This brief communication reports the efficacy outcomes of two patients who experienced dramatic tumor response on ramucirumab plus paclitaxel after progressive disease on pembrolizumab.
Abstract
Checkpoint inhibitors targeted at programmed cell death‐1 receptor (PD‐1) and its ligand (PD‐L1) can result in significant benefit to a small proportion of patients with cancer, including those with tumors of the stomach and gastroesophageal junction. These drugs are now approved for several solid tumors, including the recent accelerated approval of pembrolizumab for gastroesophageal adenocarcinomas in the third‐line setting and beyond based on the KEYNOTE‐059 phase II trial. Data are lacking on the efficacy of chemotherapy after progression on PD‐1 blockade in metastatic gastroesophageal adenocarcinoma. This report describes the exceptional response of two patients who received ramucirumab plus paclitaxel after progressive disease on pembrolizumab. This early clinical observation suggests that the sequence of administration of PD‐1 blockade and chemotherapy may be important in this disease.
Introduction
Checkpoint inhibitors targeted at the receptor programmed cell death‐1 (PD‐1) and its ligand (PD‐L1) can result in significant benefit to a small proportion of patients with cancer, including those with tumors of the stomach and gastroesophageal junction (GEJ). These drugs are now approved for several solid tumors, including the recent accelerated approval of pembrolizumab for gastric and GEJ adenocarcinomas expressing PD‐L1 in the third‐line setting and beyond based on the KEYNOTE‐059 phase II trial [1].
Recent data from non‐small cell lung cancer (NSCLC) suggest that anti‐PD‐1 therapy may enhance sensitivity to subsequent chemotherapy [2], [3]. To date, reports on treatment efficacy after failure of PD‐1 blockade in advanced gastroesophageal adenocarcinoma are lacking.
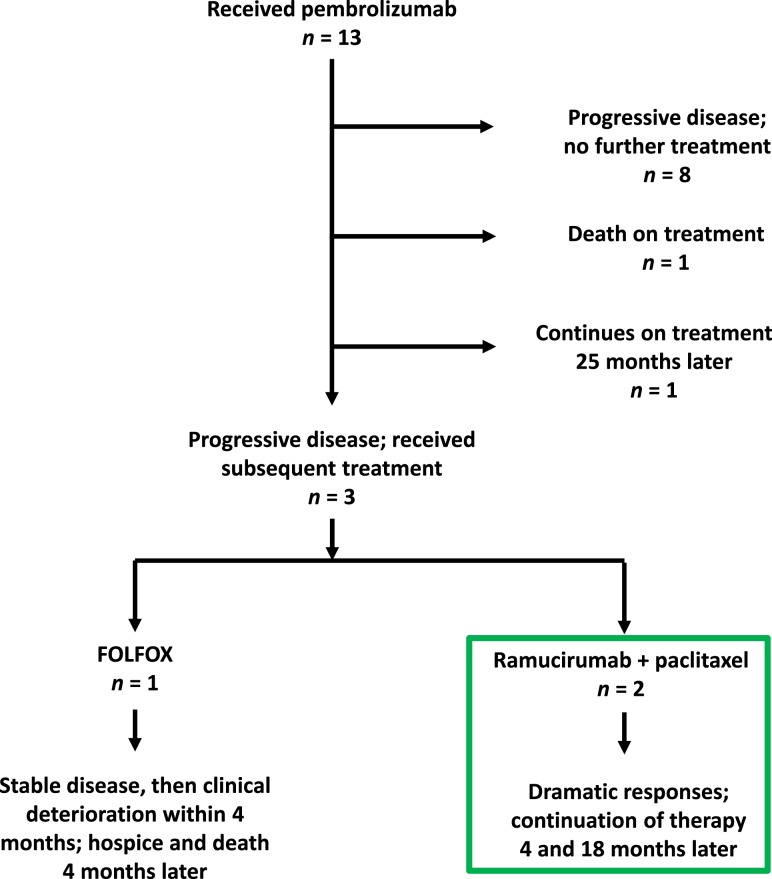
At our institution, 13 subjects enrolled in KEYNOTE‐059 (Fig. 1). Here, we report the efficacy outcomes of two patients who experienced dramatic tumor responses on ramucirumab plus paclitaxel after progressive disease (PD) on pembrolizumab.
Figure 1.
Flow diagram of patient outcomes in KEYNOTE‐059 at our institution.
Case 1
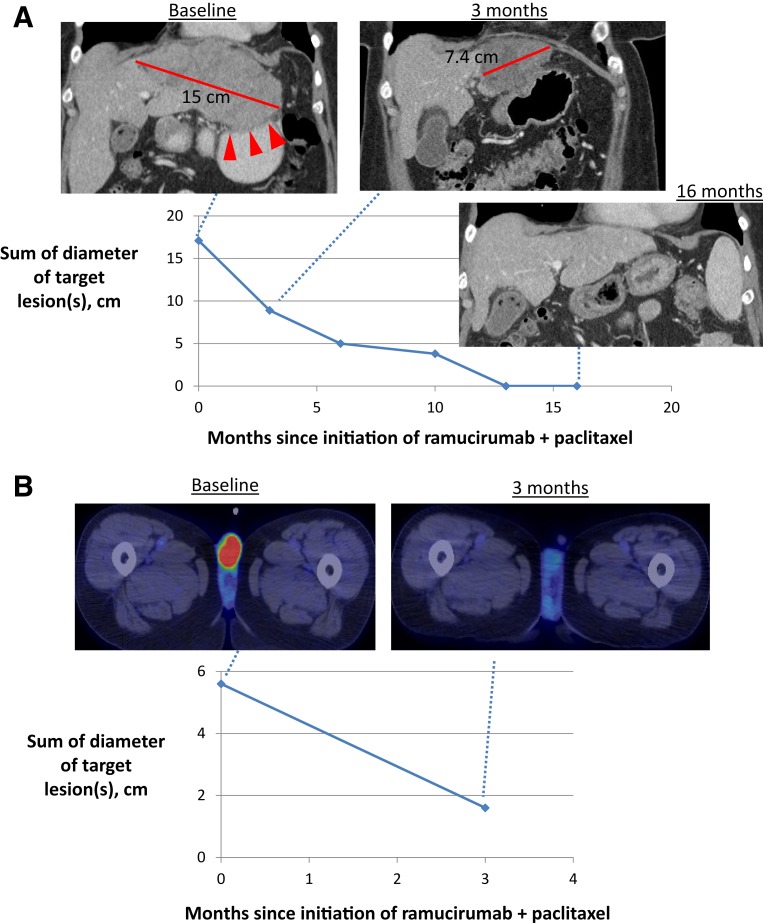
A 53‐year‐old woman presented with HER2‐negative GEJ adenocarcinoma with a liver metastasis. Epirubicin, cisplatin, and capecitabine led to a complete response, and after six cycles she was placed on observation. Three months later, PD in the liver was treated with radiofrequency ablation. With PD 4 months later, FOLFOX was initiated, leading to a partial response, followed by progression after 5 months of treatment. The patient enrolled in KEYNOTE‐059 and received pembrolizumab (200 mg every 3 weeks) as third‐line therapy. Her tumor did not shrink, and the agent was discontinued after 6 months because of symptomatic PD in the liver. Ramucirumab and paclitaxel were initiated as fourth‐line therapy. After 3 months, imaging studies showed a remarkable response (Fig. 2A), with continued improvement on subsequent scans. The patient continues on ramucirumab monotherapy without clear evidence of disease 18 months from the initiation of ramucirumab and paclitaxel.
Figure 2.
Dramatic response to ramucirumab plus paclitaxel in two patients with advanced gastroesophageal junction adenocarcinoma. (A): Case 1: Baseline computed tomography (CT) scan shows a 15‐cm confluent hepatic mass involving the left and caudate lobes. The mass abuts the lesser curvature of the stomach where the loss of the fat plane between the liver and stomach (arrowheads) corresponds to the patient's worsening epigastric discomfort and suggests direct invasion. Not shown are enlarged upper abdominal lymph nodes. Subsequent scans after treatment show shrinkage of all metastatic lesions and restoration of the fat plane, corresponding to resolution of symptoms. Complete radiographic response is evident after 13 months of therapy. (B): Case 2: Complete metabolic resolution of fluorodeoxyglucose‐avid testicular metastases on positron emission tomography and CT scan after treatment, corresponding to resolution of the metastases on clinical exam.
Case 2
A 55‐year‐old man with locally advanced, HER2‐positive GEJ adenocarcinoma underwent chemoradiation followed by surgery and relapsed 8 months later in a cytologically confirmed celiac node and a lumbar vertebral mass (L4). He received paclitaxel plus trastuzumab and progressed 4 months later. He was then treated with ramucirumab monotherapy and experienced PD within 2 months. He enrolled on KEYNOTE‐059 and received pembrolizumab. After a best response of stable disease, he was taken off therapy after 17 months because of PD in his L4 mass. He was then treated with 5‐fluorouracil plus irinotecan (FOLFIRI) plus trastuzumab, experienced PD within 2 months at L4, and developed new bilateral fluorodeoxyglucose‐avid testicular masses (up to 1.4 cm in size). Given his prolonged stable disease on KEYNOTE‐059 and the experience with Case 1, this patient was re‐exposed to pembrolizumab via a patient assistance program, with the goal of administering ramucirumab and paclitaxel afterward. After two cycles of pembrolizumab, the patient's bilateral testicular masses became palpable and grew more than twofold in size. Ramucirumab and paclitaxel were started as fifth‐line therapy, and within a few weeks the patient had complete resolution of the testicular masses. Positron emission tomography and computed tomography after 3 months of therapy showed a complete metabolic response in both testicular metastases and stability at L4 (Fig. 2B). The patient continues on ramucirumab and paclitaxel 4 months later.
Discussion
This is the first report, to our knowledge, of dramatic tumor responses with chemotherapy after progression on PD‐1 blockade in advanced gastroesophageal cancer. We describe two patients whose tumors never regressed with pembrolizumab and eventually grew, yet who experienced tumor shrinkage, including one complete response, on ramucirumab and paclitaxel as fourth‐ or fifth‐line treatment. Both patients remain on ramucirumab treatment (with or without paclitaxel) 4 months and 18 months later. The largest study of ramucirumab and paclitaxel, as second‐line therapy, was associated with a response rate of only 28% (95% confidence interval, 22%–33%), including a complete response in <1%, with a median duration of response of 4.4 months (interquartile range 2.8–7.5) [4].
Our clinical observation raises the hypothesis that the sequential administration of anti‐PD‐1 therapy followed by chemotherapy may enhance efficacy of the latter. In support of this, patients with NSCLC who were randomized to front‐line pembrolizumab followed by investigator‐choice cytotoxic therapy, which was paclitaxel in 19% of patients, had a longer time to progression after the initiation of second‐line therapy compared with patients randomized to front‐line cytotoxic therapy followed by investigator‐choice therapy, which usually consisted of PD‐1 blockade (KEYNOTE‐024) [2]. A similar pattern was observed in a separate NSCLC study, which showed that salvage chemotherapy was associated with a response rate of 27% among patients with prior exposure to PD‐1/PD‐L1 inhibitors versus 7% without prior exposure [3].
Preclinical data from our laboratory suggest an enhanced antitumor effect when PD‐1 blockade is administered prior to chemotherapy. In a B16F10 mouse melanoma model, treatment with PD‐1 blockade followed 3 days later by paclitaxel/carboplatin led to a significantly greater suppression of tumor growth and prolonged survival compared with concurrent PD‐1 blockade and paclitaxel/carboplatin [5]. Although the precise mechanism remains to be elucidated, pembrolizumab and other checkpoint inhibitors may restore immune competence crucial for the activity of chemotherapy [6]. In some studies, enhanced efficacy using the opposite treatment sequence has also been reported [6]. In a phase II, double‐blind, randomized study in patients with advanced NSCLC, paclitaxel and carboplatin followed by ipilimumab improved immune‐related progression‐free survival compared with concurrent chemotherapy plus ipilimumab [7]. The sequence of chemotherapy followed by immunotherapy is currently being tested in lung (NCT02684461), bladder (NCT02500121), ovarian (NCT02520154), and other solid tumors.
In our very small sample, the observed responses after progression on pembrolizumab occurred only with ramucirumab and paclitaxel, not with FOLFIRI (Case 2) or FOLFOX (Fig. 1). The immune‐stimulatory effects of paclitaxel are well described [8]. Paclitaxel was one of 15 agents (out of 54 agents screened in a murine dendritic cell [DC] line XS106) that delivered DC maturation signals at concentrations that caused only marginal DC death. In contrast, irinotecan primarily inhibited DC growth, and 5‐fluorouracil and oxaliplatin caused DC death or no substantial change [9]. Accumulating evidence indicates antitumor cooperativity when immune checkpoint and angiogenesis inhibition are combined, which may indicate a particular role for ramucirumab in our two patients [10], [11]. Recent data indicate that vascular endothelial growth factor modulates antitumor immunity on multiple levels: it induces regulatory T‐cell proliferation and differentiation, promotes the expansion of myeloid‐derived suppressor cells, inhibits antigen‐presenting cell maturation, inhibits effector T‐cell development, and inhibits the infiltration of T cells into the tumor microenvironment [12]. The combination of immunotherapy and antiangiogenesis is being explored in a variety of solid tumor types, including colon and lung cancer.
In conclusion, these early clinical observations suggest that sequential administration of PD‐1 blockade followed by chemotherapy could augment response to subsequent ramucirumab and paclitaxel in advanced gastric or GEJ adenocarcinoma. It may be beneficial to examine the efficacy of subsequent lines of therapy as planned secondary analysis in ongoing and future trials of PD‐1 inhibition and to consider a predefined sequence of therapies at progression. If confirmed, these findings can affect clinical decision making by identifying a better approach for sequencing immunotherapy with other anticancer therapies.
Footnotes
For Further Reading: Valerie Lee, Adrian Murphy, Dung T. Le et al. Mismatch Repair Deficiency and Response to Immune Checkpoint Blockade. The Oncologist 2016;21:1200–1211; first published on July 13, 2016.
Implications for Practice: Mismatch repair deficiency has contributed to our understanding of carcinogenesis for the past 2 decades and now identifies a subgroup of traditionally chemotherapy‐insensitive solid tumors as sensitive to PD‐1 blockade. This article seeks to educate oncologists regarding the nature of mismatch repair deficiency, its impact in multiple tumor types, and its implications for predicting the responsiveness of solid tumors to immune checkpoint blockade.
Author Contributions
Conception/design: Harry H. Yoon
Collection and/or assembly of data: Sakti Chakrabarti, Haidong Dong, Harshita R. Paripati, Helen J. Ross
Data analysis and interpretation: Sakti Chakrabarti, Harry H. Yoon
Manuscript writing: Sakti Chakrabarti, Haidong Dong, Harshita R. Paripati, Helen J. Ross, Harry H. Yoon
Final approval of manuscript: Sakti Chakrabarti, Haidong Dong, Harshita R. Paripati, Helen J. Ross, Harry H. Yoon
Disclosures
Harry H. Yoon: Merck, Lilly (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Fuchs CS, Doi T, Jang RWJ et al. KEYNOTE‐059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35(suppl 15):4003A. 29040031 [Google Scholar]
- 2. Brahmer JR, Rodriguez‐Abreu D, Robinson AG et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD‐L1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE‐024. J Clin Oncol 2017;35(suppl 15):9000A. [Google Scholar]
- 3. Leger PD, Rothschild S, Castellanos E et al. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non‐small cell lung cancer. J Clin Oncol 2017;35(suppl 15):9084A. [Google Scholar]
- 4. Wilke H, Muro K, Van Cutsem E et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): A double‐blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 5. Yan Y, Cao S, Liu X et al. CX3CR1 identifies PD-1 therapy-responsive CD8 T cells that withstand chemotherapy during cancer chemo-immunotherapy. JCI Insight 2018;3:e97828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bracci L, Schiavoni G, Sistigu A et al. Immune‐based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale‐based combined treatments against cancer. Cell Death Differ 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lynch TJ, Bondarenko I, Luft A et al. Ipilimumab in combination with paclitaxel and carboplatin as first‐line treatment in stage IIIB/IV non‐small‐cell lung cancer: Results from a randomized, double‐blind, multicenter phase II study. J Clin Oncol 2012;30:2046–2054. [DOI] [PubMed] [Google Scholar]
- 8. Ramakrishnan R, Assudani D, Nagaraj S et al. Chemotherapy enhances tumor cell susceptibility to CTL‐mediated killing during cancer immunotherapy in mice. J Clin Invest 2010;120:1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanaka H, Matsushima H, Mizumoto N et al. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res 2009;69:6978–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yasuda S, Sho M, Yamato I et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti‐tumour effect in vivo. Clin Exp Immunol 2013;172:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodi FS, Lawrence D, Lezcano C et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014;2:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin Z, Yoon HH. Antiangiogenic therapy in gastroesophageal cancer. Hematol Oncol Clin North Am 2017;31:499–510. [DOI] [PubMed] [Google Scholar]