This brief communication reports a case of delayed autoimmune hepatitis that occurred 8 months after discontinuation of nivolumab therapy in a patient with metastatic melanoma.
Abstract
Treatment with anti‐programmed cell death protein 1 (PD‐1) antibodies has demonstrated clinical efficacy in a whole range of malignancies including advanced melanoma, renal cell cancer, bladder cancer, and non‐small cell lung cancer. Immune‐related adverse events are a unique side effect of checkpoint regulator therapy including anti‐PD‐1 antibodies. Treatment‐related autoimmunity can occur in any organ system, with the median onset usually within 5–15 weeks from the commencement of therapy, depending on the organ system involved. This study describes for the first time a case of delayed autoimmunity occurring 8 months after discontinuing treatment with the anti‐PD‐1 antibody nivolumab in a patient with metastatic melanoma. The case highlights the need for ongoing surveillance of patients treated with immune checkpoint inhibitors even after cessation of therapy, especially as patients increasingly stop treatment after achieving durable responses.
Introduction
Treatment with the anti‐programmed cell death protein 1 (PD‐1) antibodies nivolumab and pembrolizumab has shown impressive and durable antitumor responses in melanoma and a whole range of other malignancies, resulting in both agents obtaining regulatory approval for several treatment indications [1]. A feature of immunotherapy with monoclonal antibodies blocking the programmed death receptor 1 (PD‐1) pathway from conventional cancer therapies is the potential development of autoimmunity as a treatment side effect (immune‐related adverse events [irAEs]). Treatment‐related autoimmunity can occur in any organ system, with the most commonly involved being skin, bowel, liver, and endocrine glands [2]. Overall, single‐agent anti‐PD‐1 therapy is well tolerated, with the overall frequency of irAEs being around 40% and the frequency of high‐grade events requiring immunosuppressive treatment less than 5% [3]. The rate of severe irAEs is significantly higher with the anti‐cytotoxic T‐cell lymphocyte‐associated protein 4 (CTLA‐4) antibody ipilimumab or anti‐CTLA‐4/anti‐PD‐1 combination therapy [4]. We report on a case of delayed autoimmune hepatitis that occurred 8 months after discontinuation of nivolumab therapy in a patient with metastatic melanoma.
Case Report
A 77‐year‐old diabetic female presented with a 3.1‐mm nonulcerated melanoma of the left temple. Two years after resection of her primary, the patient developed a recurrence in the left submandibular region, with no evidence of distant metastases on positron emission tomography‐computed tomography (PET‐CT). She underwent a left parotidectomy and level II neck dissection, with the histopathology demonstrating metastatic melanoma. Molecular testing did reveal a BRAF wild‐type status. The patient subsequently received adjuvant radiotherapy.
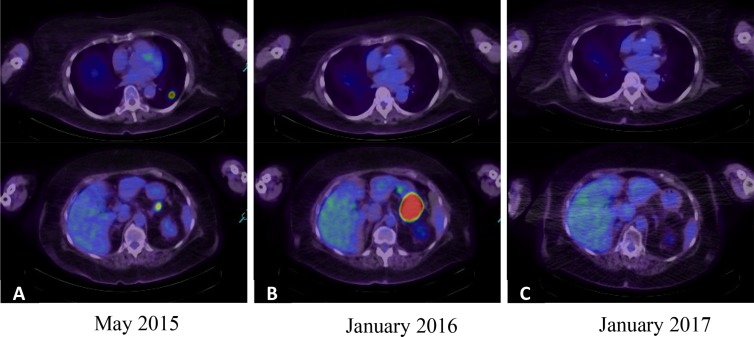
The patient was found to have a solitary right frontal brain metastasis after presenting with a single tonic‐clonic seizure almost 12 months after completing radiotherapy. The patient underwent resection of the solitary metastasis but soon after developed pulmonary, nodal, and left adrenal metastases. At the time, no anti‐PD‐1 agents were available due to lack of regulatory approval, and the patient received first‐line treatment with ipilimumab in a dose of 3 mg/kg intravenously (IV) every 3 weeks for a total of four doses. The patient achieved a partial response to treatment that was maintained for 12 months and did not develop any immune‐related adverse events. Due to further progression in existing sites of disease, her treatment was switched in May 2015 to nivolumab 3 mg/kg IV every 2 weeks. Apart from grade 1 fatigue, treatment was well tolerated, and 4 months after commencement of nivolumab in January 2016, the patient experienced a resolution of all sites of disease apart from a solitary progression of a left adrenal gland metastasis. Radiotherapy to a total dose of 36 Gy given in six fractions, while still continuing on nivolumab, was administered. Repeat imaging 2 months after completion of radiotherapy confirmed a complete response with no evidence for any residual metastatic disease on PET‐CT scan (Fig. 1). The patient continued on nivolumab for another 5 months, completing almost 11 months of treatment, after which the patient requested treatment to be ceased. Subsequent PET imaging performed every 3 months showed an ongoing complete remission.
Figure 1.
Positron emission tomography‐computed tomography images. (A): Sites of metastatic disease prior to commencing nivolumab. (B): Four months after commencement of nivolumab, a complete response was seen in all sites of disease apart from a solitary progression of a left adrenal gland metastasis. (C): The patient maintained a complete response after completion of radiotherapy to the left adrenal gland.
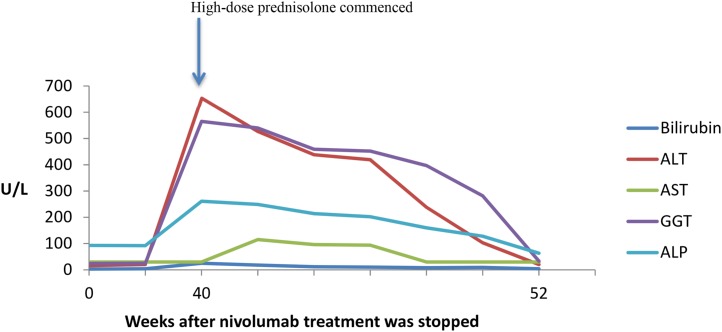
Almost 8 months after cessation of therapy, the patient was admitted to hospital with generalized weakness and unwitnessed falls at home secondary to weakness. No infective symptoms were reported, and neurological examination did not reveal any acute pathology. On admission, the patient was on her long‐term medications—metformin, gliclazide, and warfarin—for a previous pulmonary embolus, with no recent change or new medications. Blood tests on admission demonstrated highly elevated liver enzymes in keeping with an acute hepatitis: alanine aminotransferase 654 U/L, serum total bilirubin 25 µmol/L (<18 µmol/L), γ‐glutamyltransferase 565 U/L (<60 U/L), alkaline phosphatase 261 U/L (40–130 U/L), and aspartate aminotransferase 30 IU/L (<40 U/L). These continued to worsen despite stopping potential hepatotoxic medications (levetiracetam, metformin) that were part of the patient's regular medication for several years (Fig. 2). An autoimmune screen and hepatitis serology were negative, and a liver ultrasound did not reveal any structural abnormality or metastatic disease. The patient was diagnosed with delayed nivolumab‐induced autoimmune hepatitis and commenced on high‐dose methylprednisolone (2 mg/kg) with rapid improvement in liver function. Prior to discharge, the patient was switched to oral prednisolone (1 mg/kg) that was gradually weaned over several weeks with normalization of liver function tests. The patient continues to be off systemic therapy for metastatic melanoma and remains in complete remission.
Figure 2.
Time course of liver function tests.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ‐glutamyltransferase.
Discussion
Single‐agent anti‐PD‐1 therapy with nivolumab or pembrolizumab is overall well tolerated and demonstrates a favorable safety profile [3], [5], [6]. A feature of these drugs is the potential development of immune‐related adverse events. The median onset of the development of irAEs differs from the organ system that is involved with 5–15 weeks from the commencement of therapy. Late onset immune‐related adverse events are rare and have been observed in the setting of ongoing anti‐PD‐1 therapy [3]. The incidence of hepatotoxicity secondary to anti‐PD‐1 antibodies is uncommon, with grade 3/4 hepatotoxicity being reported in 1%–2% of patients [3], [7]. Hepatic dysfunction typically presents as asymptomatic laboratory abnormalities and occurs 8–12 weeks after initiation of treatment. The diagnosis of a checkpoint regulator therapy‐induced autoimmune hepatitis is a diagnosis of exclusion, and there are currently no data demonstrating that autoantibodies that are associated with idiopathic autoimmune hepatitis are helpful to establish the diagnosis. As in our case, the diagnosis requires exclusion of an infectious or medication‐related hepatitis by virus serology and careful obtaining of a medical history. Our case of nivolumab‐induced autoimmune hepatitis differs given that the patient ceased anti‐PD‐1 therapy 8 months prior to the development of the irAE, and so far, there have been no reports on delayed autoimmunity occurring months after cessation of therapy.
The mean elimination half‐life of nivolumab is 17–25 days, with high PD‐1 receptor occupancy on circulating T cells still seen 2 months after a single dose, suggesting that even when serum levels are undetectable, sufficient PD‐1 occupancy is achieved [8]. The prolonged receptor occupancy suggests that immune activation can persist for several months after completion of therapy, and the exposure to an antigenic stimulus could potentially trigger an aberrant immune reaction. In the case of autoimmune hepatitis, this may the introduction of new medications or supplements that cause subclinical liver damage and release liver antigens. Our patient had no change of her medications for a long time and denied taking any supplements.
Conclusion
Overall, our case highlights the need for ongoing surveillance of patients treated with immune checkpoint inhibitors even after cessation of therapy, especially given that patients increasingly stop treatment after achieving durable responses [9], [10].
Disclosures
The authors indicated no financial relationships.
References
- 1. Callahan MK, Postow MA, Wolchok JD. Targeting T cell co‐receptors for cancer therapy. Immunity 2016;44:1069–1078. [DOI] [PubMed] [Google Scholar]
- 2. Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weber JS, Hodi FS, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2016;35:785–792. [DOI] [PubMed] [Google Scholar]
- 4. Larkin J, Chiarion‐Sileni V, Gonzalez R et al., eds. Overall survival results from a phase III trial of nivolumab combined with ipilimumab in treatment‐naïve patients with advanced melanoma (CheckMate 067). Proceedings from the American Association for Cancer Research Annual Meeting; April 1–5, 2017; Washington, DC.
- 5. Schachter J, Ribas A, Long GV et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open‐label phase 3 study (KEYNOTE‐006). Lancet 2017;390:1853–1862. [DOI] [PubMed] [Google Scholar]
- 6. Ribas A, Hamid O, Daud A et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–1609. [DOI] [PubMed] [Google Scholar]
- 7. Hofmann L, Forschner A, Loquai C et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side‐effects of anti‐PD‐1 therapy. Eur J Cancer 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 8. Brahmer JR, Drake CG, Wollner I et al. Phase I study of single‐agent anti–programmed death‐1 (MDX‐1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ladwa R, Atkinson V. The cessation of anti‐PD‐1 antibodies of complete responders in metastatic melanoma. Melanoma Res 2017;27:168–170. [DOI] [PubMed] [Google Scholar]
- 10. Robert C, Ribas A, Hamid O et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]