, Ashley SE, et al. Good clinical response of breast cancers to neoadjuvant chemoendocrine therapy is associated with improved overall survival. Ann Oncol. 2005;16(2):267–72. doi: 10.1093/annonc/mdi049. [DOI] [PubMed] [Google Scholar]
- 9.Danforth DN, Jr, Cowan K, Altemus R, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: a prospective randomized trial. Ann Surg Oncol. 2003;10(6):635–44. doi: 10.1245/aso.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 11.Forouhi P, Dixon JM, Leonard RC, Chetty U. Prospective randomized study of surgical morbidity following primary systemic therapy for breast cancer. Br J Surg. 1995;82(1):79–82. doi: 10.1002/bjs.1800820127. [DOI] [PubMed] [Google Scholar]
- 12.Gazet JC, Ford HT, Gray R, et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Ann Oncol. 2001;12(5):685–91. doi: 10.1023/a:1011115107615. [DOI] [PubMed] [Google Scholar]
- 13.Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS) Ann Oncol. 1999;10(1):47–52. doi: 10.1023/a:1008337009350. [DOI] [PubMed] [Google Scholar]
- 14.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 15.Semiglazov VF, Topuzov EE, Bavli JL, et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann Oncol. 1994;5(7):591–5. doi: 10.1093/oxfordjournals.annonc.a058929. [DOI] [PubMed] [Google Scholar]
- 16.van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–37. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 17.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 18.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–94. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 19.Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94(10):1189–200. doi: 10.1002/bjs.5894. [DOI] [PubMed] [Google Scholar]
- 20.Krishnamurthy S, Sneige N, Bedi DG, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95(5):982–8. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 21.Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244(3):464–70. doi: 10.1097/01.sla.0000234897.38950.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303–12. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 23.Buchholz TA, Lehman CD, Harris JR, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008;26(5):791–7. doi: 10.1200/JCO.2007.15.0326. [DOI] [PubMed] [Google Scholar]
- 24.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46(18):3219–32. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]


