Abstract
Background:
Head and neck (H&N) cancers account for about 5% of all malignant tumours. Pain is one of the most feared consequences of H&N neoplasms and is experienced by up to 80% of patients and worsens their quality of life inhibiting speaking, eating, drinking or swallowing. Nevertheless, pain is still often underestimated and undertreated.
Objectives:
The role of opioids in cancer pain has been well established but evidences about the role and the relative effectiveness of opioids such as fentanyl in the context of H&N cancer pain remains unclear.
Methods:
A literature review based on the guidance of the Centre for Reviews and Dissemination was conducted. An iterative approach was used starting with an electronic search in the MEDLINE database. The search terms ((‘Neoplasms’[Mesh]) AND ‘Head and Neck Neoplasms’[Mesh]) AND ‘Fentanyl’[Mesh] were used.
Results:
A total of 18 publications were found by the first performed search on PubMed. Other publications concordant with our aim were found by cross-reference. Considering inclusion and exclusion criteria for our review, eight papers resulted eligible for analysis.
Conclusion:
Fentanyl transdermal therapeutic system (TTS) seems to be an important option, thanks to the way of administration, the good safety and tolerability profiles to control baseline pain. For breakthrough cancer pain (BTcP), several formulations of transmucosal fentanyl are available. All the formulations seem to be active and safety but we lack head-to-head studies of fentanyl versus other strong opioids, as well as with different formulation of fentanyl, particularly for BTcP where H&N cancer population is very poorly represented.
Keywords: Head and neck cancer, pain, fentanyl, breakthrough pain, opioids
Introduction
Head and neck (H&N) cancers account for about 5% of all malignant tumours, but are a major cause of morbidity and mortality in oncologic patients.1–5 In 2017, it is estimated that about 63,030 new cases of oral cavity, pharyngeal and laryngeal cancers will occur, which account for about 3.7% of new cancer diagnoses in the United States.6
Surgery, radiotherapy (RT) and systemic treatments (chemotherapy (CT) or biotherapy) represent today the three most important interventions, from curative to palliative setting.
H&N cancers have a devastating impact on patient’s lives as both disease and treatment can affect the ability to speak, swallow and breathe. Patients frequently experience several cancer-related problems, such as mucositis, difficult to swallow or chew, odynophagia, malabsorption, vomiting, infections or severe constipation.7–10 These conditions limit the oral intake of food and drugs, impacting patient’s quality of life.11
Pain is one of the most feared consequences of carcinoma and is experienced by up to 80% of patients suffering from malignancies of the H&N region. The management of pain is often a real challenge in this setting of patients.
Pain is very common, affecting most patients at any stage. It may be disease or treatment related, acute and/or immediate or persistent and/or lifelong. The application of the international guidelines for cancer-related pain in this particular setting is not easy. The stepwise approach based on the three levels of pain treatment (level 1: Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), level 2: weak opioids and level 3: opioids) is limited by reduction in oral intake and the rapid modification of pain during the treatments.12–14 Alternative routes of administration, like the transdermal one, are strongly recommended.15 The same route is also indicated in patients with poor compliance to medications and in patients who are already taking many drugs orally.
Moreover, H&N cancer patients are often susceptible of breakthrough cancer pain (BTcP), considered as any transitory pain exacerbation despite a controlled baseline pain management regimen in place. BTcP may arise either unpredictably or predictably, possibly trigged by movement, exercise or other kind of activities.16–18
Thus, pain management must be individualized taking into consideration availability of drugs, patient’s clinical situation and intensity of pain. The pharmacologic pain management should be based on an ‘around-the-clock’ (ATC) treatment to control baseline pain and additional drugs for BTcP. Opioid therapies represent the best choice to control pain for the majority of cancer patients, both for baseline and BTcP, depending on their relative duration and onset of analgesia.19–21
The choice of drug and formulation depends on whether the patient can swallow, is vomiting or has a nasogastric (NG) or gastrostomy tube in situ.
Fentanyl is a synthetic opioid pain medication with a rapid onset and short duration of action. It is a potent agonist for μ-opioid receptors in the brain. Fentanyl is 50 to 100 times more potent than morphine.22
One of the advantages of fentanyl is that it is available in different ways of administration: transdermal patch, sublingual tablet, intranasal spray, pectin-based nasal spray and so on.23–29 Each way of administration responds to the need for cover baseline pain or control BTcP.
In this review, we report the use of fentanyl-based drugs for the management of baseline or BTcP in H&N cancer patients reported in the literature.
Methods
A literature review based on the guidance of the Centre for Reviews and Dissemination was conducted.30
An iterative approach was used starting with an electronic search in the MEDLINE database (via PubMed – customized range date until April 2017)
The search terms ((‘Neoplasms’[Mesh]) AND ‘Head and Neck Neoplasms’[Mesh]) AND ‘Fentanyl’[Mesh] were used.
The Medical Subject Headings (MeSH) thesaurus was adopted to perform a more refined search strategy.
Citation tracking and search for all related eligible articles in PubMed were performed.
Study inclusion and exclusion criteria
Inclusion criteria were as follows: patients with H&N cancer older than 18 years; all types of cancer pain due to the neoplasm or as a result of active treatments (surgery, RT, systemic therapies); fentanyl as primary treatment for cancer pain; and systematic review, pooled analysis, randomized controlled trials and controlled trials published in the last 10 years.
Despite the paucity of randomized controlled trials (level of evidence 1) or other clinical trials with these topics, we excluded case reports, case series, non-systematic reviews, guidelines, consensus, Delphi studies and letters to the editor from our analysis because of the high risk of bias. We also excluded papers about post-operative pain and upper gastrointestinal (GI) tumours. The aim of this review was to analyse the effective use of fentanyl formulation among patients with H&N cancer for background and breakthrough pain. The full-text versions of the search results were obtained and analysed. Assessment of the literature was performed by two of the authors (M.M. and M.F.).
Data extraction
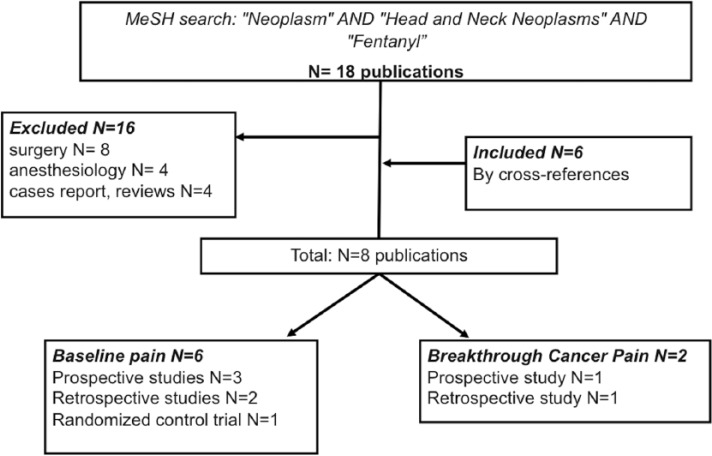
Considering the paucity and the low evidence of reported data, the use of the Centre for Reviews and Dissemination’s criteria for data extraction was not applicable.30 Instead, the following information was extracted in an Excel sheet by one author and checked by a second author: title of the paper, reference, first author, year of publication, resource, type of study and level of evidence. A total of 18 publications were found by the first performed search on PubMed. Other publications concordant with our aim were found by cross-reference. Considering inclusion and exclusion criteria for our review, eight papers resulted eligible for analysis (Figure 1). Two articles were about managing BTcP in H&N cancer patients: one retrospective study and one prospective study. Six publications were about managing baseline pain in H&N cancer patients: three prospective studies, two retrospective studies and one randomized control trial. The characteristics of the publications included in this review are summarized in Table 1.
Figure 1.
Diagram of studies founded by MeSH in this review.
Table 1.
Characteristics of all eligible publications.
| Title | Authors | Type | N patients | Endpoint/conclusions | Total number of study |
|---|---|---|---|---|---|
| Use of fentanyl for BTcP in H&N cancer patients | |||||
| Fentanyl pectin nasal spray for painful mucositis in head and neck cancers during intensity-modulated radiation therapy with or without chemotherapy | Mazzola et al. | Retrospective study | 40 | Efficacy, tolerability satisfaction | |
| Fentanyl pectin nasal spray as treatment for incident predictable breakthrough pain (BTP) in oral mucositis induced by chemoradiotherapy in head and neck cancer | Bossi et al. | Prospective study | 17 | Efficacy when swallowing | |
| Total BTcP | 2 | ||||
| Use of fentanyl for baseline pain in H&N cancer patients | |||||
| Transdermal fentanyl in the long-term treatment of cancer pain: a prospective study of 50 patients with advanced cancer of the gastrointestinal tract or the head and neck region | Grond et al. | Prospective study | 50 (22 H&N cancer) | Efficacy and safety | |
| Methadone is superior to fentanyl in treating neuropathic pain in patients with head-and-neck cancer | Haumann et al. | Randomized controlled trial | 52 | Efficacy and safety | |
| Transdermal fentanyl for pain caused by radiotherapy in head and neck cancer patients treated in an outpatient setting: a multicenter trial in Taiwan | Chang et al. | Prospective study | 88 | Efficacy, safety, QoL, Long term tolerance |
|
| Transdermal fentanyl for pain due to chemo-radiotherapy-induced oral mucositis in nasopharyngeal cancer patients: evaluating efficacy, safety, and improvement in quality of life | Guo et al. | Prospective study | 78 | Efficacy, safety, QoL | |
| Pain management during radiotherapy and radiochemotherapy in oropharyngeal cancer patients: single-institution experience | Konopka-Filippow et al. | Retrospective study | 42 | Efficacy | |
| Opioid prescribing for cancer pain during the last 3 months of life: associated factors and 9-year trends in a nationwide United Kingdom cohort study | Higginson and Gao | Retrospective study | 29,825 (1541 H&N) |
Time trends and characteristics of opioid analgesic prescribing | |
| Total baseline pain | 6 | ||||
| Total studies considered | 8 | ||||
Fentanyl in baseline pain for H&N cancer patients
To control baseline pain for patient suffering from H&N cancer with ATC treatment, fentanyl is a possible treatment, especially by transdermal (TTS) patch. Several studies were published in this field, with some data hereafter reported.
The experience of Higginson and Gao31 aimed to determine time trends and characteristics associated with opioid analgesic prescriptions to patients with cancer who are approaching the end of life in the United Kingdom. The analysed data were from 29,825 patients, including 1541 (5.2%) H&N cancer patients. Higginson stated that in the last 3 months of life, 43.6% of patients received at least one prescription of opioids: fentanyl was used in the 10.2% of patients, more frequently for H&N patients. The authors concluded that transdermal preparations might be more employed in H&N cancer because of difficulties with oral medication, as usually observed in clinical practice.
Another prospective study conducted by Grond et al.32 evaluated the combination of initial dose titration with patient-controlled analgesia (PCA) and long-term treatment with transdermal fentanyl among 50 cancer patients (44% H&N, 56% GI tract) requiring opioids for severe pain. All the patients were already in treatment with opioid for baseline pain; the previous opioids were discontinued and PCA with intravenous fentanyl was started. The dose of self-administered intravenous fentanyl in the first 24 hours was used to calculate the dose of fentanyl TTS, followed by a titration period of 1 week. The TTS was changed in 72 hours. Pain intensity was measured four times per day using self-assessment numeric scale (0 = no pain to 100 = pain as bad as could be). Mean pain intensity decreased from 45 ± 21 to 19 ± 15 in the titration phase and 15 ± 11 during long-term treatment. No severe side-effects were observed during long-term treatment. Moreover, activity, general state of health, mobility and mood were improved in comparison with the pre-study situation and the authors concluded that PCA is useful for initial dose finding of fentanyl TTS.
An interesting randomized controlled trial, focused on neuropathic pain, was conducted by Haumann and colleagues. Authors compared oral methadone with transdermal fentanyl in treating neuropathic pain in patients with H&N cancer.33 The specific choice of methadone was based on the additional effect of methadone on the N-methyl-D-aspartate (NMDA) receptors, besides an opioid receptor–mediated effect. A total of 52 strong opioid-naïve patients with pain score (Numerical Rating Scale (NRS) > 4) and a neuropathic pain component (Douleur Neuropathique 4 (DN4) > 4) were included. Half of the study population was treated with methadone and half with fentanyl. The results described a better reduction in NRS with methadone versus fentanyl, but the difference was significant only at first and third weeks. An important limitation of the study, in addition to the small sample size, was the significant loss of patients to follow-up in only 5 weeks.
A multicentre prospective study was conducted by Chang et al.34 on 163 H&N cancer patients assessing the efficacy, safety and long-term tolerance of transdermal fentanyl as treatment for baseline pain. All patients were in treatment with RT or RT + CT. The strength of this study is the multicentre characteristic, the ample sample and the long observation time. The limitations are that the cancer treatments were not standardized and only 88 patients completed the study. Also, there was a significant dispersion of the sample over the time. Transdermal fentanyl revealed to be effective and relatively easy to use in this setting.
To evaluate efficacy, safety and improvement in quality of life (QoL) of the transdermal fentanyl for the treatment of baseline pain due to oral mucositis in 78 patients diagnosed with nasopharyngeal cancer, a single-centre prospective study was conducted by Su-Ping Guo.35 All patients were in treatment with same RT + CT protocol. Pain and QoL were systematically evaluated through the NRS pain scale before and after the analgesic treatment and the Karnofsky Performance Status (KPS) and SPAASMS (Score for pain, physical activity levels, additional pain medication, additional physician/emergency room visits, sleep, mood and side-effects) before and 3 days after the start of the protocol. Notwithstanding, the study is limited by the presence of a single centre and by the absence of a control group, the pain relief comparing before/after treatment scores was significant with the use of the transdermal patch. The last analysed study is a retrospective single-institution experience written by Konopka-Filippow and colleagues. The primary endpoint of the study was the efficiency of the analgesic treatment in oropharyngeal cancer patients undergoing RT + CT.36 In total, 42 patients were assessed: they were, therefore, treated with different analgesic protocols that made the different groups of subjects non-comparable. In 21 patients, strong opioids were used and only in 11 patients a TTS delivering buprenorphine or fentanyl was used. TTS was found to be a slightly more popular way of administering strong opioids, probably because of the convenient method of application and the assurance of constant pain control.
Fentanyl in BTcP for H&N cancer patients
The management of acute, uncontrolled BTcP may require parenteral opioids, such as morphine, given their rapid onset of analgesia.37 Moreover, a lot of rapid-onset fentanyl-based drugs are now available to manage BTcP. The collective group of rapid-acting fentanyl-based products is referred to transmucosal immediate-release fentanyl (TIRF), including sublingual tablets, sublingual spray, intranasal spray, pectin-based nasal spray, buccal tablets and buccal soluble films. TIRFs have an immediate onset of action within minutes and 1–2 hours duration of action.38 The lists of available TIRFs and the route of administration are reported in Table 2.
Table 2.
List of transmucosal immediate-release fentanyl available and way of administration.
| Sublingual | Intranasal | Buccal | Oral/buccal |
|---|---|---|---|
| Fentanyl citrate tablet | Intranasal fentanyl | Fentanyl effervescent tablet | Fentanyl citrate lozenge |
| Fentanyl spray | Fentanyl citrate pectin spray | Fentanyl soluble film |
BTcP in patients with H&N cancer can be unpredictable, but a lot of conditions linked to the clinical situation can lead to a BTcP episode.
RT is often the first choice in the treatment of this type of malignancies. Patients undergoing RT can experience several predictable pain situations: the necessity to lie still on a treatment table for a sustained time during therapy, wearing of the customized immobilization mask, and odynophagia and pain during swallowing related to mucositis.39,40 The uncontrolled BTcP can lead to interruption of treatment schedule with a potentially negative impact on outcomes.41 Several studies investigated the role of immediate-release fentanyl to control predictable pain in H&N cancer patients.
A retrospective study conducted by Mazzola et al. evaluated the effectiveness and tolerability of rapid-onset opioid (especially fentanyl pectin nasal spray (FPNS)) in a cohort of H&N patients suffering from painful mucositis affecting swallowing function during RT ± CT. Results from 40 patients were analysed. The type of background pain was neuropathic in all patients, frequently associated to nociceptive component (n = 11). The most effective route of administration was judged to be transmucosal intranasal route. In fact, in patients with H&N cancer, oral transmucosal administration may be an issue because of sticky saliva, xerostomia, infection or oral ulcerations, thus nasal products could be preferred. It is important to underline that in this study, the authors declared that the BTcP was not present at the baseline, but it appeared approximately after 3 weeks from the beginning of RT treatment. FPNS showed an acceptable safety activity profile in predictable BTcP due to painful mucositis during CT-RT treatment. FPNS was also effective in reducing the mucositis sequelae and allowing the completion of RT scheduled scheme. Moreover, patients declared satisfaction in terms of ease of use. Patients’ body mass index (BMI) was stable during treatment. Otherwise, the number of meals per day significantly decreased after 3 weeks (simultaneously with the appearance of symptoms), from 3 to 2 meals/day, but after introducing FPNS, patients returned at the same number of baseline.42
Similarly, Bossi and colleagues conducted a prospective study on 17 patients assessing the efficacy of the FPNS as treatment for incident predictable BTcP in oral mucositis induced by RT-CT in H&N cancer. The analgesic drug was administered before eating or drinking, actions causing predictable pain in the patients. The study showed a mean reduction in incidental BTcP intensity after FPNS of 3.1 points on a 11-point scale.
Nevertheless, it presented several limitations: small sample size, no presence of a control group and short observation period (3 days).43
Conclusion
Supportive care is becoming an increasingly important area of focus for both investigators and clinicians who care for H&N cancer patients. This is due to both the success of recent therapeutic approaches and their associated toxicities. Treatment for H&N cancer involves single or multimodal therapy employing surgery, systemic therapies and/or radiation (RT), all of which can damage somatic tissues and nerves.
Pain is common for the people who are diagnosed with H&N cancer. Pain may arise due to tissue damage from multiple sources such as mucosal injury, invasion of the tumour into somatic tissue (skin, muscle, bone) with inflammation or ischaemia, and nerve infiltration or compression. To reach an optimum in analgesia, the major problems to consider are difficulties in intake of oral drugs, acute pain led by treatments (i.e. mucositis, dysphagia) and presence of neuropathic pain due to tumour infiltration.
To control baseline pain, fentanyl TTS seems to be an important option, thanks to the way of administration, the good safety and tolerability profiles, and the easy way to use.
For BTcP, several formulations of transmucosal fentanyl are available. Particularly, FPNS was studied in this setting, because it avoids oral cavity; therefore, all the formulations seem to be active and safety.
However, there are some points to underline. In H&N cancer patients, only one randomized controlled trial was conducted using fentanyl versus methadone for analgesia and was negative, though all the reported limitations of the study. We lack head-to-head studies of fentanyl versus other strong opioids, as well as with different formulation of fentanyl, particularly for BTcP.
Moreover, in the most important studies about BTcP,44,45 the H&N cancer population is very poorly represented.
Pain management may require a specific tailored plan to each patient, with the understanding that H&N cancer is a dynamic process and may make the patient’s pain to vary dramatically from one moment to another. All professionals caring for H&N cancer patients should assess palliative and supportive care needs in initial treatment planning, and throughout the illness, seeking for pain management specialists in the most difficult cases.
Transdermal preparations of fentanyl have theoretical and practical attractions for stable background pain, particularly if there is morphine intolerance (e.g. sedation and dysphoria) or in case of renal failure. For breakthrough pain, new preparations of buccal, sublingual or intranasal fentanyl are recommended, especially for incident pain due to planned treatment.
In conclusion, fentanyl represents a good choice to control pain in H&N patients. Although it is largely used in clinical practice, we need more specific studies among this population to understand the real role of fentanyl in this setting.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Higginson IJ, Hearn J, Murtagh F. Epidemiology of cancer pain. In: Sykes N, Bennett MI, Yuan C-S. (eds) Clinical pain management: cancer pain, 2nd edn. London: Hodder Arnold, 2008, pp. 13–26. [Google Scholar]
- 2. Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer: SAGE study group. Systematic assessment of geriatric drug use via epidemiology. JAMA 1998; 279(23): 1877–1882. [DOI] [PubMed] [Google Scholar]
- 3. Portenoy RK. Treatment of cancer pain. Lancet 2011; 377(9784): 2236–2247. [DOI] [PubMed] [Google Scholar]
- 4. Van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18: 1437–1449. [DOI] [PubMed] [Google Scholar]
- 5. O’Connor P, Bisson J, Asplin P, et al. Retrospective analysis of self-reporting pain scores and pain management during head and neck IMRT radiotherapy: a single institution experience. Radiography 2017; 23(2): 103–106. [DOI] [PubMed] [Google Scholar]
- 6. Baxi S, Fury M, Ganly I, et al. Ten years of progress in head and neck cancers. J Natl Compr Canc Netw 2012; 10(7): 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elting LS, Keefe DM, Sonis ST, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008; 113(10): 2704–2713. [DOI] [PubMed] [Google Scholar]
- 8. Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003; 66: 253–262. [DOI] [PubMed] [Google Scholar]
- 9. Schindler A, Denaro N, Russi EG, et al. Dysphagia in head and neck cancer patients treated with radiotherapy and systemic therapies: literature review and consensus. Crit Rev Oncol Hematol 2015; 96(2): 372–384. [DOI] [PubMed] [Google Scholar]
- 10. Yazbeck VY, Villaruz L, Haley M, et al. Management of normal tissue toxicity associated with chemoradiation (primary skin, esophagus, and lung). Cancer J 2013; 19(3): 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong JA, McCaffrey R. The effects of mucositis on quality of life in patients with head and neck cancer. Clin J Oncol Nurs 2005; 10: 53–56. [DOI] [PubMed] [Google Scholar]
- 12. Grond S, Zech D, Schug SA, et al. Validation of World Health Organization guidelines for cancer pain relief during the last days and hours of life. J Pain Symptom Manage 1991; 6(7): 411–422. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. Cancer pain relief, 2nd edn. Geneva: World Health Organization, 1996. [Google Scholar]
- 14. Carlson CL. Effectiveness of the World Health Organization cancer pain relief guidelines: an integrative review. J Pain Res 2016; 9: 515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kestenbaum MG, Vilches AO, Messersmith S, et al. Alternative routes to oral opioid administration in palliative care: a review and clinical summary. Pain Med 2014; 15(7): 1129–1153. [DOI] [PubMed] [Google Scholar]
- 16. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain 1990; 41(3): 273–281. [DOI] [PubMed] [Google Scholar]
- 17. Mercadante S. Pharmacotherapy for breakthrough cancer pain. Drugs 2012; 72: 181–190. [DOI] [PubMed] [Google Scholar]
- 18. Bhatnagar S, Upadhyay S, Mishra S. Prevalence and characteristics of breakthrough pain in patients with head and neck cancer: a cross-sectional study. J Palliat Med 2010; 13: 291–295. [DOI] [PubMed] [Google Scholar]
- 19. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13(2): e58–e68. [DOI] [PubMed] [Google Scholar]
- 20. Ripamonti CI, Santini D, Maranzano E, et al. Management of cancer pain: ESMO clinical practice guidelines. Ann Oncol 2012; 23(suppl 7): vii139–vii154. [DOI] [PubMed] [Google Scholar]
- 21. Benedetti C, Brock C, Cleeland C, et al. NCCN practice guidelines for cancer pain. Oncology 2000; 14(11A): 135–150. [PubMed] [Google Scholar]
- 22. Hess R, Stiebler G, Herz A. Pharmacokinetics of fentanyl in man and the rabbit. Eur J Clin Pharmacol 1972; 4(3): 137–141. [DOI] [PubMed] [Google Scholar]
- 23. Fallon M, Reale C, Davies A, et al. Efficacy and safety of fentanyl pectin nasal spray compared with immediate-release morphine sulfate tablets in the treatment of breakthrough cancer pain: a multicenter, randomized, controlled, double-blind, double-dummy multiple-crossover study. J Support Oncol 2011; 9: 224–231. [DOI] [PubMed] [Google Scholar]
- 24. Gourlay GK, Kowalski SR, Plummer JL, et al. The efficacy of transdermal fentanyl in the treatment of postoperative pain: a double-blind comparison of fentanyl and placebo systems. Pain 1990; 40(1): 21–28. [DOI] [PubMed] [Google Scholar]
- 25. Miser AW1, Narang PK, Dothage JA, et al. Transdermal fentanyl for pain control in patients with cancer. Pain 1989; 37: 15–21. [DOI] [PubMed] [Google Scholar]
- 26. Alberts DS, Smith CC, Parikh N, et al. Fentanyl sublingual spray for breakthrough cancer pain in patients receiving transdermal fentanyl. Pain Manag 2016; 6(5): 427–434. [DOI] [PubMed] [Google Scholar]
- 27. Mercadante S, Portenoy RK. Breakthrough cancer pain: twenty-five years of study. Pain 2016; 157(12): 2657–2663. [DOI] [PubMed] [Google Scholar]
- 28. Schug SA, Ting S. Fentanyl formulations in the management of pain: an update. Drugs 2017; 77(7): 747–763. [DOI] [PubMed] [Google Scholar]
- 29. Mystakidou K, Katsouda E, Parpa E, et al. Oral transmucosal fentanyl citrate for the treatment of breakthrough pain in cancer patients: an overview of its pharmacological and clinical characteristics. Am J Hosp Palliat Care 2005; 22: 228–232. [DOI] [PubMed] [Google Scholar]
- 30. Systematic reviews: CRD’s guidance for undertaking reviews in healthcare. York: Centre for Reviews and Dissemination, 2009. [Google Scholar]
- 31. Higginson IJ, Gao W. Opioid prescribing for cancer pain during the last 3 months of life: associated factors and 9-year trends in a nationwide United Kingdom cohort study. J Clin Oncol 2012; 30: 4373–4379. [DOI] [PubMed] [Google Scholar]
- 32. Grond S, Zech D, Lehmann KA, et al. Transdermal fentanyl in the long-term treatment of cancer pain: a prospective study of 50 patients with advanced cancer of the gastrointestinal tract or the head and neck region. Pain 1997; 69: 191–198. [DOI] [PubMed] [Google Scholar]
- 33. Haumann J, Geurts JW, van Kuijk SM, et al. Methadone is superior to fentanyl in treating neuropathic pain in patients with head-and-neck cancer. Eur J Cancer 2016; 65: 121–129. [DOI] [PubMed] [Google Scholar]
- 34. Chang JT, Lin CY, Lin JC, et al. Transdermal fentanyl for pain caused by radiotherapy in head and neck cancer patients treated in an outpatient setting: a multicenter trial in Taiwan. Jpn J Clin Oncol 2010; 40(4): 307–312. [DOI] [PubMed] [Google Scholar]
- 35. Guo SP, Wu SG, Zhou J, et al. Transdermal fentanyl for pain due to chemoradiotherapy-induced oral mucositis in nasopharyngeal cancer patients: evaluating efficacy, safety, and improvement in quality of life. Drug Des Devel Ther 2014; 12(8): 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Konopka-Filippow M, Zabrocka E, Wójtowicz A, et al. Pain management during radiotherapy and radiochemotherapy in oropharyngeal cancer patients: single-institution experience. Int Dent J 2015; 65(5): 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simon SM, Schwartzberg LS. A review of rapid-onset opioids for breakthrough pain in patients with cancer. J Opioid Manage 2014; 10: 207–215. [DOI] [PubMed] [Google Scholar]
- 38. Chang A, Roeland EJ, Atayee RS, et al. Transmucosal immediate-release fentanyl for breakthrough cancer pain: opportunities and challenges for use in palliative care. J Pain Palliat Care Pharmacother 2015; 29(3): 247–260. [DOI] [PubMed] [Google Scholar]
- 39. Pignon T, Fernandez L, Ayasso S, et al. Impact of radiation oncology practice on pain: a cross-sectional survey. Int J Radiat Oncol Biol Phys 2004; 60: 1204–1210. [DOI] [PubMed] [Google Scholar]
- 40. D’Ambrosio DJ, Li T, Horwitz EM, et al. Does treatment interruption affect outcome after radiotherapy for prostate cancer? Int J Radiat Oncol Biol Phys 2008; 72: 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kwong DL, Sham JS, Chua DT, et al. The effect of interruptions and prolonged treatment time in radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1997; 39: 703–710. [DOI] [PubMed] [Google Scholar]
- 42. Mazzola R, Ricchetti F, Fiorentino A, et al. Fentanyl pectin nasal spray for painful mucositis in head and neck cancers during intensity-modulated radiation therapy with or without chemotherapy. Clin Transl Oncol 2017; 19(5): 593–598. [DOI] [PubMed] [Google Scholar]
- 43. Bossi P, Locati L, Bergamini C, et al. Fentanyl pectin nasal spray as treatment for incident predictable breakthrough pain (BTP) in oral mucositis induced by chemoradiotherapy in head and neck cancer. Oral Oncol 2014; 50(9): 884–887. [DOI] [PubMed] [Google Scholar]
- 44. Mercadante S, Marchetti P, Cuomo A, et al. Breakthrough pain and its treatment: critical review and recommendations of IOPS (Italian Oncologic Pain Survey) expert group. Support Care Cancer 2016; 24(2): 961–968. [DOI] [PubMed] [Google Scholar]
- 45. Mercadante S, Adile C, Cuomo A, et al. Fentanyl buccal tablet vs. oral morphine in doses proportional to the basal opioid regimen for the management of breakthrough cancer pain: a randomized, crossover, comparison study. J Pain Symptom Manage 2015; 50(5): 579–586. [DOI] [PubMed] [Google Scholar]