Abstract
Lessons Learned.
Treatment with BEZ235 has not been shown to demonstrate increased efficacy compared with everolimus and may be associated with a poorer tolerability profile.
The hypothesis of dual targeting of the phosphatidylinositol 3‐kinase and mammalian target of rapamycin pathways in patients with advanced pancreatic neuroendocrine tumors may warrant further study using other agents.
Background.
This phase II study investigated whether targeting the phosphatidylinositol 3‐kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway via PI3K, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) inhibition using BEZ235 may be more effective than mTORC1 inhibition with everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET) who are naïve to mTOR inhibitor therapy.
Methods.
Patients with advanced pNET were randomized (1:1) to oral BEZ235 400 mg twice daily or oral everolimus 10 mg once daily on a continuous dosing schedule. The primary endpoint was progression‐free survival (PFS). Secondary endpoints included safety, overall response rate (ORR), overall survival (OS), and time to treatment failure.
Results.
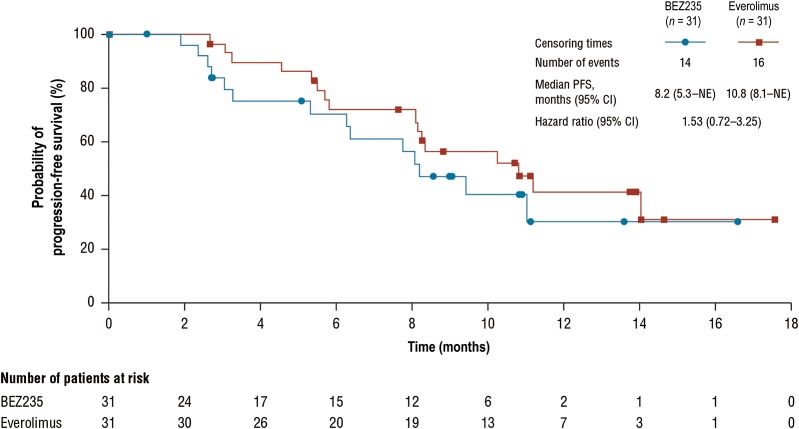
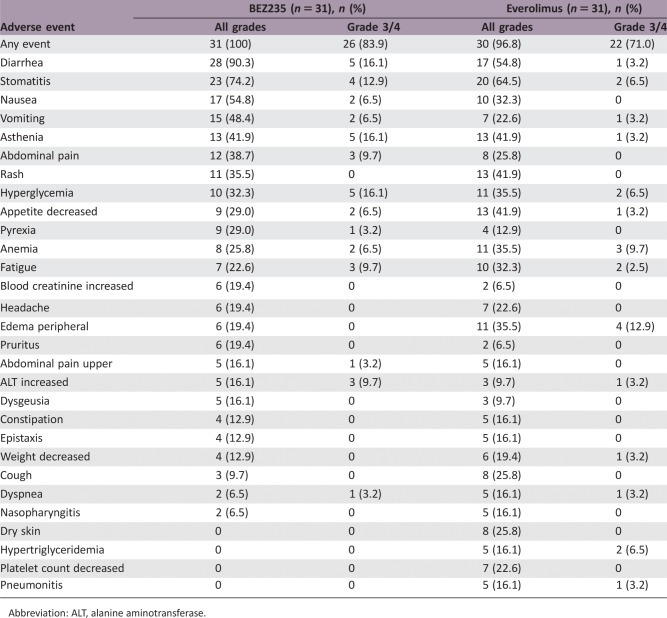
Enrollment in this study was terminated early (62 enrolled of the 140 planned). The median PFS was 8.2 months (95% confidence interval [CI]: 5.3 to not evaluable [NE]) with BEZ235 versus 10.8 months (95% CI: 8.1–NE) with everolimus (hazard ratio 1.53; 95% CI: 0.72–3.25). The most commonly reported all‐grade adverse events (>50% of patients regardless of study treatment relationship) with BEZ235 were diarrhea (90.3%), stomatitis (74.2%), and nausea (54.8%).
Conclusion.
BEZ235 treatment in mTOR inhibitor‐naïve patients with advanced pNET did not demonstrate increased efficacy compared with everolimus and may be associated with a poorer tolerability profile.
Abstract
经验总结
• 与依维莫司相比,BEZ235 治疗未显示出疗效增加,可能与耐受性较差相关。
• 晚期胰腺神经内分泌肿瘤患者中磷脂酰肌醇3‐激酶和哺乳动物雷帕霉素靶蛋白通路的双靶向假设可能需要使用其他药物进行进一步研究。
摘要
背景.该II期研究考察的内容是,在未接受过哺乳动物雷帕霉素靶蛋白(mTOR)抑制剂治疗的晚期胰腺神经内分泌肿瘤(pNET)患者中,使用BEZ235靶向通过磷脂酰肌醇3‐激酶(PI3K)、mTOR复合物1(mTORC1)和mTOR复合物2(mTORC2)抑制的PI3K/mTOR通路是否比使用依维莫司的mTORC1抑制效果更好。
方法.晚期pNET患者随机以连续给药方案(1:1)口服BEZ235 400 mg每天两次或口服依维莫司10 mg每天一次。主要终点为无进展生存期(PFS)。次要终点包括安全性、总缓解率(ORR)、总生存期(OS)和至治疗失败的时间。
结果.本研究的入组提前终止(计划入组140名,实际入组62名)。BEZ235组的中位PFS为8.2个月[95%置信区间(CI):5.3至无法评估(NE)],而依维莫司组为10.8个月(95% CI:8.1‐NE)(风险比 1.53;95% CI:0.72‐3.25)。BEZ235最常报告的所有等级不良事件(>50%的患者,不考虑研究治疗关系)为腹泻(90.3%)、口腔炎(74.2%)和恶心(54.8%)。
结论.未接受过mTOR抑制剂治疗的晚期pNET患者的BEZ235治疗未显示出与依维莫司相比增强的疗效,这可能与耐受性较差相关。
Discussion
This phase II study aimed to investigate whether targeting the PI3K/mTOR pathway via PI3K, mTORC1, and mTORC2 adenosine triphosphate (ATP) site inhibition using BEZ235 is more effective than mTORC1 allosteric inhibition with everolimus in patients with advanced pNET who are naïve to mTOR inhibition therapy. However, emerging data suggesting an unfavorable safety profile and unpredictable bioavailability led to the sponsor's decision to halt the development of BEZ235 in all oncology indications including pNET, and enrollment in this study was terminated before the planned 70 patients had been randomized in each treatment arm and before a preplanned primary analysis after 70 disease progression events was reached.
The results of the study suggest that the efficacy of BEZ235 did not surpass that of everolimus in this setting (Fig. 1). The median PFS of 10.8 months observed with everolimus in this study is comparable to prior data in this setting (RADIANT‐3 trial; [1]). The median PFS of 8.2 months observed with BEZ235 exceeded that of 4.6 months in the placebo arm of RADIANT‐3, indicating some degree of efficacy [1]. ORR (9.7%) was similar in both groups, suggesting that a small degree of tumor shrinkage was observed with both treatments. Disease control rate was substantially lower with BEZ235 (61.3%) than with everolimus (90.3%), although the high rate of unknown tumor responses among patients in the BEZ235‐treated group (25.8%) versus the everolimus‐treated group (6.5%) precludes any meaningful comparison of disease stabilization between the groups. A small numerical difference in the estimated 6‐month OS rate was observed with BEZ235 (96.6%) versus everolimus (90.3%), which should be interpreted with caution due to the early termination of the study, limited number of patients, and very few on‐study deaths during the trial.
Figure 1.
Kaplan‐Meier plot of progression‐free survival per local radiologic review.
Abbreviations: CI, confidence interval; NE, not evaluable; PFS, progression‐free survival.
All adverse events (AEs) observed with BEZ235 and everolimus during the study were consistent with their known safety profiles and no unexpected events were reported [1], [2], [3], [4]. However, this study indicated that BEZ235 was potentially less well tolerated than everolimus. More grade 3/4 AEs were reported with BEZ235 (83.9%) versus everolimus (71.0%) and discontinuations due to AEs were twice as frequent with BEZ235 versus everolimus (38.7 vs. 16.1%, respectively). The poor tolerability of BEZ235 versus everolimus may explain why patients randomized to this treatment were exposed to study medication for almost half the duration of time as those randomized to everolimus (22.9 vs. 39.4 weeks, respectively). This short duration of treatment time may have negatively impacted BEZ235 efficacy outcomes.
mTOR inhibitor‐naïve patients with advanced pNET on treatment with BEZ235 did not demonstrate superior efficacy compared with everolimus and may have a poorer tolerability profile. However, the hypothesis of dual targeting of the PI3K and mTOR pathways in patients with advanced pNET may still warrant further study using other agents with more favorable safety profiles.
Trial Information
- Disease
Neuroendocrine
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
2 prior regimens
- Type of Study – 1
Phase II
- Type of Study – 2
Randomized
- Primary Endpoint
Progression‐free survival
- Secondary Endpoint
Safety
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Time to treatment failure
- Additional Details of Endpoints or Study Design
- The main analysis was originally intended to be performed after 70 patients had experienced progression of the disease or death due to any cause. Based on this, an indication for a longer PFS under BEZ235 would be achieved when the following two efficacy criteria are both met: (a) the estimated hazard ratio (HR) is ≤0.75 and (b) the posterior probability that the HR is <1 is at least 90%: Ppost (HR <1) ≥0.9. But due to early enrollment study termination on October 29, 2013, the 62 patients randomized were analyzed mainly for descriptive purpose after 6 months after the last patient started study treatment.
- Investigator's Analysis
Poorly tolerated/not feasible
Drug Information for Phase II Everolimus
- Drug 1
- Generic/Working Name
Everolimus
- Trade Name
Afinitor
- Company Name
Novartis Pharmaceuticals Corporation
- Drug Type
Targeted therapy
- Drug Class
m‐TOR
- Dose
10 mg per flat dose
- Route
p.o.
- Schedule of Administration
Once daily, every day (28‐day cycles)
Drug Information for Phase II BEZ235
- Drug 1
- Generic/Working Name
BEZ235
- Trade Name
Dactolisib
- Company Name
Novartis Pharmaceuticals Corporation
- Drug Type
Small molecule
- Drug Class
PI3 kinase
- Dose
400 mg per flat dose
- Route
p.o.
- Schedule of Administration
Twice daily, every day (28‐day cycles)
Patient Characteristics for Phase II Everolimus
- Number of Patients, Male
15
- Number of Patients, Female
16
- Age
Median: 57
- Performance Status: ECOG
-
0 — 20
1 — 11
2 — 0
3 —
Unknown —
Patient Characteristics for Phase II BEZ235
- Number of Patients, Male
17
- Number of Patients, Female
14
- Age
Median: 56 years
- Performance Status: ECOG
-
0 — 20
1 — 10
2 — 1
3 —
Unknown —
Primary Assessment Method for Phase II Everolimus
- Title
Total patient population
- Number of Patients Screened
31
- Number of Patients Enrolled
31
- Number of Patients Evaluable for Toxicity
31
- Number of Patients Evaluated for Efficacy
31
- Evaluation Method
Response Evaluation Criteria in Solid Tumors (RECIST) 1.0
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 3 (9.7%)
- Response Assessment SD
n = 25 (80.6%)
- Response Assessment PD
n = 1 (3.2%)
- Response Assessment OTHER
n = 2 (6.5%)
- (Median) Duration Assessments PFS
10.8 months
- (Median) Duration Assessments Duration of Treatment
39.4 weeks
Primary Assessment Method for Phase II BEZ235
- Title
Total patient population
- Number of Patients Screened
31
- Number of Patients Enrolled
31
- Number of Patients Evaluable for Toxicity
31
- Number of Patients Evaluated for Efficacy
31
- Evaluation Method
RECIST 1.0
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 3 (9.7%)
- Response Assessment SD
n = 16 (51.6%)
- Response Assessment PD
n = 4 (12.9%)
- Response Assessment OTHER
n = 8 (25.8%)
- (Median) Duration Assessments PFS
8.2 months
- (Median) Duration Assessments Duration of Treatment
22.9 weeks
Treatment‐Emergent Adverse Events (>15%)
Abbreviation: ALT, alanine aminotransferase.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Toxicity
- Investigator's Assessment
Poorly tolerated/not feasible
Two targeted agents have been approved for the treatment of locally advanced and metastatic pancreatic neuroendocrine tumors (pNET) based on improvements in progression‐free survival versus placebo: everolimus, a mammalian target of rapamycin (mTOR) inhibitor, and sunitinib, a multitargeted tyrosine kinase inhibitor. Everolimus therapy has demonstrated considerable clinical benefit in the treatment of advanced pNET. However, de novo and acquired resistance to therapy have been observed. Such resistance may stem from enhanced activation of the phosphatidylinositol 3‐kinase (PI3K)/mTOR pathway [5], [6], [7]. Everolimus specifically inhibits the mTOR complex 1 (mTORC1) but does not block mTOR complex 2 (mTORC2)‐mediated activation of AKT. It is postulated that activation of AKT by way of insulin‐like growth factor 1/insulin‐like growth factor 1 receptor and mTORC2 signaling activation due to the inhibition of S6 kinase (S6K) negative feedback are mechanisms of everolimus treatment resistance [1], [8]. Samples from patients with advanced solid tumors and neuroendocrine tumors have shown that treatment with everolimus led to increases in activated AKT through the silencing of an S6K‐dependent negative feedback‐induced loop, especially in patients with clinical responses [9], [10]. Therefore, it is possible that targeting the PI3K/mTOR pathway via PI3K, mTORC1, and mTORC2 inhibition may circumvent treatment resistance and improve outcomes for patients with pNET compared with inhibition of mTORC1 alone.
These observations provided impetus for the development of dual PI3K/mTOR inhibitors such as dactolisib (BEZ235), SAR245409 (XL765), BGT226, and apitolisib (GDC‐0980), which have subsequently been evaluated in a variety of solid tumors [2], [12], [13], [14], [15]. BEZ235 is a novel pan‐class I PI3K inhibitor that also inhibits mTORC1 and mTORC2 [16]. BEZ235 inhibits kinase activity by binding to the ATP‐binding cleft of these enzymes, which occurs through a different mechanism to allosteric inhibition by everolimus, thus avoiding potential rapamycin complex binding site mutations [17].
In preclinical studies, BEZ235 was shown to have potent antitumor activity resulting in Gap 1 phase (G1) phase cell cycle arrest in vivo that was synergistic with the activity of other anticancer drugs, including everolimus [18], [19]. Furthermore, BEZ235 overcame long‐term acquired everolimus resistance in human pNET cell lines, leading to further clinical evaluation of this compound in clinical trials [20]. In the clinical setting, a phase I study involving patients with advanced solid tumors treated with BEZ235 as a single agent demonstrated both clinical activity (45% had stable disease) and acceptable tolerability [2].
The safety and tolerability profile of BEZ235 observed in this study was consistent with the known experience, with no new signal identified. However, treatment with BEZ235 single agent 400 mg bid was accompanied with toxicity requiring frequent treatment modifications and discontinuations in this population with advanced pNET. Adverse events that were frequently reported in phase I studies with the other dual PI3K/mTOR inhibitors in development (XL765, GDC‐0980, GSK2126458, and PF‐04691502) were similar to those seen in this phase I trial of BEZ235, including nausea, vomiting, diarrhea, anorexia, and skin disorders [2]. Taken together, dual inhibition of PI3K‐mTOR was associated with higher incidences of toxicity [21]. The shorter duration of treatment of BEZ235 (22.9 weeks) compared with everolimus (39.4 weeks) because of poor tolerability may have negatively impacted BEZ235 efficacy outcomes. Efficacy may also have been restricted by the high intra‐ and interpatient pharmacokinetic variability with BEZ235 administration observed in previous clinical studies [2], [4], [22], [23], and bioavailability issues [24]. In conclusion, the results of this study suggest that the modest efficacy and poor tolerability of pan‐PI3K inhibitors and dual PI3K/mTOR inhibitors may limit further clinical development of these compounds.
Acknowledgments
We thank the patients enrolled in this study and their families, in addition to Nabanita Mukherjee for support with statistical analyses. Medical editorial assistance was provided by Sai Krishna Arepalli, Ph.D. (Novartis Pharmaceuticals Corporation).
Footnotes
ClinicalTrials.gov Identifier: NCT01628913
Sponsor(s): Novartis Pharmaceuticals Corporation
Principal Investigator: James C. Yao
IRB Approved: Yes
Disclosures
Ramon Salazar: Novartis (C/A, H); Rocio Garcia-Carbonero: Ipsen, Novartis, AAA Pharmaceutical Inc., Pfizer (C/A), Ipsen, Novartis, Pfizer (RF); Andrew E. Hendifar: Ipsen, Novartis, Abbvie (C/A); Catherine Lombard-Bohas: Ipsen, Novartis, Pfizer (C/A, SAB); Heinz-Josef Klümpen: Ipsen (C/A), Bayer, Novartis (RF); Jaume Capdevila: Novartis, Ipsen, Pfizer (C/A, RF, H); Nicholas Reed: Novartis, Ipsen, AAA Pharmaceutical Inc., Eisai (C/A, H), Novartis, Ipsen, Eisai (RF); Annemiek Walenkamp: Novartis (C/A, RF, H, SAB); Oliver Kong: Novartis (E, OI); Herve Salomon: Novartis (E); Ranjana Tavorath: Novartis (E); James C. Yao: Novartis (C/A, RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Yao JC, Shah MH, Ito T et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bendell JC, Kurkjian C, Infante JR et al. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Invest New Drugs 2015;33:463–471. [DOI] [PubMed] [Google Scholar]
- 3. Yao JC, Lombard‐Bohas C, Baudin E et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J Clin Oncol 2010:28;69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fazio N, Buzzoni R, Baudin E et al. A phase II study of BEZ235 in patients with everolimus‐resistant, advanced pancreatic neuroendocrine tumours. Anticancer Res 2016;36:713–719. [PMC free article] [PubMed] [Google Scholar]
- 5. Fazio N. Neuroendocrine tumors resistant to mammalian target of rapamycin inhibitors: A difficult conversion from biology to the clinic. World J Clin Oncol 2015;6:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tijeras‐Raballand A, Neuzillet C, Couvelard A et al. Resistance to targeted therapies in pancreatic neuroendocrine tumors (PNETs): Molecular basis, preclinical data, and counteracting strategies. Target Oncol 2012;7:173–181. [DOI] [PubMed] [Google Scholar]
- 7. Fonseca PJ, Uriol E, Galván JA et al. Prolonged clinical benefit of everolimus therapy in the management of high‐grade pancreatic neuroendocrine carcinoma. Case Rep Oncol 2013;6:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolin EM. PI3K/AKT/mTOR pathway inhibitors in the therapy of pancreatic neuroendocrine tumors. Cancer Lett 2013;335:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Tabernero J, Rojo F, Calvo E et al. Dose‐ and schedule‐dependent inhibition of the mammalian target of rapamycin pathway with everolimus: A phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008;26:1603–1610. [DOI] [PubMed] [Google Scholar]
- 10. Meric‐Bernstam F, Akcakanat A, Chen H et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res 2012;18:1777–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blackwell K, Burris H, Gomez P et al. Phase I/II dose‐escalation study of PI3K inhibitors pilaralisib or voxtalisib in combination with letrozole in patients with hormone‐receptor‐positive and HER2‐negative metastatic breast cancer refractory to a non‐steroidal aromatase inhibitor. Breast Cancer Res Treat 2015;154:287–297. [DOI] [PubMed] [Google Scholar]
- 12. Markman B, Tabernero J, Krop I et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol‐3‐kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol 2012;23:2399–2408. [DOI] [PubMed] [Google Scholar]
- 13. Papadopoulos KP, Tabernero J, Markman B et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res 2014;20:2445–2456. [DOI] [PubMed] [Google Scholar]
- 14. Powles T, Lackner MR, Oudard S et al. Randomized open‐label phase II trial of apitolisib (GDC‐0980), a novel inhibitor of the PI3K/mammalian target of rapamycin pathway, versus everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2016;34:1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wise‐Draper TM, Moorthy G, Salkeni MA et al. A Phase Ib study of the dual PI3K/mTOR inhibitor dactolisib (BEZ235) combined with everolimus in patients with advanced solid malignancies. Target Oncol 2017;12:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maira SM, Stauffer F, Brueggen J et al. Identification and characterization of NVP‐BEZ235, a new orally available dual phosphatidylinositol 3‐kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther 2008;7:1851–1863. [DOI] [PubMed] [Google Scholar]
- 17. Thomas HE, Mercer CA, Carnevalli LS et al. mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Sci Transl Med 2012;4:139ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu CX, Li Y, Yue P et al. The combination of RAD001 and NVP‐BEZ235 exerts synergistic anticancer activity against non‐small cell lung cancer in vitro and in vivo. PLoS One 2011;6:e20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maira SM, Pecchi S, Huang A et al. Identification and characterization of NVP‐BKM120, an orally available pan‐class I PI3‐kinase inhibitor. Mol Cancer Ther 2012;11:317–328. [DOI] [PubMed] [Google Scholar]
- 20. Vandamme T, Beyens M, de Beeck KO et al. Long‐term acquired everolimus resistance in pancreatic neuroendocrine tumours can be overcome with novel PI3K‐AKT‐mTOR inhibitors. Br J Cancer 2016;114:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway–beyond rapalogs. Oncotarget 2010;1:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burris H, Rodon J, Sharma S et al. First‐in‐human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol 2010;28(suppl 15):3005a. [Google Scholar]
- 23. Gil‐Martin M, Fumoleau P, Isambert N et al. A dose‐finding phase Ib study of BEZ235 in combination with paclitaxel in patients with HER2‐negative, locally advanced or metastatic breast cancer. Presented at: 38th Annual San Antonio Breast Cancer Symposium; December 10–14, 2013; San Antonio:P2‐16‐22a. [Google Scholar]
- 24. Peyton JD, Rodon Ahnert J, Burris H et al. A dose‐escalation study with the novel formulation of the oral pan‐class I PI3K inhibitor BEZ235, solid dispersion system (SDS) sachet, in patients with advanced solid tumors. J Clin Oncol 2011;29(suppl 15):3066a. [Google Scholar]