Abstract
Noninvasive imaging of demyelination and remyelination is critical for diagnosis and clinical management of demyelinating diseases. Positron emission tomography (PET) has the potential to complement magnetic resonance imaging (MRI) by providing a quantitative measure specific to demyelination. In Brugarolas et al’s study1, we describe the development of the first PET tracer for voltage-gated K+ channels based on a clinically approved drug for multiple sclerosis that can be used for imaging demyelination in animal models.
Keywords: advances in PET/SPECT probes, imaging in neuroscience, molecular imaging of neuro-degenerative diseases
Myelin is essential for proper functioning of the central nervous system. Its main role is to speed up the propagation of electrical impulses along myelinated fibers, and it also provides protection and nutrients to neurons. In many diseases, the myelin sheath is damaged, which can cause a wide range of motor, sensory, and cognitive symptoms. Disruption to the myelin sheath may be genetic (eg, leukodystrophies),2 immunological (eg, multiple sclerosis),3 traumatic (eg, traumatic brain injury and spinal cord injuries),4,5 ischemic (eg, stroke), or degenerative (eg, Alzheimer disease or even normal aging).6 Quantitative imaging of changes in myelin content is critical to understanding these diseases and monitoring their progression.
Myelin is composed of lipids (70% dry weight) and proteins. Given its high lipid content, water molecules within myelin have limited diffusion, which can be detected using magnetic resonance imaging (MRI). Lipid itself also changes the relaxation properties of nearby water protons, most prominently by shortening the T1 relaxation time constant. Consequently, MRI is very sensitive to demyelinating lesions. In practice, however, various tissue properties and pathologies can give rise to similar findings on MRI, and as a result, it can be challenging to distinguish demyelination from other potentially coexisting processes such as inflammation and axonal loss, solely by MRI. In addition, the physics of MRI are such that the signal is not fully quantitative, which limits our ability to monitor changes in myelination over time.
Positron emission tomography (PET) uses radioactive molecules to detect pathological changes in live subjects. Because PET scanners detect radiation emitted directly by the radiotracer, they can provide quantitative images of the radioactive source within the subject. As such, a tracer that preferentially localizes to demyelinated lesions could provide a quantitative measure of demyelination.
Current PET tracers for demyelination bind to myelin,7 which has the limitation that small areas of demyelination are masked due to the high abundance of surrounding myelin. This is particularly problematic when the structures of interest, such as in multiple sclerosis (MS) lesions, are small (often on the order of 0.5 mm in diameter or less). In addition, since myelin is mostly made of lipids, tracers for myelin are very lipophilic, which typically results in tracers with high background due to nonspecific binding.
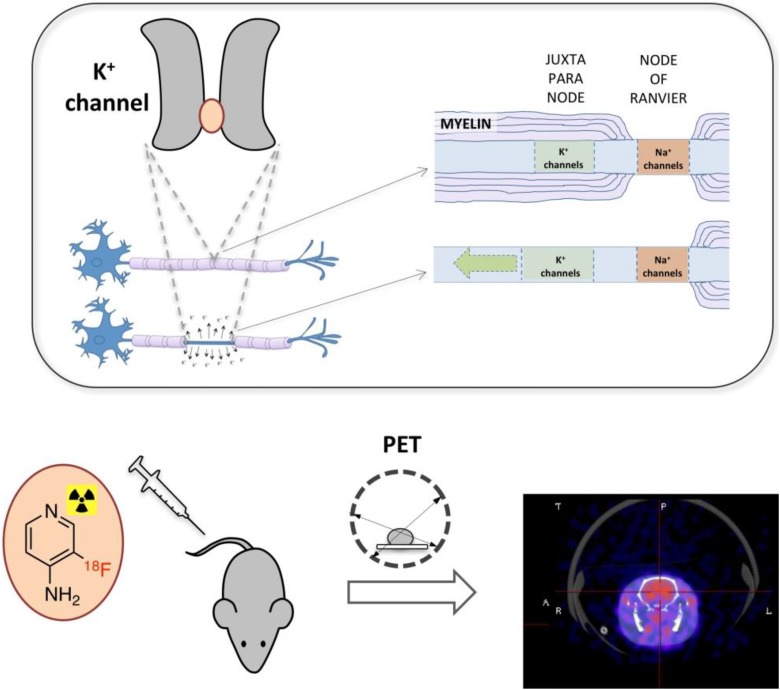
We took a different approach. It has long been known that demyelination alters the distribution of axonal potassium channels.8-11 In normally myelinated axons, sodium channels concentrate at the nodes of Ranvier (also known as myelin-sheath gaps) and potassium channels at the neighboring juxtaparanodes beneath the myelin sheath.10 During demyelination, K+ channels become exposed, migrate through the demyelinated segment and increase in expression. This exposure of K+ channels results in leakage of intracellular potassium ions, which in turn impairs propagation of electrical impulses. This process is the basis for the mechanism of action of the multiple sclerosis drug, 4-aminopyridine (4AP, dalfampridine). 4-aminopyridine is a small cationic molecule that binds inside the pore of voltage-gated K+ channels (Kv1 family), blocking the passage of K+ ions. Based on this mechanism, we proposed that a radiolabeled form of 4AP could potentially serve to detect demyelinated lesions (Figure 1).
Figure.
Imaging K+ channels from demyelinated axons. Upon demyelination, voltage-gated K+ channels accumulate at the demyelinated segment. [18F]3F4AP, a radiofluorinated analog of the multiple sclerosis drug 4-aminopyridine, binds to these channels and can be used to trace demyelinated lesions noninvasively using positron emission tomography.
In order to test our hypothesis, we commissioned the production of 14C-labeled 4AP for autoradiography experiments. Because binding of 4AP requires the channels to be open, something that only occurs during membrane depolarization, incubation of tissue sections with the radiolabeled drug gave no significant binding. We then performed autoradiography after administering the drug to live mouse models of demyelination and found that there was greater binding in demyelinated areas, where there is high concentration of accessible K+ channels, than in normally myelinated areas, where there is low concentration of accessible K+ channels. We also saw significant binding in the gray matter, which is not surprising since there are many K+ channels in those regions, with little myelin to block access of the tracer. After confirming that 4AP preferentially binds to areas with low-myelinated over well-myelinated areas, we set out on a quest for a fluorine-containing derivative that could be used for PET imaging. With the help of Dr Pancho Bezanilla and his laboratory at the University of Chicago (experts in the electrophysiology of ion channels), we found that 3-fluoro-4-aminopyridine, 3F4AP, has similar capacity to block K+ channels as 4AP. Additional efforts and collaboration with University of Wisconsin–Madison radiochemistry professor, Dr Onofre DeJesus, led to the successful labeling of 3F4AP with F-18.12 With [18F]3F4AP on hand, we performed microPET/CT imaging in rodents with demyelinated lesions at the University of Chicago, aided by Dr Chin-Tu Chen and colleagues, and demonstrated that [18F]3F4AP can be used to detect demyelination by PET in rodents. Finally, a collaboration with the NIH Intramural Research Program led to the improvement in the radiolabeling method by Rolf Swenson and colleagues13 and to the testing of the tracer in nonhuman primates conducted by Peter Herscovitch. Primate studies showed that [18F]3F4AP has excellent properties for brain imaging, including rapid entry into the brain, slow to moderate clearance, and high signal in the brain (SUVmax 3-6, depending on the brain region).
An important challenge that we faced in this project was how to convince ourselves (and the reviewers) that the signal was specific for K+ channels. Since 4AP and 3F4AP target K+ channels that are directly involved in neuronal function, it is not possible to perform blocking studies by saturating the receptors with cold drug as this would cause fatal seizures to the animals. We believe it may be possible to partially block the signal, but this will require careful titration of the cold drug while measuring the concentration of the tracer and radiometabolites in blood as well as mathematically modeling the data. Nevertheless, several indirect pieces of evidence point to high degree of specific binding, including the facts that binding is lowest in white matter (which typically shows highest nonspecific binding in PET studies), that no binding is observed when the drug is applied directly to tissue sections, where most receptors that could give rise to nonspecific binding remain available and most importantly that there were significant differences between demyelinated animals and controls in 3 different murine models of demyelination. These models include Shiverer mice that lack compact myelin due to a null mutation of the myelin basic protein gene,14,15 lysolecithin-injected mice, which display chemically induced focal demyelination,16 and DTA mice, which are a genetically engineered strain in which tamoxifen injections induce death of oligodendrocytes and consequent demyelination.17,18 The results in DTA mice are particularly exciting, as they suggest that our tracer is sensitive to demyelination and remyelination.
Many questions remain. For example, since Kv1 channels are involved in many other processes such as cell migration and proliferation, regulation of cell volume, and immune cell activation, how do other potentially coexisting processes, such as inflammation, affect the PET signal? It is known that inflammation also occurs in MS and that K+ channels (such as Kv1.3) are expressed in microglia, so it is important to look at this in the future. The fact that [18F]3F4AP targets a protein as ubiquitous as K+ channels may provide additional applications for the tracer. In the past, there have been tracers developed with 1 application in mind, which have found application for other purposes. For example, [18F]flurodeoxyglucose, [18F]FDG, was developed to monitor brain activity, but it has found its most enduring use in the imaging of tumors and metastases. Similarly, a tracer for K+ channels could potentially have applications for cancer, inflammation, neurodegenerative diseases, and kidney diseases.
In addition, longitudinal studies comparing PET, MRI, clinical scores, and histology in MS models will provide invaluable information for the successful translation of 3F4AP to humans. If proven sensitive and specific for demyelination, this tracer has the potential to solve a significant challenge in the field, namely, how to detect and quantify remyelination in clinical trials of myelin repair drugs, which is currently a major focus in MS drug development.19
In summary, the article describes step-by-step the process of developing the first PET tracer for voltage-gated K+ channels, including validating the target in mouse models of MS, developing fluorinated small molecules amenable to F-18 labeling, and imaging them in rodent models of MS and monkeys. As with any new concept, many unanswered questions remain regarding [18F]3F4AP for those willing to explore them.
The work took 6 years and a large team of people, which included experts in neuroscience, animal models, synthetic chemistry, electrophysiology, and PET imaging from the University of Chicago, the University of Wisconsin at Madison, Case Western University, and the National Institutes of Health. It was supported by the National Multiple Sclerosis Society, the National Institute for Neurological Disorders and Stroke, the National Institute for Biomedical Imaging and Bioengineering, the Chicago Innovation Exchange (now known as the Polsky Center), and the Adelson Medical Research Foundation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The University of Chicago has filed a patent application related to this technology where P.B., and B.P. are listed as inventors. D.S.R. wishes to disclose funding from MRF and Vertex to develop methods for imaging demyelination using MRI.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by NIH/NIBIB K99EB020075 (P.B.), NIH/NINDS R21NS084382 and the National Multiple Sclerosis Society through special funds from the Illinois Lottery (B.P.), an Innovation Fund Award from the Chicago Innovation Exchange (P.B. and B.P.), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (D.S.R. and B.P.). D.S.R. is supported by the Intramural Research Program of NINDS.
References
- 1. Brugarolas P, Sanchez-Rodriguez JE, Tsai HM, et al. Development of a PET radioligand for potassium channels to image CNS demyelination. Sci Rep. 2018;8(1):607 2018/01/14. doi:10.1038/s41598- 017-18747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheon JE, Kim IO, Hwang YS, et al. Leukodystrophy in children: a pictorial review of MR imaging features. Radiographics. 2002;22(3):461–476. doi:10.1148/radiographics.22.3.g02ma01461. [DOI] [PubMed] [Google Scholar]
- 3. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi:10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: clues to axon and myelin repair capacity. Experimental Neurol. 2016;275(pt 3):328–333. doi:10.1016/j.expneurol.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 5. Waxman SG. Demyelination in spinal cord injury. J Neurol Sci. 1989;91(1-2):1–14. [DOI] [PubMed] [Google Scholar]
- 6. Gouw AA, Seewann A, Vrenken H, et al. Heterogeneity of white matter hyperintensities in Alzheimer’s disease: postmortem quantitative MRI and neuropathology. Brain. 2008;131(pt 12):3286–3298. doi:10.1093/brain/awn265. [DOI] [PubMed] [Google Scholar]
- 7. Faria Dde P, Copray S, Buchpiguel C, Dierckx R, de Vries E. PET imaging in multiple sclerosis. J Neuroimmune Pharmacol. 2014;9(4):468–482. 2014/05/09. doi:10.1007/s11481-014-9544-2. [DOI] [PubMed] [Google Scholar]
- 8. Coman I, Aigrot MS, Seilhean D, et al. Nodal, paranodal, and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 2006;129(pt 12):3186–3195. 2006/06/13. doi:10.1093/brain/awl144. [DOI] [PubMed] [Google Scholar]
- 9. Arroyo EJ, Sirkowski EE, Chitale R, et al. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J Comp Neurol. 2004;479(4):424–434. 2004/10/30. doi:10.1002/cne.20321. [DOI] [PubMed] [Google Scholar]
- 10. Rasband MN, Trimmer JS, Schwarz TL, et al. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci. 1998;18(1):36–47. 1998/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980;283(5747):570–572. 1980/02/07. [DOI] [PubMed] [Google Scholar]
- 12. Brugarolas P, Freifelder R, Cheng S-H, et al. Synthesis of meta-substituted [18F]3-fluoro-4-aminopyridine via direct radiofluorination of pyridine N-oxides. Chem Commun (Camb). 2016;52(44):7150–7152. doi:10.1039/C6CC02362B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basuli F, Zhang X, Brugarolas P, Reich DS, Swenson RE. An efficient new method for the synthesis of 3-[18F]Fluoro- 4-aminopyridine via yamada-curtius rearrangement. J Labelled Comp Radiopharm. 2018;61(2):112–117. doi:10.1002/jlcr.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popko B, Puckett C, Lai E, et al. Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell. 1987;48(4):713–721. 1987/02/27. [DOI] [PubMed] [Google Scholar]
- 15. Privat A, Jacque C, Bourre JM, Dupouey P, Baumann N. Absence of the major dense line in myelin of the mutant mouse “shiverer”. Neurosci Lett. 1979;12(1):107–112. [DOI] [PubMed] [Google Scholar]
- 16. Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25(3):216–228. [DOI] [PubMed] [Google Scholar]
- 17. Traka M, Podojil JR, McCarthy DP, Miller SD, Popko B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat Neurosci. 2016;19(1):65–74. doi:10.1038/nn.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Traka M, Arasi K, Avila RL, et al. A genetic mouse model of adult-onset, pervasive central nervous system demyelination with robust remyelination. Brain. 2010;133(10):3017–3029. doi:10.1093/brain/awq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kremer D, Kury P, Dutta R. Promoting remyelination in multiple sclerosis: current drugs and future prospects. Mult Scler. 2015;21(5):541–549. doi:10.1177/1352458514566419. [DOI] [PubMed] [Google Scholar]