Abstract
Consumption of herbal and dietary supplements (HDS) has increased worldwide as potential treatment for weight reduction and metabolic enhancement. However, it has been reported that HDS can cause liver injury which accounts for 20% of hepatotoxicity in the United States. Prevention of HDS induced liver injury remains a challenge due to difficulties in identifying the hepatotoxins in these preparations and lack of federal regulations for dietary supplements. We report a case of acute severe hepatic necrosis presumably due to consumption of nutritional supplement advertised to boost vitality and stem cells in human body.
Keywords: Liver, green tea extract, acute severe hepatic necrosis, nutritional supplements
Introduction
Recently published prospective study of drug-induced liver injury (DILI) demonstrated that herbal and dietary supplements (HDS) accounted for 16% of all cases of hepatotoxicity in the United States. Furthermore, the frequency of HDS increased from 7% in 2004 to 19% by 2012.1 Interestingly, prevalence of liver injury due to HDS continues to rise and was reported to be 20% in 2014.1 Several lines of evidence suggest that among HDS ingredients, green tea extract (GTE) represents a potential hepatotoxic agent. In our case, we suspect the active ingredient of HDS consumed by the patient was most likely GTE.
Case
A 50-year-old Caucasian woman presented to her primary care physician with a 2-week history of diminished appetite, night sweats, weakness, and truncal pruritis. Patient had a past history of systemic lupus erythematosus (SLE) controlled with medications. Home medications included Vitamin D and folic acid. Physical examination was remarkable for severe scleral icterus and a soft non-distended non-tender abdomen. Patient was well nourished with a body mass index (BMI) of 24.4 and no evidence of obesity. Laboratory data were significant for total bilirubin (0.1-1.2 mg/dL) elevated to 38 mg/dL and the direct bilirubin (<0.3 mg/dL) to 32 mg/dL. Markedly elevated aspartate aminotransferase (AST) (10-40 U/L) to 1657 U/L and alanine aminotransferase (ALT) (7-56 U/L) to 1170 U/L were noted. Alkaline phosphatase (ALP) (44-147 IU/L) was normal at 113 IU/L. Ultrasound of the abdomen was unremarkable. Magnetic resonance cholangiopancreatography (MRCP) showed edema surrounding the distal common bile duct (CBD) without signs of a stricture. An endoscopic retrograde cholangio-pancreatography (ERCP) evaluation showed a normal biliary tree with possible constriction of the distal CBD. A sphincterotomy was performed and a stent was placed in the distal CBD which was removed 2 weeks later. She was prescribed ursodeoxycholic acid (ursodiol) and cholestyramine to treat pruritus.
However, in following 2 days, the patient developed increasing nausea and vomiting resulting in admission to the hospital for further management. Lab findings were significant for total bilirubin 37 mg/dL, direct bilirubin 31 mg/dL, AST 669 U/L, ALT 558 U/L, and ALP was normal. Additional lab data included a negative acetaminophen level, Ethyl Alcohol, and Urinary Toxicology. Serologic markers of autoimmune hepatitis (filamentous actin, antinuclear antibodies [ANA], and Liv/Kid antibodies), hepatitis A (HAV Immunoglobulin M [IgM] and Immunoglobulin G [IgG]), hepatitis B (HBsAg, IgG anti-HBc, anti-HBc IgM), hepatitis C (anti-HCV, HCV RNA by PCR), and hepatitis E (HEV IgM and IgG), HIV, herpes simplex virus (HSV), Wilson’s disease (ceruloplasmin and copper assessment in 24 hour urine) and alpha-1-antitrypsin deficiency were all negative. However, smooth muscle antibody was weakly positive at 1:320. A computed tomography (CT)-guided liver biopsy revealed an acute hepatocellular injury event with intact basic architecture of the liver (Figures 1 and 2). She was empirically started on prednisone therapy 40 mg for resolution of jaundice and intense pruritus. Her labs over the month period are summarized in Table 1.
Figure 1.
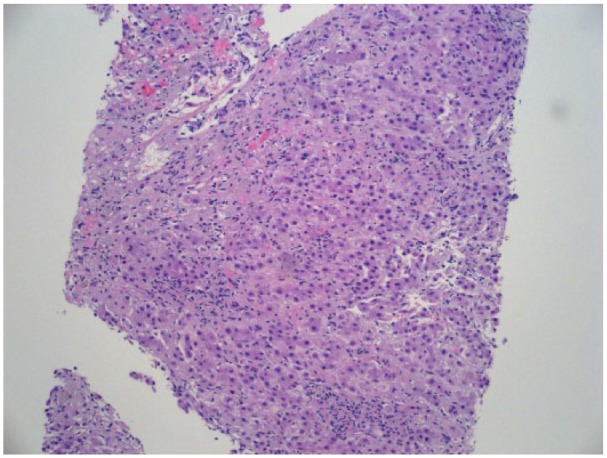
Liver biopsy with histology shows acute hepatocellular injury pattern. Hepatocytes are reactive with prominent ballooning degeneration and individual cell necrosis.
Figure 2.

Liver trichrome stain shows acute hepatocellular injury pattern.
Table 1.
Summary of labs table.
| March 24, 2017 | April 5, 2017 | April 6, 2017 | April 7, 2017 | April 8, 2017 | April 9, 2017 | May 9, 2017 | |
|---|---|---|---|---|---|---|---|
| Total bilirubin (mg/dL) | 38.4 | 37 | 31 | 24.6 | 19.1 | 15.9 | 1.5 |
| Direct bilirubin (mg/dL) | 32 | 31.1 | 26.8 | 20.2 | 14.3 | 11.9 | |
| Alkaline phosphate (U/L) | 113 | 92 | 88 | 83 | 91 | 132 | 92 |
| Aspartate aminotransferase (U/L) | 1657 | 659 | 468 | 338 | 287 | 311 | 21 |
| Alanine aminotransferase (U/L) | 1170 | 558 | 467 | 382 | 340 | 425 | 27 |
 |
 |
||||||
| Prednisone 40 mg one a day | Liver biopsy | ||||||
On further questioning, the patient admitted to taking over-the-counter nutritional supplements (Vital Stem) containing GTE daily for 1 month. After excluding other potential causes of acute liver injury, it was presumed that her severe hepatic necrosis was most likely due to GTE. The R factor for liver injury was calculated with a score of 31.06 consistent with hepatocellular damage. On a follow-up visit at 4 weeks, she was noted to have marked symptomatic improvement and resolution of pruritus and jaundice (Figures 3 and 4). She was eventually tapered off prednisone over the next few weeks. Roussel Uclaf Causality Assessment Method (RUCAM) was done and the total score was 4 suggesting a possible cause. The informed consent for description of the case report was obtained from the patient.
Figure 3.

Line with markers showing impact of idiosyncratic drug-induced liver injury on total and direct bilirubin levels.
Figure 4.

Line with markers showing impact of idiosyncratic drug-induced liver injury on AST and ALT levels. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
Although the real prevalence of HDS is unknown, it is estimated that more than 50% of the US adult population consumes HDS.2 Drug-induced liver injury (DILI) is a common cause for hepatotoxicity but the diagnosis is often delayed or missed when patients and clinicians do not consider herbal and nutritional supplements as drugs.
Our case illustrates acute severe hepatic necrosis presumably due to consumption of nutritional supplement (Vital Stem). “Vital Stem” claims to contain stem cell enhancing blend (L-leucine, blueberry powder, GTE, L-carnosine and Vitamin D3) per 1400 mg. The patient consumed half tea spoon of “Vital Stem” in pomegranate juice every day for a period of 1 month prior to her onset of current illness. The effect of pomegranate juice on cytochrome P450 (CYP450) present in human liver microsomes in vitro studies has been well studied. It was reported that pomegranate juice inhibits human cytochrome P450 3A (CYP3A) metabolism of carbamazepine. This was also confirmed in rat model.3 Based on her clinical history, biochemical findings, histologic observation, and subsequent clinical course, we suspect that the patient developed acute severe hepatic necrosis due to GTE. Similar cases of hepatotoxicity have been reported in the literature demonstrating hepatotoxicity of GTE.4 The United States Pharmacopeia (USP) has evaluated the safety data for green tea products and has compiled 34 reports of liver injury attributed to consumption of a variety of GTE preparations utilizing the Naranjo scale which has low specificity to DILI. Subsequently from those studies it was concluded that safety of GTE preparations was not updated with a safety label cautioning the public.5 The spectrum of liver injury extended from acute hepatitis to fulminant liver failure with 1 patient requiring liver transplantation.1 Our patient had acute severe hepatic necrosis and responded to prednisone therapy with complete resolution of normal liver function tests. Subsequently, the USP had recommended, a notice, expressing side effects of liver damage be placed on GTE products.1 Our case report suggests physicians should enquire from patients’ history of nutritional supplements. This report also should sound an alarm to practicing primary care physicians about GTE toxicity leading to liver failure. This is particularly a matter of concern for all health care providers due to rising intake of green tea preparations in the general population.
Green tea extract is derived from the plant Camellia sinensis, which is considered to have beneficial medicinal properties. It contains catechins mainly epicatechin, epicatechin-3-gallate, epigallotocatechin, and epigallocatechin-3-gallate (EGCG). In vitro studies, catechins have been demonstrated to express antioxidant activity, lipogenesis inhibition, and increase in several metabolic pathways (ie, Increased mRNA expression of acyl-CoA oxidase). In human liver microsome studies done in vitro, it has been shown that GTE inhibit CYP isoforms.6 Catechin EGCG is responsible for competitive inhibition of CYP2B6 and CYP2C8 and non-competitive inhibition of CYP2C19 and CYP3A.6 The molecular mechanism of DILI due to GTE is linked to disruption of intracellular calcium homeostasis and actin filaments.7 Furthermore, GTE activates apoptotic pathways by tumor necrosis factor alpha (TNF-alpha) and inhibition of mitochondrial function by dual effect on beta oxidation and respiratory chain enzymes leading to accumulation of lactate and reactive oxygen species.7 These metabolites may be responsible for hepatic cell injury. Interestingly, a randomized control trial was undertaken to test if GTE had liver toxicity and reduced cardiovascular disease (CVD) in healthy men (17 treatment group, 16 control group). The blood samples were collected 3 weeks later after they were given 6 capsules of GTE preparation throughout the day (equivalent to 714 mg/d green tea polyphenols) or placebo.8 The trial concluded no health benefits on CVD risk biomarker other than total: high-density lipoprotein (HDL) cholesterol ratio with no change in liver biomarkers in both the groups.8
Available evidence suggests GTE-induced reactions are idiosyncratic and not dose dependent.9 Recent investigations of genome-wide association studies (GWASs) have suggested new relations, between human leucocyte antigen (HLA) class I and II alleles and DILI.9 Recent investigations have identified risk alleles with substantially higher risk ratios for susceptibility to DILI.9 However, the precise mechanism of GTE-induced liver injury remains poorly defined. Further investigations are needed to identify molecular biomarkers associated with induction and perpetuation of cascades of metabolic pathways leading to hepatic necrosis.
Acknowledgments
We thank the patient for sharing her details, and thank Dr Padmanabhan Nair, Laila Philips, and Dr Kweku Hayford for advice. The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: BKS involved in drafting the manuscript or revising it critically for important intellectual content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SD agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Given final approval of the version to be published. RV involved in drafting the manuscript or revising it critically for important intellectual content. JJ made substantial contributions to conception and design of case report and took consent from patient. ML involved in drafting the manuscript or revising it critically for important intellectual content.
References
- 1. Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65:363–373. 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: a tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:537 10.3390/ijms17040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hidaka M, Okumura M, Fujita KI, et al. Effects of pomegranate juice on human cytochrome P450 3A (CYP3A) and carbamazepine pharmacokinetics in rats. Drug Metab Dispos. 2005;33:644–648. 10.1124/dmd.104.002824. [DOI] [PubMed] [Google Scholar]
- 4. Patel SS, Beer S, Kearney DL, Phillips G, Carter BA. Green tea extract: a potential cause of acute liver failure. World J Gastroenterol. 2013;19:5174–5177. 10.3748/wjg.v19.i31.5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liss G, Lewis JH. Drug-induced liver injury: what was new in 2008? Expert Opin Drug Metab Toxicol. 2009;5:843–860. 10.1517/17425250903018904. [DOI] [PubMed] [Google Scholar]
- 6. Teschke R, Zhang L, Melzer L, Schulze J, Eickhoff A. Green tea extract and the risk of drug-induced liver injury. Expert Opin Drug Metab Toxicol. 2014;10:1663–1676. 10.1517/17425255.2014.971011. [DOI] [PubMed] [Google Scholar]
- 7. Kotchen TA. Harrisons Principle of Internal Medicine 18th Edition. Harrisons principles of Internal Medicine. 2015. 10.1016/B978-141604485-7.50022-6 [DOI]
- 8. Frank J, George TW, Lodge JK, et al. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 2008;139:58–62. 10.3945/jn.108.096412. [DOI] [PubMed] [Google Scholar]
- 9. Kullak-Ublick GA, Andrade RJ, Merz M, et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66:1154–1164. 10.1136/gutjnl-2016-313369. [DOI] [PMC free article] [PubMed] [Google Scholar]


