Abstract
Pulmonary arterial hypertension (PAH) is a deadly vascular disease, characterized by increased pulmonary arterial pressures and right heart failure. Considering prior non-US studies of atrial arrhythmias in PAH, this retrospective, regional multi-center US study sought to define more completely the risk factors and impact of paroxysmal and non-paroxysmal forms of atrial fibrillation and flutter (AF/AFL) on mortality in this disease. We identified patients seen between 2010 and 2014 at UPMC (Pittsburgh) hospitals with hemodynamic and clinical criteria for PAH or chronic thromboembolic pulmonary hypertension (CTEPH) and determined those meeting electrocardiographic criteria for AF/AFL. We used Cox proportional hazards regression with time-varying covariates to analyze the association between AF/AFL occurrence and survival with adjustments for potential cofounders and hemodynamic severity. Of 297 patients with PAH/CTEPH, 79 (26.5%) suffered from AF/AFL at some point. AF/AFL was first identified after PAH diagnosis in 42 (53.2%), identified prior to PAH diagnosis in 27 (34.2%), and had unclear timing in the remainder. AF/AFL patients were older, more often male, had lower left ventricular ejection fractions, and greater left atrial volume indices and right atrial areas than patients without AF/AFL. AF/AFL (whether diagnosed before or after PAH) was associated with a 3.81-fold increase in the hazard of death (95% CI 2.64–5.52, p < 0.001). This finding was consistent with multivariable adjustment of hemodynamic, cardiac structural, and heart rate indices as well as in sensitivity analyses of patients with paroxysmal versus non-paroxysmal arrhythmias. In these PAH/CTEPH patients, presence of AF/AFL significantly increased mortality risk. Mortality remained elevated in the absence of a high burden of uncontrolled or persistent arrhythmias, thus suggesting additional etiologies beyond rapid heart rate as an explanation. Future studies are warranted to confirm this observation and interrogate whether other therapies beyond rate and rhythm control are necessary to mitigate this risk.
Keywords: atrial fibrillation, atrial flutter, pulmonary arterial hypertension
Introduction
Pulmonary arterial hypertension (PAH) is characterized by increased pulmonary arterial pressures that, over time, may precipitate right heart failure. In this population, both functional status and survival have been shown to be dependent on hemodynamics of the right ventricle, with significant changes in function impacting survival.1 While targeted therapies for PAH have made for significant improvement in functionality and mortality, the clinical course of the disease remains fraught with complications that also increase risk of mortality.2,3 One such complication is the development of atrial arrhythmias in the forms of atrial fibrillation and flutter (AF/AFL).4
Prior studies in small, predominantly European cohorts with PAH found that AF/AFL were detected in 11–13% within 1 year of PAH diagnosis and in up to 25.1% after 5 years.5,6 In these studies, AF/AFL arrhythmias were associated with poor prognosis and worse right atrial pressure and functional class.7,8 It has been suggested that these findings may be explained by a reliance on organized atrial activity (“atrial kick”) in the setting of right heart failure; persistent loss of coordinated atrial contraction may worsen an already fragile hemodynamic balance.9 Indeed, past studies found that restoration of sinus rhythm was associated with better long-term survival in patients with PAH.5,10
However, in the absence of a high burden of uncontrolled heart rate or persistent atrial arrhythmias, it is unclear whether isolated diagnoses of paroxysmal, rather than more permanent, AF/AFL may still be associated with worsened mortality. Furthermore, the clinical characteristics of these atrial arrhythmias in a more contemporary era of PAH therapies and management have not been studied in depth, nor have they been evaluated in a North American population. In this regional multi-center retrospective cohort study, we sought to define those clinical characteristics of AF/AFL in PAH and a related form of pulmonary hypertension (PH), chronic thromboembolic pulmonary hypertension (CTEPH), and to determine whether both paroxysmal and non-paroxysmal forms of these arrhythmias are associated with poor survival.
Methods
Study design and population
We conducted this retrospective cohort study using data collected as part of routine clinical care across all UPMC-affiliated hospitals (31 in total). All procedures were approved by the Institutional Review Board at the University of Pittsburgh (PRO11070366) and conformed to the standards of the Declaration of Helsinki.
We included all patients seen in participating centers who met World Health Organization (WHO) Pulmonary Hypertension Classification criteria for Group 1 (PAH) or Group 4 (CTEPH), a form of PH where distinct parallels of molecular pathogenesis exist with PAH and similar targeted pulmonary vasodilators are used to improve morbidity and mortality.11 We first identified patients who met hemodynamic criteria for pre-capillary PAH via the use of right heart catheterization performed at any time from January 2010 through December 2014, where pre-capillary PAH was defined as mean pulmonary artery pressure (mPAP) ≥ 25 mmHg, pulmonary capillary wedge pressure ≤ 15 mmHg, and pulmonary vascular resistance > 3 Wood Units.12 Next, our study team identified those who met criteria for WHO PH Group 1 or Group 4, based on review of clinical notes and relevant studies. The majority of patients had been evaluated by PH specialists adhering to standardized clinical practices to determined etiology of PH. Furthermore, our study team comprised PH specialists who offered an additional independent layer of adjudication of diagnosis. Patients with CTEPH who had undergone curative thromboembolectomy were not included. Thus, this study population comprised prevalent cases who were followed over time and were analyzed retrospectively.
Exposures and outcomes
AF/AFL arrhythmias served as both an exposure and an outcome in this study. We initially screened for AF/AFL using ICD codes and then confirmed the presence or absence of either of these atrial arrhythmias via chart review of available electrocardiograms (ECGs), rhythm strips, and cardiologist documentation. We determined the type of AF/AFL (notably, distinguishing paroxysmal versus non-paroxysmal arrhythmias) as well as duration and treatment strategies via chart review and standardized definitions.13 As a surrogate reflection of chronic left and right atrial pressures, respectively, we reviewed echocardiogram reports that were closest in time to the date of first right heart catheterization (RHC) and recorded the left atrial volume index (LAVI) and right atrial area (RAA). We also recorded echocardiographic measures of systolic and diastolic left heart dysfunction, including left ventricular ejection fraction (LVEF), ratio of early mitral inflow to average early mitral annular velocity (E/E’), and mitral flow deceleration time. If primary images were available, they were reviewed as necessary to fill in missing data. After additional review was completed, we had obtained LVEF in 99% of patients, E/E’ in 88%, mitral flow deceleration time in 68%, RAA in 92%, and LAVI in 90%. Tricuspid annular plane systolic excursion could not be obtained in a large proportion of patients, so we were unable to use this as an objective measure. Furthermore, we felt qualitative echocardiographic characterizations of right ventricular function were not suitable for the study, given the subjective nature of these retrospectively collected data. For every patient where serial heart rate recordings were available (which included the vast majority of all subjects), we also cataloged each heart rate documented in the medical record for both inpatient and outpatient encounters starting from the time of first RHC after January 2010 in the non-AF/AFL group and from date of first recorded atrial arrhythmia in the AF/AFL group. For patients without AF/AFL, 213 (out of 218) patients had serial heart rates measured, with a mean of 153 (71, 204; first, third quartiles) heart rate recordings per individual. For the non-paroxysmal AF/AFL patients, 35 (out of 39) patients had serial heart rates measured, with a mean of 204 (151, 259; first, third quartiles) heart rate recordings per individual. For paroxysmal AF/AFL patients, 37 (out of 40) patients had serial heart rates measured, with a mean of 184 (112, 241; first, third quartiles) heart rate recordings per individual. For 90.7% of patients, heart rate recordings were followed for at least 6 months after the above start dates, and for 83.5% of patients, heart rates were followed for at least 1 year.
Statistical analysis
We summarized continuous variables as mean ± SD and categorical variables as frequency (percentage). We compared characteristics of participants with and without AF/AFL using Student’s t-tests for continuous variables and chi-squared tests for categorical variables. Lastly, we used Cox proportional hazards regression with time-varying covariates (when appropriate) to (a) identify risk factors for the development of AF/AFL after PAH diagnosis and (b) assess the association between AF/AFL and 3-year survival. In the models assessing risk factors for the development of AF/AFL, participants who were diagnosed with AF/AFL before PAH (or for whom the timing of the diagnosis was unknown) were censored immediately, while the 42 patients developing AF/AFL after their diagnosis of PAH were followed until the development of arrhythmia for calculation of the time-to-event. In the models examining the association between atrial arrhythmia and 3-year survival, AF/AFL was treated as a time-varying covariate, meaning that participants were analyzed as unexposed before the first documentation of AF/AFL and as exposed after the first documentation of AF/AFL. In addition, we used separate Cox regression models to determine whether paroxysmal AF/AFL alone and non-paroxysmal AF/AFL alone were associated with reduced survival; we included permanent and persistent AF/AFL in the non-paroxysmal group. Finally, to evaluate the possibility that participants who died shortly after first developing paroxysmal AF/AFL could be inflating the association with reduce survival, we performed two additional sensitivity analyses, repeating the Cox regression after excluding participants who died within 7 days of AF/AFL diagnoses and excluding those who died within 30 days of AF/AFL diagnoses. For all regression models, we selected covariates, based on univariable analyses of characteristics, hemodynamic parameters, and echocardiographic measures that we considered to be potential confounders based on previous research and biological plausibility. Multiple imputation was used to allow the inclusion of patients with missing data for one or more of the covariates in multivariable regression models. We performed all statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Study population
Between January 2010 and December 2014, 691 patients met hemodynamic criteria for pre-capillary PAH; 297 of these were determined to have either Group 1 PAH (n = 266) or CTEPH (n = 31). Descriptive characteristics of the study population are shown in Table 1 and Table S1. An electrocardiographic diagnosis of atrial fibrillation or flutter was noted in 79 (26.5%) of all participants. As displayed in Table S1, approximately half of the atrial arrhythmias (50.6%) were determined to be paroxysmal in nature. Atrial fibrillation was observed in 46 participants (58.2%), atrial flutter in 25 participants (31.6%), and instances of both were seen in eight participants (10.1%).
Table 1.
Demographics & clinical characteristics of PAH patients.
| Full cohort | No AF/AFL | AF/AFL | p-value | |
|---|---|---|---|---|
| # Patients | 297 | 218 | 79 | |
| Descriptive characteristics | ||||
| Age | 57.6 ± 14.7 | 56.0 ± 14.6 | 61.8 ± 14.0 | <0.01 |
| Gender (male) | 97 (32.7%) | 55 (25.2%) | 42 (53.2%) | <0.01 |
| Body Mass Index | 28.9 ± 7.01 | 29.2 ± 7.16 | 28.1 ± 6.56 | 0.26 |
| Smoking status | 0.20 | |||
| Never | 204 (68.7%) | 156 (71.6%) | 48 (60.8%) | |
| Former | 80 (26.9%) | 53 (24.3%) | 27 (34.2%) | |
| Current | 13 (4.4%) | 9 (4.1%) | 4 (5.1%) | |
| Systolic blood pressure (SBP) | 121 ± 18.9 | 120 ± 19.4 | 124 ± 17.3 | 0.05 |
| Diastolic blood pressure (DBP) | 72.8 ± 10.7 | 72.9 ± 11.3 | 72.7 ± 9.09 | 0.91 |
| Congestive heart failure (CHF) | 49 (16.5%) | 31 (14.2%) | 18 (22.8%) | 0.07 |
| Diabetes mellitus | 37 (12.5%) | 26 (11.9%) | 11 (13.9%) | 0.64 |
| Chronic obstructive Pulmonary Disease (COPD) | 32 (10.8%) | 17 (7.8%) | 15 (19.0%) | <0.01 |
| Obstructive sleep apnea (OSA) | 39 (13.1%) | 25 (11.5%) | 14 (17.7%) | 0.15 |
| Vasodilator (any) | 275 (92.6%) | 204 (93.6%) | 71 (89.9%) | 0.22 |
| ERA | 68 (22.9%) | 51 (23.4%) | 17 (21.5%) | 0.72 |
| Prostacyclin | 100 (33.7%) | 79 (36.2%) | 21 (26.6%) | 0.11 |
| PDE5 | 234 (78.8%) | 171 (78.4%) | 63 (79.7%) | 0.86 |
| sGC Agonist | 11 (3.7%) | 8 (3.7%) | 3 (3.8%) | 0.96 |
| RHC-derived hemodynamics | ||||
| mPAP (mm Hg) | 45.8 ± 12.0 | 46.5 ± 12.3 | 44.0 ± 11.3 | 0.11 |
| PCWP (mm Hg) | 10.0 ± 3.15 | 9.79 ± 3.16 | 10.8 ± 3.03 | 0.02 |
| sPAP (mm Hg) | 75.3 ± 20.3 | 75.7 ± 20.4 | 74.2 ± 20.0 | 0.56 |
| TPG (mm Hg) | 35.8 ± 12.1 | 36.7 ± 12.4 | 33.2 ± 10.9 | 0.02 |
| CO (L/min) | 4.63 ± 1.43 | 4.60 ± 1.45 | 4.72 ± 1.37 | 0.51 |
| PVR (wood units) | 8.54 ± 4.29 | 8.87 ± 4.49 | 7.63 ± 3.53 | 0.02 |
| Echocardiography parameters | ||||
| LVED (cm) | 4.18 ± 0.77 | 4.16 ± 0.79 | 4.25 ± 0.70 | 0.39 |
| LVES (cm) | 2.77 ± 0.73 | 2.73 ± 0.74 | 2.87 ± 0.66 | 0.17 |
| SWT (mm) | 1.14 ± 0.27 | 1.13 ± 0.26 | 1.19 ± 0.27 | 0.10 |
| PWT (mm) | 1.10 ± 0.25 | 1.08 ± 0.25 | 1.15 ± 0.23 | 0.03 |
| LAD (cm) | 3.79 ± 0.82 | 3.68 ± 0.79 | 4.10 ± 0.84 | <0.01 |
| Left ventricular ejection fraction (LVEF) | 52.6 ± 8.63 | 53.9 ± 6.47 | 49.0 ± 12.1 | <0.01 |
| RAA | 21.5 ± 7.88 | 20.5 ± 7.08 | 24.2 ± 9.22 | <0.01 |
| LAVI | 28.7 ± 13.3 | 25.5 ± 10.5 | 37.1 ± 16.0 | <0.01 |
| TRJet | 3.79 ± 0.74 | 3.80 ± 0.74 | 3.76 ± 0.76 | 0.72 |
| PASP | 70.1 ± 22.8 | 70.2 ± 23.0 | 69.9 ± 22.3 | 0.91 |
| E | 81.0 ± 29.9 | 77.5 ± 27.6 | 90.3 ± 33.9 | <0.01 |
| Mitral flow deceleration time | 229 ± 72.4 | 234 ± 75.5 | 218 ± 62.9 | 0.15 |
| E’ | 8.74 ± 3.29 | 8.65 ± 3.23 | 8.98 ± 3.44 | 0.48 |
| E/E’ | 10.5 ± 5.83 | 10.2 ± 5.72 | 11.1 ± 6.12 | 0.25 |
| Diastolic dysfunction grade | 0.66 | |||
| 0 | 246 (82.8%) | 178 (81.7%) | 68 (86.1%) | |
| 1 | 3 (1.0%) | 2 (0.9%) | 1 (1.3%) | |
| 2 | 42 (14.1%) | 34 (15.6%) | 8 (10.1%) | |
| 3 | 6 (2.0%) | 4 (1.8%) | 2 (2.5%) |
ERA: endothelin-receptor antagonist; PDE5: phosphodiesterase-5 inhibitor; sGC: soluble guanylyl cyclase; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; sPAP: systolic pulmonary artery pressure; TPG: transpulmonary gradient; CO: cardiac output; PVR: pulmonary vascular resistance. LVED: left ventricular end-diastolic diameter; LVES: left ventricular end-systolic diameter; SWT: septal wall thickness; PWT: posterior wall thickness; LAD: left atrial diameter; RAA: right atrial area; LAVI: left atrial volume index; PASP: pulmonary artery systolic pressure.
Risk factors associated with AF/AFL in PAH and CTEPH patients
Compared with participants without AF/AFL, those with AF/AFL were older, more often male, had higher systolic blood pressures (SBPs), had lower LVEFs, and more often carried an ICD code for chronic obstructive pulmonary disease (COPD) in univariable analyses (Table 1). In addition, those with AF/AFL had altered hemodynamic and echocardiographic measures. In the multivariable Cox regression model, older age (OR 1.03, 95% CI 1.01–1.05, p-value < 0.001), male gender (OR 1.91, 95% CI 1.16–3.13, p-value 0.01), lower LVEF (OR 0.97, 95% CI 0.95–0.99, p-value 0.02), greater LAVI (37.1 ± 16.0 vs. 25.5 ± 10.5, p < 0.01) and greater RAA (24.2 ± 9.2 vs. 20.5 ± 7.1, p < 0.01) remained independently associated with the presence of atrial arrhythmias in PAH patients. By definition, AF/AFL patients displayed longer E wave filling as compared with those with organized atrial activity. Patients with these atrial arrhythmias tended to have higher pulmonary capillary wedge pressure (PCWP), consequently accompanied by decreased transpulmonary gradient (TPG) and pulmonary vascular resistance (PVR). Notably, no significant differences were noted in mean or systolic pulmonary arterial pressure (mPAP) or cardiac output (CO) among PAH/CTEPH patients with or without atrial arrhythmias. Importantly, there was also no difference in the use of pulmonary vasodilators between these groups, and over 90% of all PAH/CTEPH patients were treated with such medications.
Of the participants where the timing of their first AF/AFL diagnosis could be confirmed, 27 (34.2%) were diagnosed with AF/AFL prior to being diagnosed with PAH by RHC, while 42 (53.2%) were diagnosed with atrial arrhythmia at some point after the diagnosis of PAH was established (Table S2). Among those 42 participants, male gender, lower LVEF, and presence of ICD code for congestive heart failure (CHF) were associated with AF/AFL diagnosis in univariable analyses regardless of timing of PAH diagnosis. Older age, greater LAVI, greater RAA, and higher PCWP were associated with AF/AFL before, rather than after, PAH diagnosis.
AF/AFL treatment strategies are summarized in Table S3. The majority (86%) was managed with AV nodal blocking and/or anti-arrhythmic agent(s). Interventions attempting to restore sinus rhythm were performed in 31 patients (14 received cardioversion, 17 received ablation).
Increased mortality in PAH/CTEPH patients diagnosed with AF/AFL
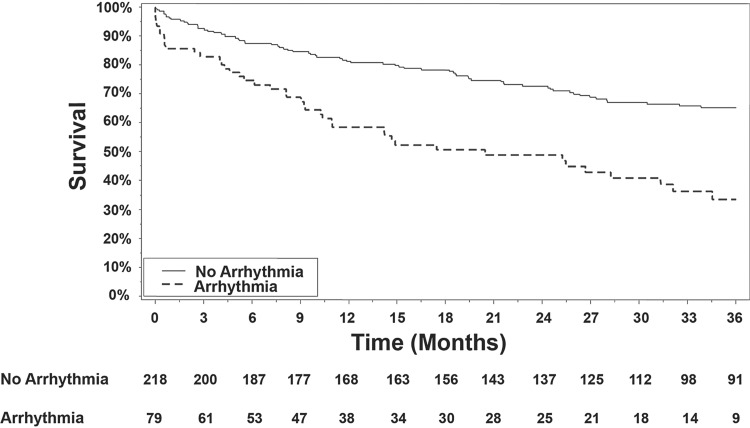
As displayed in Fig. 1, estimated survival was worse for PAH/CTEPH participants who were diagnosed with AF/AFL than for those without a diagnosis of atrial arrhythmia. In unadjusted Cox regression that treated AF/AFL as a time-varying exposure, a diagnosis of AF/AFL was associated with a 3.81-fold increase in the hazard of death (95% CI 2.64–5.52, p < 0.001). This association remained strong after adjustment for selections of clinical covariates, hemodynamic parameters, and echocardiographic measures (Table 2), indicating that after accounting for multiple combinations of potential confounders, the presence of atrial arrhythmia portends significantly worse prognosis in patients with PAH or CTEPH. These findings were generally consistent in sensitivity analyses analyzing PAH participants alone (i.e. excluding CTEPH participants). Of note, in additional exploratory analyses besides those reported, there was no single variable (clinical, structural, or hemodynamic) which significantly attenuated the main effect of AF/AFL on mortality.
Fig. 1.
Survival in PAH/CTEPH patients with AF/AFL compared with those without those atrial arrhythmias. By analysis of Kaplan–Meier curves, mortality after diagnosis of either atrial fibrillation or flutter was significantly elevated (17% at 90 days; 41% at 1 year), as compared with those without (7% at 90 days; 17% at 1 year). When evaluated as time-varying exposure, diagnosis of AF or AFL was associated with significantly increased mortality in PAH/CTEPH patients (unadjusted HR = 3.81, 95% CI 2.64–5.52, p < 0.001).
Table 2.
Associations between AF/AFL and 3-year survival in PAH/CTEPH patients.
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| Unadjusted | 3.81 | (2.64, 5.52) | <0.001 |
| Adjusted for clinical covariates* | 3.75 | (2.51, 5.59) | <0.001 |
| Adjusted for RHC parameters** | 4.08 | (2.77, 6.00) | <0.001 |
| Adjusted for echo parameters*** | 4.17 | (2.75, 6.31) | <0.001 |
| Adjusted for median follow-up heart rate**** | 3.90 | (2.69, 5.65) | <0.001 |
| Adjusted for max follow-up heart Rate**** | 3.93 | (2.71, 5.69) | <0.001 |
Adjusted for age, gender, BMI, smoking status, SBP, CHF, Diabetes, COPD, OSA
Adjusted for mPAP, PCWP, sPAP, CO, and PVR
Adjusted for LVEF, RAA, LAVI, E/E’, Mitral flow deceleration time, and DD grade
Adjusted for follow-up heart rate
Sensitivity analysis #1: PAH only (excluding CTEPH)
Unadjusted HR = 4.05, 95% CI (2.74–5.99), p < 0.001
Sensitivity analysis #2: Paroxysmal AF/AFL only
Unadjusted HR = 4.67, 95% CI (2.83–7.70), p < 0.001
Sensitivity analysis #3: Non-paroxysmal AF/AFL only
Unadjusted HR = 3.11, 95% CI (1.96–4.93), p < 0.001
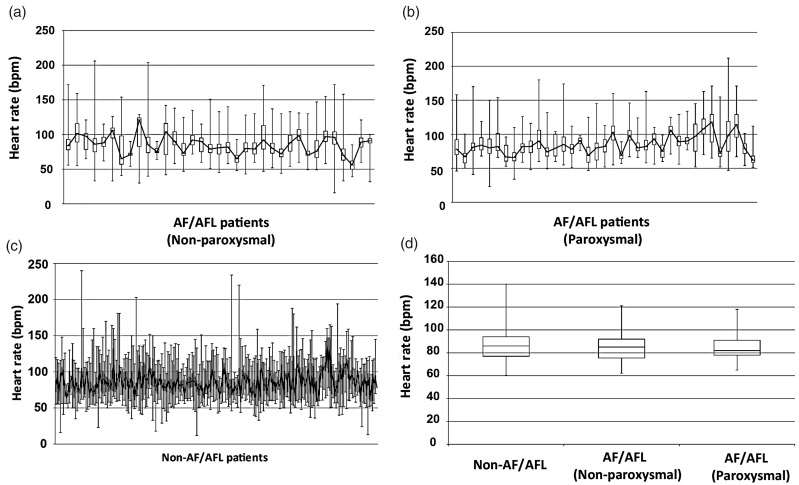
The presence of paroxysmal AF/AFL alone was significantly associated with increased mortality (HR = 4.67, 95% CI 2.83–7.70, p < 0.001), as was the presence of non-paroxysmal atrial arrhythmia alone (HR = 3.11, 95% CI 1.96–4.93, p < 0.001). A significant increase in both LAVI (37.1 ± 16.0 vs. 25.5 ± 10.5, p < 0.01) and RAA (24.2 ± 9.2 vs. 20.5 ± 7.1, p < 0.01) was noted in those patients with AF/AFL. However, the impact of AF/AFL on morality remained consistent with adjustment for these variables. This was also the case with adjusting either for median or maximum follow-up heart rates. Notably, median heart rates did not significantly differ between those with AF/AFL and those without (84.7 ± 13.3 bpm vs. 85.9 ± 12.5 bpm, median ± SD, p = 0.49) and did not differ between paroxysmal and non-paroxysmal AF/AFL patients (84.6 ± 13.4 bpm vs. 84.9 ± 13.2 bpm, median ± SD, p = 0.93) (Figure 2).
Fig. 2.
Longitudinal follow-up of clinically recorded heart rates of PAH patients with non-paroxysmal AF/AFL (35 patients) (a), with paroxysmal AF/AFL (37 patients) (b), and without atrial arrhythmias (213 patients) (c). Box and whisker plots contain center bars (connected by solid black line) representing median heart rates, bottom and top of boxes representing first and third quartile, respectively, and error bars representing maximum and minimum recorded heart rates. For each patient, documented heart rates for both inpatient and outpatient encounters were included, starting from the time of first RHC after January 2010 in the non-AF/AFL group and from date of first recorded atrial arrhythmia in the AF/AFL group. (d) As displayed in box and whisker plots, average median heart rates did not significantly differ among these three cohorts (p > 0.05).
Finally, to rule out an explanation that PAH/CTEPH patients who died shortly after initial “paroxysmal” arrhythmia could be inflating the mortality increase, two additional sensitivity analyses were performed (Table 2). Of 40 participants diagnosed with paroxysmal AF/AFL, 7 died within 7 days of first event while 13 died within 30 days of first event. In separate Cox regression models that excluded these participants, the associations of between paroxysmal AF/AFL and reduced survival remained (HR = 3.96, 95% CI 2.31–6.77, p < 0.001 and HR = 2.95, 95% CI 1.61–5.42, p < 0.001, respectively), suggesting that the association between AF/AFL and increased mortality was not merely a function of a few patients developing AF/AFL immediately before death.
Discussion
In this regional multi-center North American cohort of 297 PAH/CTEPH patients, AF and AFL were found to be common atrial arrhythmias, occurring in over 25% of the population over a 4-year period of study. In multivariable analysis, older age, male gender, lower LVEF, and greater LAVI and RAA were significant risk factors for developing AF/AFL in the PAH/CTEPH population. Furthermore, these rhythms were associated with a high risk of mortality. Importantly, this risk persisted after adjusting for potential confounders, including clinical covariates, hemodynamic severity of PH, as well as atrial and left ventricular structural indices as reflected by echocardiographic measures. Finally, both non-paroxysmal as well as paroxysmal AF/AFL were associated with higher mortality risk, even after controlling for patients who died within 30 days of first atrial arrhythmia episode and ruling out any substantial differences in heart rate control. Thus, elevated mortality persisted—even in the absence of uncontrolled or persistent arrhythmias—indicating other potential etiologies beyond rapid heart rate may exist to explain the risk conferred by AF/AFL.
Prior studies have reported an increased mortality risk of supraventricular tachycardias in PAH patients in Europe and China.5,6,10,14 Geographic, cultural, and financial discrepancies among countries worldwide drive stark differences in medical care and could influence the mortality risk seen in PAH patients with AF/AFL. Previous studies have reported significant demographic differences between PAH populations in the USA and those in Europe.15 There are also notable differences in the guidelines for management of atrial arrhythmias between the major US cardiology societies and the European Society of Cardiology.16 Despite those differences, however, our findings demonstrated that atrial arrhythmias are associated with increased mortality in this American cohort as well, thus adding to a growing body of evidence, given its consistency despite known and often confounding variations in management practices and guidelines of PAH worldwide.17
One proposed theory put forth in prior studies for this observation of increased mortality among PAH patients with AF/AFL argues that the acute disruption of the already fragile hemodynamics by these rhythms—either via prolonged episodes of AF/AFL or via rapid ventricular rates—worsens right heart failure.4 Consistent with this model, prior studies found that survival was particularly reduced in patients suffering from permanent supraventricular arrhythmias.5,10 Our findings also demonstrated a significant increase in mortality in PAH/CTEPH patients with non-paroxysmal, and thus permanent or persistent, episodes of these rhythms. Importantly, however, our study also found an elevated mortality rate in those with paroxysmal AF/AFL and thus a presumably lower arrhythmic burden. This association persisted after excluding immediate death following first arrhythmia episode and, notably, after confirming well-controlled heart rates that were not substantially different than the non-paroxysmal AF/AFL group, as well as those patients without history of atrial arrhythmia.
Thus, at least for some patients, such as those with paroxysmal AF/AFL, another etiology besides prolonged or rapid arrhythmic disturbances of normal hemodynamics may underlie the association with increased mortality. For instance, paroxysmal episodes of atrial arrhythmias may signify an environment prone to arrhythmogenic foci, which could predispose to more catastrophic arrhythmias. In a recent observational study of patients with heart failure with preserved ejection, those with concomitant atrial fibrillation were found to be at increased risk for sudden cardiac death.18 Alternatively, elevated pulmonary vasculature remodeling with resultant increased afterload placed on the right ventricle over time leads to remodeling in both the ventricles and atria, a known risk factor for conduction system disturbance.19,20 Finally, abnormal atrial rhythms may be the result of advanced, long-standing right heart overload or independent right heart dysfunction, which itself represents driving features of severity of disease and mortality in PAH/CTEPH. This is supported by prior observations that atrial arrhythmias attributed to PAH generally do not manifest until an average of 3.5 years after initial PAH diagnosis.4,6 Similarly, roughly half of our cohort were first diagnosed with AF/AFL sometime after the diagnosis of PAH. This is also consistent with our finding that RAA, which has been suggested as a reasonable marker of right ventricular dysfunction, was significantly increased in the AF/AFL group, and with a recent study that found right atrial size to predict supraventricular tachycardia in PAH.21,22 However, our data also suggest that overwhelming right heart dysfunction in AF/AFL is unlikely to be the sole explanation for increased mortality, particularly since adjusting for RAA did not mitigate the mortality risk (Table 2) and since patients with atrial arrhythmias in our study displayed less severe pulmonary vascular hemodynamic compromise as compared with those without AF/AFL (as reflected by TPG and PVR, Table 1). Yet, it should be acknowledged that with retrospective data, all of these notions remain speculative, and future prospective studies will be necessary to reveal more definitive mechanistic explanations that drive this mortality risk independent of heart rate control per se.
Our analyses also demonstrated that AF/AFL are common arrhythmias diagnosed in US PAH/CTEPH patients, with specific risk factors for manifestation including older age, male gender, elevated LAVI and RAA, and modest depressions of LVEF (Table 1). The association of male gender to AF/AFL in PAH/CTEPH patients is notable, especially in the context of increased mortality associated with these arrhythmias and the clinical observation that, while women are more prone to develop PAH, women display enhanced survival compared with males, yet AF/AFL has not been included consistently as a covariate in those survival analyses. Thus, a higher predisposition to AF/AFL in men with PAH/CTEPH males could offer a key insight and direction for a molecular explanation for this so-called “gender paradox.” In addition, prior studies implicated indices of more severe hemodynamic stress as risk factors for developing AF/AFL such as elevations of right ventricular diameter and left atrial area by echocardiography and higher right atrial pressure and PVR by invasive hemodynamics.10 Our data demonstrated that some, but not all, of these indices of hemodynamic compromise were risk factors for AF/AFL. Yet, as expected with known functional connections between increased left atrial pressure and size with these atrial arrhythmias, we found that patients with AF/AFL tended to have higher PCWP and LAVI (Table 1). These inter-study discrepancies could be explained by potential differences in severity of PAH in our cohort as compared with prior studies, particularly given geographical differences of our study, the contemporary advances in pulmonary vasodilator therapy, and consequent alteration of the composition of PAH patient severity in the USA.15
It is worth noting that some clinical covariates seen in this PAH population are known to be independently associated with an increased risk of mortality but predispose to PH subtypes beyond the accepted clinical definition of PAH or CTEPH—specifically left heart disease.24 To ensure accuracy in our diagnoses of PAH/CTEPH, we utilized two layers of adjudication. First, WHO PH Group 1 or 4 patients were identified by clinical notes of treating physicians, many of whom were PH specialists well versed in determining classification in WHO Group 1 or Group 4 PH. To that point, the vast majority of these patients were treated appropriately with vasodilator therapy. Second, our study team also comprised independent PH specialists who corroborated those diagnoses. Notably, however, a degree of diastolic dysfunction was observed in a small portion of both PAH and CTEPH groups (Table 1), thus reflecting a degree of phenotypic impurity in the cohort that we feel is more representative of the real-world PAH population where coexisting comorbidities exist. While such diastolic dysfunction did not carry strong attenuating effects on the association of AF/AFL with mortality in this population (Table 2), such phenotypic overlap highlights the need for improved means of classification beyond patient characteristics and hemodynamics.
Limitations of this study and future directions of investigation are worth noting. These include its retrospective design, which cannot determine causality, and small sample size, which can increase the possibility of type II error. Similarly, due to limitations in sample size, we were unable to comment on associations and outcomes with specific etiologies in the Group 1 PAH patients, which would be an interesting point of future inquires. Although including multiple centers, this was a regional study and may not reflect the demographics of PAH/CTEPH populations in other areas of the USA. PH etiology was determined based on treating physician documentation and diagnosis, as opposed to independent adjudication of the available data by a PH specialist not affiliated directly with that patient’s clinical management. However, we feel the likelihood of incorrect categorization was low, as the majority of the documents reviewed were authored by PH specialists at UPMC who adhere to standard guidelines for the determination of PH etiology and performed the appropriate diagnostic testing to do so.
Importantly, because of the limited sample size for both PAH patients and particularly CTEPH patients, there was insufficient power to discern potentially differential associations between mortality and various rate versus rhythm AF/AFL treatment strategies in this population, or whether rate/rhythm control alone are associated with reduced mortality risk in AF/AFL patients. Such analysis was precluded by the fact that confounding strategies besides rate control tend to be used in patients with more refractory or poorly controlled disease. Specifically, within our study, attempts to restore or maintain sinus rhythm were performed in 31 participants. Yet, although prior studies suggested that conversion of sinus rhythm was associated with significantly improved functional status and survival in PAH patients, there are no existing data regarding the type of rhythm control strategy best suited for this population, either via ablation, early cardioversion, or anti-arrhythmic agents.5,6,14 Alternatively, beyond rhythm control, future studies should also address the question of whether use of atrioventricular (AV) nodal blocking agents would be helpful or harmful in these patients for rate control. Notably, the use of AV nodal blocking agents has long been the standard of care for rate control in AF/AFL, but these agents often also have negative ionotropic effects, and it has been proposed that use of these agents may impair right heart function.4 However, more contemporary data have indicated that AV nodal agents such as carvedilol are safe in PAH patients, and the potential utility of such drugs, particularly in cases where rate control would be useful such as AF/AFL, is being reconsidered for this patient population.25
In summary, our findings offer evidence demonstrating both the prevalence and mortality risk of PAH/CTEPH patients with atrial arrhythmias. When considered with prior small studies in Europe and China, these data together argue that AF/AFL are associated with reduced survival in PAH/CTEPH. Interventional trials are needed to determine whether aggressive rate and rhythm control improves survival for PAH patients with AF/AFL. In addition, further investigation is warranted to evaluate potential mechanisms underlying the association between AF/AFL and reduced survival. Such studies could lead to specialized recommendations regarding the management of these clinical conditions, which currently do not exist in detail in Europe or the USA.26,27 Consequently, PAH patients with AF/AFL may benefit from referral to centers specializing in PAH. In that context, larger prospective studies in the future may be more feasible to answer these questions regarding the molecular and pathophysiologic etiology of these atrial arrhythmias in this clearly susceptible PAH and CTEPH population.
Acknowledgments
We thank M. Saul and O. Marroquin for expert guidance in extraction of clinical bioinformatic data from the electronic health record system at the University of Pittsburgh Medical Center.
Author Contributions
B.S., A.D.A., and S.Y.C. conceived the study. B.S., A.H., T.G., A.D.A., M.V.G., and S.Y.C. designed the analyses. A.H. and F.W.T. performed the computational extraction of clinical bioinformatic data. For verification of bioinformatic data B.S., A.K., E.C., C.M.H., R.H.Z., S.Y.C., and M.V.G. performed manual chart review and adjudication. B.S., A.D.A., and S.Y.C. wrote the manuscript. All authors participated in interpreting the results and revising the manuscript.
Conflict of interest
SYC has served as a consultant for Actelion (Significant), Gilead, Pfizer, and Vivus (Modest). The authors declare no other conflicts of interest.
Funding
This work was supported by NIH grants R01 HL124021, HL 122596, HL 138437, UH2 TR002073, and AHA grant 18EIA33900027 (S.Y.C.) as well as T32 HL083825 (M.V.G.).
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 2.Liu HL, Chen XY, Li JR, et al. Efficacy and safety of pulmonary arterial hypertension-specific therapy in pulmonary arterial hypertension: A meta-analysis of randomized controlled trials. Chest 2016; 150: 353–366. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004; 126: 78S–92S. [DOI] [PubMed] [Google Scholar]
- 4.Rajdev A, Garan H, Biviano A. Arrhythmias in pulmonary arterial hypertension. Prog Cardiovasc Dis 2012; 55: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsson KM, Nickel NP, Tongers J, et al. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol 2013; 167: 2300–2305. [DOI] [PubMed] [Google Scholar]
- 6.Tongers J, Schwerdtfeger B, Klein G, et al. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J 2007; 153: 127–132. [DOI] [PubMed] [Google Scholar]
- 7.Corciova FC, Arsenescu-Georgescu C. Prognostic factors in pulmonary hypertension. Maedica (Buchar) 2012; 7: 30–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Rottlaender D, Motloch LJ, Schmidt D, et al. Clinical impact of atrial fibrillation in patients with pulmonary hypertension. PLoS One 2012; 7: e33902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Showkathali R, Tayebjee MH, Grapsa J, et al. Right atrial flutter isthmus ablation is feasible and results in acute clinical improvement in patients with persistent atrial flutter and severe pulmonary arterial hypertension. Int J Cardiol 2011; 149: 279–280. [DOI] [PubMed] [Google Scholar]
- 10.Wen L, Sun ML, An P, et al. Frequency of supraventricular arrhythmias in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol 2014; 114: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DT, Hemnes AR. Current concepts in the pathogenesis of chronic thromboembolic pulmonary hypertension. Pulm Circ 2016; 6: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 13.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Cano MJ, Gonzalez-Mansilla A, Escribano P, et al. Clinical implications of supraventricular arrhythmias in patients with severe pulmonary arterial hypertension. Int J Cardiol 2011; 146: 105–106. [DOI] [PubMed] [Google Scholar]
- 15.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: How REVEAL differs from historic and non-US Contemporary Registries. Chest 2011; 139: 128–137. [DOI] [PubMed] [Google Scholar]
- 16.Andrade JG, Macle L, Nattel S, et al. Contemporary atrial fibrillation management: A comparison of the current AHA/ACC/HRS, CCS, and ESC guidelines. Can J Cardiol 2017; 33: 965–976. [DOI] [PubMed] [Google Scholar]
- 17.Maron BA, Ryan JJ. Treatment differences in pulmonary arterial hypertension management. Pulm Circ 2016; 6: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okin PM, Bang CN, Wachtell K, et al. Relationship of sudden cardiac death to new-onset atrial fibrillation in hypertensive patients with left ventricular hypertrophy. Circ Arrhythm Electrophysiol 2013; 6: 243–251. [DOI] [PubMed] [Google Scholar]
- 19.Archer S, Rich S. Primary pulmonary hypertension: A vascular biology and translational research ‘Work in progress’. Circulation 2000; 102: 2781–2791. [DOI] [PubMed] [Google Scholar]
- 20.Farber H, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004; 351: 1655–1665. [DOI] [PubMed] [Google Scholar]
- 21.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. quiz 86–88. [DOI] [PubMed] [Google Scholar]
- 22.Waligora M, Tyrka A, Miszalski-Jamka T, et al. Right atrium enlargement predicts clinically significant supraventricular arrhythmia in patients with pulmonary arterial hypertension. Heart Lung 2018; 47: 237–242. [DOI] [PubMed] [Google Scholar]
- 23.Foderaro A, Ventetuolo CE. Pulmonary arterial hypertension and the sex hormone paradox. Curr Hypertens Rep 2016; 18: 84. [DOI] [PubMed] [Google Scholar]
- 24.Wijeratne DT, Lajkosz K, Brogly SB, et al. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: A population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes 2018; 11: e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perros F, de Man FS, Bogaard HJ, et al. Use of beta-blockers in pulmonary hypertension. Circ Heart Fail 2017, pp. 10. [DOI] [PubMed] [Google Scholar]
- 26.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 2016; 69: 177. [DOI] [PubMed] [Google Scholar]
- 27.Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest 2014; 146: 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]