Abstract
Lessons Learned.
Ablation therapy appears to be a reasonably safe and effective approach to obtain a significant treatment‐free interval for a subset of patients with limited sites of metastatic disease for which systemic control can be obtained with six cycles of chemotherapy.
Background.
Metastatic sarcoma often becomes resistant to treatment by chemotherapy. There is sometimes prolonged stable disease from active chemotherapy that provides a window of opportunity for an intervention to prolong disease‐free survival.
Materials and Methods.
We performed a phase II study in patients with metastatic sarcoma who had been stable on six cycles of chemotherapy who then received ablation therapy to their residual disease. Histologies captured in this study included leiomyosarcoma, malignant peripheral nerve sheath tumor, pleiomorphic rhabdomyosarcoma, and myxoid liposarcoma. Sites ablated included lung metastases and retroperitoneal metastatic deposits. In this study, up to three lesions were ablated in any given interventional radiology session. After ablation, patients were not treated with any further therapy but were followed by surveillance imaging to determine progression‐free rate (PFR).
Results.
Although terminated early because of slow accrual, this study demonstrated a 3‐month PFR of 75% for this cohort of eight patients treated with ablation performed after completion of six cycles of chemotherapy with stable disease. Median progression‐free survival (PFS) was 19.74 months, and the median overall survival (OS) was not reached.
Conclusion.
Our data are the first prospective study to suggest that ablation therapy in selected patients who are stable on chemotherapy can provide a significant progression‐free interval off therapy and warrants further study in a randomized trial.
Abstract
经验总结
消融疗法似乎是一种适度安全且有效的方法,能够使转移性病灶有限的患者亚群(已通过六周期化疗得到全身控制)获得有意义的无治疗间期。
摘要
背景.转移性肉瘤通常会对化疗治疗产生耐药性。积极化疗有时可延长病情稳定期,可为延长无病生存期的干预措施提供时机窗口。
材料和方法.我们对转移性肉瘤患者进行了II期研究,这些患者经过六周期化疗病情稳定后针对残留病灶接受了消融治疗。本研究中收集的组织学结果包括平滑肌肉瘤、恶性外周神经鞘瘤、多形性横纹肌肉瘤和粘液样脂肪肉瘤。消融部位包括肺转移瘤和腹膜后转移性沉积。在此研究中,在任何给定的介入放射治疗中最多消融三处病灶。消融后,患者未接受任何进一步的治疗,但随后接受监测成像以确定无进展率(PFR)。
结果.虽然由于招募工作进展缓慢而提前终止,但该研究表明,在完成六个化疗周期且病情稳定后进行消融治疗的八例患者组成的队列,3个月PFR为75%。中位无进展生存期(PFS)为19.74个月,未达到中位总生存期(OS)。
结论.我们的数据是首项前瞻性研究,表明针对经过化疗病情稳定的选择性患者进行的消融治疗可以提供有意义的无进展无治疗间期,需要在随机试验中进行进一步研究。
Discussion
Sarcomas are rare cancers that encompass a group of an estimated 70 different histologic subtypes with varying biology [1]. Given the diversity of these tumors, a single drug therapy is not likely to be successful across all subtypes [2].
In this phase II trial of patients with metastatic soft tissue sarcoma, we demonstrate a 3‐month PFR of 75% after ablation. Based on prior studies, this degree of response certainly supports the hypothesis that ablation after stability on chemotherapy can serve as a well‐tolerated maintenance therapy and provide a significant PFS along with a chemotherapy‐free interval for patients with metastatic soft tissue sarcoma [7], [8].
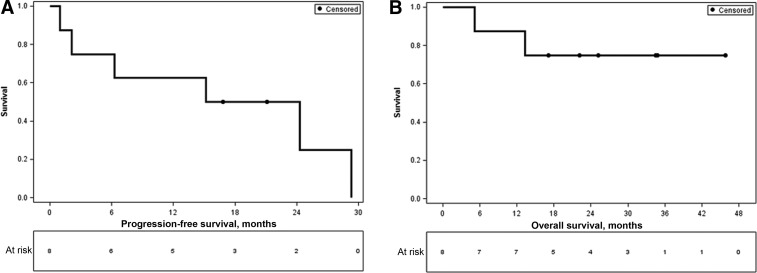
Unfortunately, this study was closed early because of low accrual at a single center. Nonetheless, most patients on trial did very well, and median overall survival had not been reached at the time of manuscript preparation (Figure 1). Furthermore, we report a median PFS of 19.7 months compared with the 13.4 months reported for pulmonary metastasectomy in sarcoma, suggesting that ablation therapy is a viable option to a surgical metastasectomy [3], [4], [5], [9], [10]. Additionally, ablation, which has a quick recovery time, can be used on lesions such as bone metastases, liver metastases, and various visceral sites that may pose more of a challenge for surgical intervention, especially in cases in which more than one organ site is involved in the same patient [4].
Figure 1.
Kaplan‐Meier curves for progression‐free and overall survival. (A): Progression‐free survival. (B): Overall survival.
In conclusion, we have shown a 75% PFR with a median PFS of 19.74 months for patients stable on chemotherapy who then underwent ablation of residual sites of disease, strongly supporting ablation as a potential form of maintenance therapy for soft tissue sarcomas (Figure 1).
Trial Information
- Disease
Sarcomas – Adult
- Stage of Disease/Treatment
Metastatic/Advanced
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Progression‐free rate (PFR)
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Quality of life
- Additional Details of Endpoints or Study Design
- Patients
- This study was performed under an active Human Studies Protocols approved by the Institutional Review Boards at Washington University in St. Louis (201309108) in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This trial was registered on ClinicalTrials.gov (NCT01986829). Patients with histologically or cytologically confirmed high‐grade metastatic sarcoma that had been stable on 6–12 cycles of one chemotherapeutic regimen (cytotoxic or biologic) were recruited from the Washington University Sarcoma Clinic. Patients had to be at least 18 years of age. Other patient entry criteria included measurable disease defined as lesions that could be accurately measured in at least one dimension (longest diameter to be recorded) as >10 mm with CT, positron emission tomography/CT, or magnetic resonance imaging; Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and normal bone marrow and organ function. The patients could have no more than 10 treatable lesions, as evaluated by an experienced interventional radiologist for eligibility and technical accessibility. The lesions had to be amenable to a safe, ultrasound/computed tomographic guided percutaneous approach, as assessed by one of the three interventional radiologists involved in the study. The targeted metastases had to be sufficiently separateable from the central nervous system, major peripheral motor nerves, bowel, and bladder. Key exclusion criteria were: (a) history of another malignancy within 5 years, (b) known brain metastasis, (c) patients receiving other investigational agents, (d) intercurrent illness that would limit compliance with the study, (e) pregnancy, and (f) patients wishing to receive chemotherapy after ablation.
- Study Design and Procedures
- Patients whose disease was stable on six cycles of standard‐of‐care chemotherapy and who met the above inclusion criteria were offered the option of enrolling in this trial.
- Eligible patients could have had their lesions ablated using cryoablation, radiofrequency ablation, or microwave ablation. These techniques are thought to be equivalent. The choice of the procedure was based on the expertise of the interventional oncologic radiologist and the site of metastasis. Cryoablation was used on all eight patients in the current study.
- Cryoablation of the soft tissue and parenchymal metastases were performed under CT guidance with cryoprobes from Endocare Inc. (Irvine, CA) or Galil Medical (Arden Hills, MN). For cryoablation, a single freeze‐thaw‐freeze cycle was performed for each lesion. The freezing portions of the cycle varied depending on size of the ice ball and adequacy of coverage and proximity to adjacent critical structures. Nonenhanced computed tomography was performed every 3 to 5 minutes, with soft tissue windows throughout the freezing cycle to monitor growth of the ice ball. After completion of the final freeze cycle of the cryoablation procedure, the cryoprobes were actively heated with helium until temperature was above 20°C, and then the probe(s) was (were) withdrawn.
- Ablations were performed according to the manufacturer's recommendations in the package insert. After completion of ablation, tract ablation occasionally was performed as the probe was withdrawn. Procedures were performed on an outpatient basis, but patients were admitted for observation after the procedure as deemed appropriate by the treating interventional radiologist. Patients were generally under moderate conscious sedation with continuous nurse monitoring of pulse oximetry, blood pressure, cardiac rhythm and rate, and respirations. One percent lidocaine alone or a 1:1 mixture of 1% lidocaine and 0.25% bupivicaine or 0.5% ropivicaine was used for local anesthesia.
- Chemotherapy was stopped at initiation of ablation therapy, and ablation therapy was completed within 3 weeks of enrollment. Patients were followed with CT scans every 9 weeks for the first year, every 12 weeks for the second year, and then every 6 months until a new biopsy‐proven lesion or a previously ablated lesion grew by 20% in size. Additionally, patients completed a quality of life assessment the 7‐item functional assessment of cancer therapy‐general (FACT‐G7) prior to ablation, 1 month after ablation, and at progression.
- Study Outcome and Statistical Analysis
- The primary outcome was to determine the PFR at 3 months. Three‐month PFR was defined as the percentage of patients with no progression (local recurrence of an ablated lesion or the appearance of a new lesion) at 3 months after ablation. Secondary outcomes included overall survival and quality‐of‐life measures.
- Our power analysis was based on the following hypotheses. The null hypothesis assumed that the 3‐month progression‐free survival (PFS) for treatment with ablation therapy would be overall 20% for patients with metastatic sarcoma. We hypothesized in the alternative hypothesis that the 3‐month PFS would be at least 40% with ablation therapy. This was based on data from a clinical trials database used to provide reference values for conducting phase II studies in sarcoma with PFR as the principal endpoint [14]. An enrollment of 36 patients was intended to achieve at least 80% power to reject the null hypothesis based on a two‐sided one‐sample proportion test at a significance level of 0.05. This trial was closed early, however, because of slow accrual.
- Descriptive statistics were used to describe the characteristics and adverse events of the patients. OS was defined as the time from prior to chemotherapy to death from any cause, and PFS was defined as the time prior to ablation to documented disease progression or death. The Kaplan‐Meier product limit method was applied to estimate the empirical survival probabilities for OS and PFS. Median survival times were estimated. The Wilcoxon signed‐rank test was used to determine whether there was an improvement in quality of life after ablation versus baseline. All p values were two‐sided, and significance was claimed at the 5% level. All statistical analyses were performed using the statistical software SAS (version 9.4, SAS Institute, Cary, NC).
- Investigator's Analysis
Activity suggested and should be further pursued
Patient Characteristics
- Number of Patients, Male
4
- Number of Patients, Female
4
- Stage
Metastatic
- Age
Median (range): 60 years
- Number of Prior Systemic Therapies
Median (range): 1
- Performance Status: ECOG
-
0 — 8
1 —
2 —
3 —
Unknown —
- Cancer Types or Histologic Subtypes
- leiomyosarcoma
- malignant peripheral nerve sheath tumor
- pleiomorphic rhabdomyosarcoma
- myxoid liposarcoma
Primary Assessment Method for Phase II Control
- Title
Total Patient Population
- Number of Patients Screened
9
- Number of Patients Enrolled
8
- Number of Patients Evaluable for Toxicity
8
- Number of Patients Evaluated for Efficacy
8
- Evaluation Method
RECIST, version 1.1
- Response Assessment CR
n = 6 (75%)
- (Median) Duration Assessments PFS
19.74 months
Adverse Events
Adverse Events Legend
Two patients experienced adverse events, which are summarized in the above table. One patient developed a pneumothorax and a small pleural effusion that resolved. The second patient developed a hemopneumothorax and died 1 month after the procedure. The second patient who experienced an adverse event had required two ablation procedures because she had lesions in both lungs. Although this patient had stable disease at the end of six cycles of chemotherapy, she was found to have progressive disease (rapid increase in size of one of the nodules to be ablated) at the time of the second ablation procedure and afterwards continued to experience rapid progression of her disease.
Abbreviation: NC/NA, no change from baseline, no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated reason
Did not fully accrue
- Investigator's Assessment
Activity suggested and should be further pursued
Sarcomas encompass a group of an estimated 70 different histologic subtypes with varying biology [1]. There are approximately 15,000 new cases of sarcoma per year in the United States, accounting for about 1% of adult malignancies [2]. Prognosis is poor for patients with metastatic disease, with a median overall survival of only 12–14 months. Given the biological diversity of these tumors, a single drug therapy is not likely to be successful across all subtypes [3]. As such, novel and multidisciplinary approaches will be imperative to improve survival.
Cytotoxic chemotherapy is the mainstay of therapy for metastatic sarcoma. This alone, however, is very unlikely to result in a durable remission or cure. The combination of chemotherapy with resection of pulmonary metastases has been shown to increase the 3‐year overall survival in metastatic osteosarcoma from approximately 5% to 65% [4]. Similar data exist for soft tissue sarcomas as well [5], [6]. Unfortunately, not all metastases are amenable to resection.
An alternative procedural approach to treating metastatic cancer includes ablation therapy. There are several types of ablation procedures, including radiofrequency ablation, cryoablation, irreversible electroporation, and microwave ablation [7], [8], [9], [10], [11]. Each technique has its merits and disadvantages, but their results are thought to be equivalent, and the choice of which type of ablation to use is typically based on the site of metastasis and operator preference.
There are retrospective data suggesting that radiofrequency ablation is safe in patients with sarcoma with lung metastases with a 3‐year overall survival of 65%, similar to what is quoted in surgical studies [12]. Given these data, we performed this single‐arm prospective phase II trial of ablation therapy in patients with metastatic sarcoma who had fewer than 10 lesions and whose disease was stable on chemotherapy. These patients were stable on 6–12 cycles of cytotoxic chemotherapy, as this is the natural stopping point for doxorubicin‐based chemotherapy, which is the standard treatment in soft tissue sarcoma [13]. Ablation therapy then served as a form of maintenance therapy.
In this early terminated phase II trial of patients with metastatic soft tissue sarcoma, we demonstrated a 3‐month progression‐free rate (PFR) of 75% with a median PFS of 19.74 months after ablation. Based on prior studies, this magnitude of response certainly supports the hypothesis that ablation after stability on chemotherapy can serve as a well‐tolerated maintenance therapy and provide a significant PFS along with a chemotherapy‐free holiday for patients with metastatic soft tissue sarcoma [14], [15]. Furthermore, median overall survival has not been met to date, and several patients are still being monitored off any therapy after ablation (Figure 1). The antitumor mechanisms may be twofold. First, there may be direct antitumor effects associated with the ablation process. Additionally, there are data from other studies suggesting immune modulation after ablation therapy as evidenced by increases in levels of cytokines such as interleukin‐6 (IL‐6) and tumor necrosis factor (TNF), as well as tumor‐antigen‐specific T cells in the bloodstream after ablation [16], [17].
Unfortunately, this study was closed early because of low accrual at a single center. There were two major reasons for this. The first was that there was a limited population of patients with metastatic sarcoma who were able to maintain stable disease for six cycles of chemotherapy, indicating that this will not be an option for all patients. Second, given that metastatic sarcomas are not curable, most academic centers offer clinical trials for patients with metastatic disease, and most clinical trials maintain patients on therapy until progression. As such, after discussing all options, many patients chose to enroll in another clinical trial rather than pursue a standard‐of‐care regimen with the hope that they would obtain stable disease for six cycles in order to be eligible to consent for this ablation study. Nonetheless, most patients on trial did very well, and median overall survival had not been reached at the time of manuscript preparation. Furthermore, we report a median PFS of 19.7 months compared with the 13.4 months reported for pulmonary metastectomy in sarcoma, suggesting that ablation therapy is a viable option to a surgical metastectomy [4], [5], [6], [18], [19]. Additionally, ablation, which has a quick recovery time, is able to be used on lesions, such as bone metastases, liver metastases, and various visceral sites, that may pose more of a challenge for surgical intervention, especially in cases in which more than one organ site is involved in the same patient [5].
We reported two adverse events in this study. One patient developed a pneumothorax and pleural effusion that required hospitalization. That patient was treated with a chest tube and antibiotics, recovered, and had a 6‐month PFS after recovery. The second patient required two ablation procedures, which were spaced by 2 weeks. In that 2‐week period, the patient demonstrated significant progression despite having stable scans at the end of chemotherapy. She subsequently developed a hemopneumothorax after a second ablation procedure. During hospitalization, she developed rapidly progressive disease and passed away 1 month after ablation. None of the other patients experienced any adverse events. Overall, ablation is a safe and well‐tolerated procedure for sarcomas [[12], [20], 21].
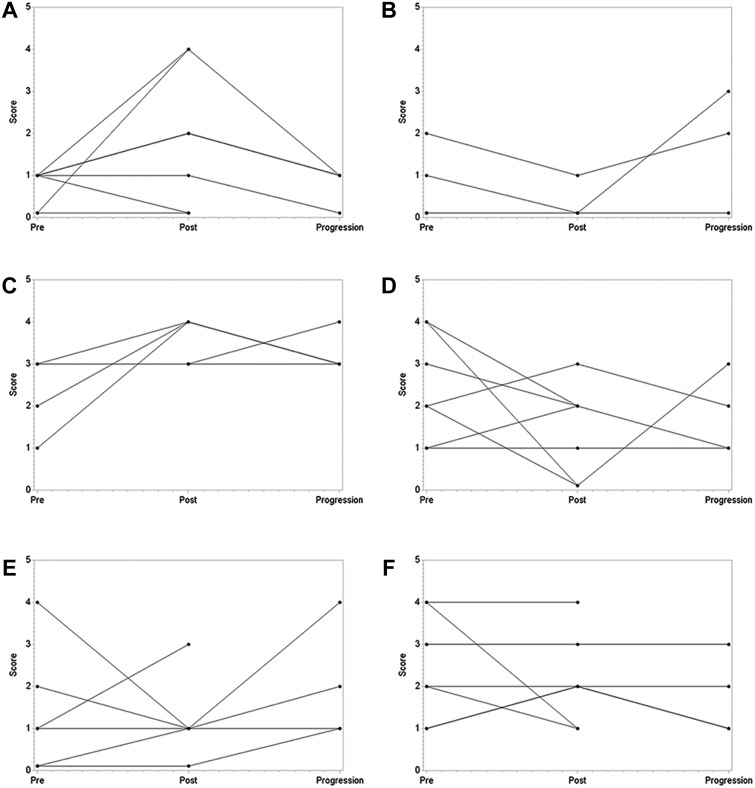
Although no statistically significant changes were reported in quality‐of‐life measures, several observations were made. Most patients reported increased pain on the survey performed after the ablation procedure. That symptom resolved in all patients assessed at their next follow‐up appointment in clinic and was thought to be because of the discomfort associated with the procedure, not a change in the pain related to their malignancy. Most patients reported a decrease in nausea, improvement in energy, decreased worry, and overall improvement in quality of life (Figure 2).
In conclusion, we report the results of a phase II trial for patients with metastatic sarcoma stable on six cycles of chemotherapy who then underwent ablation of the residual metastatic sites. This is the first prospective examination of ablation therapy in metastatic sarcoma. Furthermore, we have shown a 75% PFR with a median PFS of 19.74 months, strongly supporting this as a beneficial form of maintenance therapy.
Figures and Tables
Figure 2.
Quality of life (QOL) measurements before ablation, after ablation, and at progression. (A): Pain. (B): Nausea. (C): QOL. (D): Lack of energy. (E): Worry. (F): Sleeping well.
Acknowledgments
The authors thank the patients who participated in this study. Additionally, the authors thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes‐Jewish Hospital in St. Louis, MO, for the use of the Biostatistics Shared Resource, which provided statistical support. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA091842, Eberlein, PI. The authors also thank the Division of Oncology at Washington University for the generous support that funded this study.
Footnotes
ClinicalTrials.gov Identifier: NCT01986829
Sponsor(s): Division of Oncology, Washington University School of Medicine
Principal Investigator: Brian A. Van Tine
IRB Approved: Yes
Disclosures
Jack Jennings: Merit, Medtronic (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Wardelmann E, Schildhaus HU, Merkelbach‐Bruse S et al. Soft tissue sarcoma: From molecular diagnosis to selection of treatment. Pathological diagnosis of soft tissue sarcoma amid molecular biology and targeted therapies. Ann Oncol 2010;21(suppl 7):vii265–vii269. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer Facts and Figures 2017. Atlanta, GA: American Cancer Society; 2017. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed August 16, 2017.
- 3. Pang A, Carbini M, Maki RG. Contemporary therapy for advanced soft‐tissue sarcomas in adults: A review. JAMA Oncol 2016;2:941–947. [DOI] [PubMed] [Google Scholar]
- 4. Carter SR, Grimer RJ, Sneath RS et al. Results of thoracotomy in osteogenic sarcoma with pulmonary metastases. Thorax 1991;46:727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blackmon SH, Shah N, Roth JA et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long‐term survival. Ann Thorac Surg 2009;88:877–884; discussion 884–875. [DOI] [PubMed] [Google Scholar]
- 6. Rehders A, Hosch SB, Scheunemann P et al. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg 2007;142:70–75; discussion 76. [DOI] [PubMed] [Google Scholar]
- 7. Carrafiello G, Laganà D, Mangini M et al. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int J Surg 2008;6(suppl 1):S65–S69. [DOI] [PubMed] [Google Scholar]
- 8. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323–331. [DOI] [PubMed] [Google Scholar]
- 9. Ni Y, Mulier S, Miao Y et al. A review of the general aspects of radiofrequency ablation. Abdom Imaging 2005;30:381–400. [DOI] [PubMed] [Google Scholar]
- 10. Simon CJ, Dupuy DE, Mayo‐Smith WW. Microwave ablation: Principles and applications. Radiographics 2005;25(suppl 1):S69–S83. [DOI] [PubMed] [Google Scholar]
- 11. Tatli S, Acar M, Tuncali K et al. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol 2010;16:90–95. [DOI] [PubMed] [Google Scholar]
- 12. Palussière J, Italiano A, Descat E et al. Sarcoma lung metastases treated with percutaneous radiofrequency ablation: Results from 29 patients. Ann Surg Oncol 2011;18:3771–3777. [DOI] [PubMed] [Google Scholar]
- 13. Edmonson JH, Ryan LM, Blum RH et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–1275. [DOI] [PubMed] [Google Scholar]
- 14. Demetri GD, Blay JY, Casali PG. Advances and controversies in the management of soft tissue sarcomas. Future Oncol 2017;13:3–11. [DOI] [PubMed] [Google Scholar]
- 15. Van Glabbeke M, Verweij J, Judson I et al. Progression‐free rate as the principal end‐point for phase II trials in soft‐tissue sarcomas. Eur J Cancer 2002;38:543–549. [DOI] [PubMed] [Google Scholar]
- 16. Osada S, Imai H, Tomita H et al. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol 2007;95:491–498. [DOI] [PubMed] [Google Scholar]
- 17. Haen SP, Pereira PL, Salih HR et al. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin Dev Immunol 2011;2011:160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schur S, Hoetzenecker K, Lamm W et al. Pulmonary metastasectomy for soft tissue sarcoma–Report from a dual institution experience at the Medical University of Vienna. Eur J Cancer 2014;50:2289–2297. [DOI] [PubMed] [Google Scholar]
- 19. van Geel AN, Pastorino U, Jauch KW et al. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer‐Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 1996;77:675–682. [DOI] [PubMed] [Google Scholar]
- 20. Gronchi A, Guadagnolo BA, Erinjeri JP. Local ablative therapies to metastatic soft tissue sarcoma. Am Soc Clin Oncol Educ Book 2016;35:e566–e575. [DOI] [PubMed] [Google Scholar]