Abstract
Purpose:
To determine 1) which components of retinal function are impaired after rhegmatogenous retinal detachment, 2) which outer retinal pathways (rod- or cone-driven) are more severely affected, and 3) whether there is concomitant inner retinal dysfunction.
Methods:
We conducted a prospective observational study in a large academic institution. We performed preoperative electroretinography on eight patients to assess outer and inner retinal function. In all cases, a comparison between the eye with the detached retina and the control fellow eye was made.
Results:
Eyes with a detached retina had significantly lower a-wave and b-wave amplitudes with respect to both rod- and cone-dominated testing parameters (P < 0.05) and reduced 30 Hz flicker responses compared to fellow eyes (P < 0.05); the effect size was similar for all significantly reduced parameters (r~0.6). There were no significant differences between eyes with detached retinas and control fellow eyes with respect to b/a-wave ratios, a-wave latencies, or b-wave latencies.
Conclusion:
Patients with rhegmatogenous retinal detachment have preoperative outer retinal dysfunction equally affecting both rod- and cone-driven pathways, and they have minimal inner retinal dysfunction.
Keywords: Clinical Electrophysiology, Electroretinography, Retinal Detachment
INTRODUCTION
Retinal detachment is a clinical disorder in which the neurosensory retina separates from the underlying retinal pigment epithelium (RPE). There are three types of detachments: 1) exudative retinal detachment, associated with inflammation and neoplasms; 2) tractional retinal detachment, often secondary to proliferative diabetic retinopathy; and 3) rhegmatogenous retinal detachment (RRD). RRD is the most common form of retinal detachment, and is caused by vitreous separation with associated traction that leads to a tear in the neurosensory retina. This tear allows vitreous fluid to access the potential subretinal space, causing fluid accumulation beneath the neurosensory retina and subsequent separation from the RPE.
Typically, RRD is treated with pars plana vitrectomy, scleral buckling, pneumatic retinopexy, or a combination of these procedures, depending on the type and the severity of the detachment.[1] In many cases, appropriate surgical intervention restores some retinal function, including partial or complete recovery of central visual acuity; however, complete recovery of global retinal function is rarely achieved.[2,3,4,5] The status of the macula in RRD is also a critical factor in determining the ultimate visual acuity outcomes. In particular, involvement of the macula in RRD predicts an overall worse visual acuity outcome, especially in the absence of timely surgical intervention.[6,7]
Electroretinography (ERG) is a diagnostic technique that allows clinicians to assess retinal function in awake human subjects by measuring the retina's electrical response to various light stimuli. ERG responses have a characteristic pattern consisting of an initial negative deflection called the a-wave, followed by a positive deflection called the b-wave. The a-wave has been shown to be generated by photoreceptors in the outer retina, whereas the b-wave is generated predominantly by the inner retina, including on-bipolar and Müller cells. Numerous past studies have monitored retinal function with ERG at various time points following surgical interventions. For instance, Gong et al have reported that scleral buckling surgery leads to improvement in visual function of eyes with retinal detachments at one month and six months following surgery, although the amplitudes of various ERG responses did not reach even 60% of the amplitudes of the normal fellow eyes.[8] Similar incomplete recovery of visual function was observed in patients who underwent vitrectomy instead of scleral buckling surgery,[9] and a recent study has confirmed this incomplete recovery of retinal function following surgical intervention.[10]
Despite these studies, there is no consensus regarding which components of retinal function are affected by RRD and whether certain subcomponents of retinal function are more severely damaged by RRD. Many studies have reported outer retinal dysfunction in rod and cone photoreceptor-driven pathways after RRD;[8,9,10,11,12] other studies have suggested that there are long-lasting deficits in inner retinal function, manifesting as delayed implicit times on postsurgical follow-up[9] or changes in the b/a-wave ratio.[11] It remains unclear whether these inner retinal defects are a primary feature of RRD or are a consequence of outer retinal dysfunction. These inconsistent findings highlight the need for further electrophysiological examination of the neurosensory retina after RRD in humans as these findings may have clinical relevance for predicting visual prognosis or for informing treatment decisions.
We conducted a prospective, observational study involving preoperative electrophysiological characterization of patients with macula-involving RRD. The goal of this study was to determine 1) which components of the ERG response are impaired following RRD, 2) which of the outer retinal pathways (rod- or cone-driven) are more severely affected, and 3) whether there is concomitant preoperative inner retinal dysfunction. Overall, this study was conducted to improve our understanding of the characteristics of retinal dysfunction associated with RRD.
METHODS
Patients
We recruited ten patients from one large academic institute (Washington University in St. Louis) who were treated by six vitreoretinal surgeons. All patients gave written informed consent, and all procedures conformed to the Declaration of Helsinki and were approved by the institutional review board of the Human Research Protection Office (HRPO) of Washington University in St. Louis. Patients were included if they were 18 years or older and had been diagnosed within three weeks with primary macula-involving RRD comprising of at least three clock-hours of the retina. Patients were excluded if they had any retinal pathology in either eye that might confound ERG assessment, including severe diabetic or hypertensive retinopathy; advanced age-related macular degeneration (AMD); active retinitis or uveitis; extensive retinal scarring from any cause; proliferative vitreoretinopathy of any grade; presence of detachment or history of detachment in the fellow eye; previous detachment in the study eye; history of rheumatologic or autoimmune disease; advanced glaucoma; or any form of severe media opacity. During the study, there were 15 cases that met the above entry criteria. Five patients declined participation, yielding a total of 10 consecutive patients who met entry criteria. In the analysis, we excluded two patients for a final total of 8: one patient did not want his/her control eye tested by ERG; the other patient was excluded when we later discovered that he had a previous macula-off detachment in the fellow eye, which had required multiple operations, and a recent vitreous hemorrhage in the study eye.
Electroretinography
We performed electroretinography (ERG) on all patients preoperatively using the ColorBurst (Diagnosys LLC, Lowell, MA), which follows the International Society for Clinical Electrophysiology of Vision (ISCEV) Standard.[13] Before recording the ERG responses, we dilated the pupil of both the affected and control eyes to approximately 7-8 mm with 2.5% phenylephrine and 1.0% tropicamide after administration of 0.5% tetracaine. We then cleaned the skin of the patient's forehead with an abrasive cream and used an electrode cream to attach the neutral ground electrode to the skin of the forehead. We draped a single-use impregnated microfiber Dawson-Trick-Litzkow (DTL) electrode across the cornea above the lower eyelid and placed the reference electrode near the lateral canthus. After 20 minutes of dark adaptation, we tested rod activity by using achromatic flashes against an achromatic background and recording responses to 0.01 cd·s/m2 and 0.50 cd·s/m2 light flashes. The combined rod-cone response was obtained by recording responses to the 3.0 cd·s/m2 light flash. After 10 minutes of light adaptation in ambient light, we measured the photopic ERG response by recording light-adapted responses to 3.0 cd·s/m2 with 30 cd/m2 background illumination and 30 Hz flicker responses. The stimulus for all ERG recordings was full-field white light at 6,500 K. We recorded two responses per eye at each step to account for possible noise and blinking artifacts. An investigator masked to detachment status (JBL) manually selected the ERG waveform with less noise for quantitation. The amplitudes and the latencies of the a- and b-waves were calculated automatically by the Colorburst software (Diagnosys LLC). To calculate the b/a-wave ratio, we divided the b-wave amplitude by the a-wave amplitude for each waveform.
Statistics
We performed statistical analysis using SPSS Statistics version 23 (IBM, Armonk, NY) and Prism version 5 (GraphPad Software, La Jolla, CA). To compare ERG parameters between eyes with detached retinas and fellow eyes, we performed Wilcoxon signed-rank tests. To calculate effect sizes, we calculated r by dividing the Wilcoxon test statistic by the square root of the number of observations. To compare three groups in our subgroup analysis, we performed the Kruskal-Wallis test with Dunn's post-hoc test when appropriate. We considered P <.05 to be statistically significant.
RESULTS
Demographic and clinical characteristics of the patients are shown in Table 1. The median age of subjects was 60 years (range, 24-70 years), and there were four men and four women. The laterality of the eye with detached retina was 2 OD and 6 OS. We performed electrophysiological characterization of all subjects with full-field ERG at various flash intensities. We observed significant decreases in a- and b-wave amplitudes in eyes with detached retinas compared to those in fellow eyes at the 0.01 cd·s/m2 (Z = −2.380, P = 0.017; Z = −2.380, P = 0.017; respectively) [Figure 1a and b], the 0.50 cd·s/m2 (Z = −2.380, P = 0.017; Z = −2.240, P = 0.025; respectively) [Figure 1c and d], and the 3.0 cd·s/m2 flash intensities (Z = −2.380, P = 0.017; Z = −2.380, P = 0.017; respectively) [Figure 1e and f], indicating deficits in the rod photoreceptor-driven pathway. We also observed deficits in the cone photoreceptor-driven pathway that was manifested both as a decrease in a- and b-wave amplitudes in response to the 3.0 cd·s/m2 light under 30 cd/m2 background illumination (Z = −2.380, P = 0.017; Z = −2.240, P = 0.025; respectively) [Figure 2a and b] and as a decrease in the 30 Hz flicker amplitudes (Z = −2.380, P = 0.017) [Figure 2c]. Together, these findings suggest that RRD causes dysfunction of both rod- and cone-driven pathways.
Table 1.
Demographic and clinical characteristics of study subjects

Figure 1.

On preoperative electroretinography, eyes with detached retinas exhibited significantly reduced a- and b-wave amplitudes compared to fellow control eyes at 0.01 cd·s/m2 (a and b), 0.50 cd·s/m2 (c and d), and 3.0 cd·s/m2 (e and f) flash intensities. Open circles depict individual data points; horizontal lines depict medians (a–f) (*P < 0.05).
Figure 2.
On preoperative electroretinography, eyes with detached retinas exhibited significantly reduced a- and b-wave amplitudes compared to fellow control eyes at the 3.0 cd·s/m2 flash intensity under 30 cd/m2 background (bkgd) illumination (a and b) and decreased peak amplitudes in response to 30 Hz flicker (c). Open circles depict individual data points; horizontal lines depict medians (a–c) (*P < 0.05).
Next, we calculated b/a-wave ratios to determine whether the decreases in b-wave amplitudes, which are generated primarily by the inner retina, exceeded the decreases in a-wave amplitudes, which are generated by the outer retina. A decrease in the b/a-wave ratio would indicate a greater extent of dysfunction in inner retina versus outer retina. We did not observe any difference in the b/a-wave ratios between the eyes with detached retinas and fellow eyes in the rod-driven pathway at the 0.01 cd·s/m2 (Z = −1.260, P = 0.208) [Figure 3a], the 0.50 cd·s/m2 (Z = −0.560, P = 0.575) [Figure 3b], or the 3.0 cd·s/m2 flash intensity (Z = −0.140, P = 0.889) [Figure 3c], or in the cone-driven pathway (Z = −0.140, P = 0.889) [Figure 3d]. These results suggest that the deficits we observed in b-wave amplitudes were in proportion to outer retinal dysfunction.
Figure 3.

On preoperative electroretinography, the b/a-wave ratios in eyes with detached retinas were similar to those in fellow control eyes at 0.01 cd·s/m2 (a), 0.50 cd·s/m2 (b), and 3.0 cd·s/m2 (c) flash intensities, and at 3.0 cd·s/m2 flash intensity under 30 cd/m2 background (bkgd) illumination (d). Open circles depict individual data points; horizontal lines depict medians (a–d).
Other studies have reported increased latencies following RRD, which would suggest impaired signal transmission in the detached retinas. We did not observe any statistically significant differences in a-wave latencies at the 0.01 cd·s/m2 (Z = −0.169, P = 0.866), the 0.50 cd·s/m2 (Z = 0.000, P = 1.000), or the 3.0 cd·s/m2 flash intensity (Z = −1.289, P = 0.197), or at the 3.0 cd·s/m2 flash intensity under 30 cd/m2 background illumination (Z = −1.590, P = 0.112) [Figure 4a]. Likewise, we did not observe any statistically significant differences in b-wave latencies at the 0.01 cd·s/m2 (Z = −1.407, P = 0.159), the 0.50 cd·s/m2 (Z = −0.106, P = 0.915), or the 3.0 cd·s/m2 flash intensity (Z = −0.406, P = 0.684), or at the 3.0 cd·s/m2 flash intensity under 30 cd/m2 background illumination (Z = −1.802, P = 0.072) [Figure 4b]. These findings suggest that in the participants of this study, RRD did not affect signal transmission.
Figure 4.

. On preoperative electroretinography, there were no differences between eyes with detached retinas and fellow control eyes with respect to a- (a) or b-wave (b) latencies at 0.01 cd·s/m2, 0.50 cd·s/m2, or 3.0 cd·s/m2 flash intensities, or at 3.0 cd·s/m2 flash intensity under 30 cd/m2 background (bkgd) illumination. Open circles depict individual data points; horizontal lines depict medians (a and b).
To compare the relative magnitude of these retinal deficits, we calculated the effect size of each ERG parameter that was significantly decreased in eyes with detached retinas compared to fellow eyes [Table 2]. We observed a similar effect size for all parameters. These findings suggest that the deficits in the rod- and cone-driven pathways are similar following RRD.
Table 2.
Effect sizes of significantly reduced ERG parameters in eyes with detached retinas

Finally, we noticed a large extent of heterogeneity in the ERG amplitudes in detached eyes. Therefore, we hypothesized that different extents of detachment could explain some of this functional heterogeneity. Therefore, we performed a subgroup analysis, dividing study eyes into two groups based on the extent of the detachment: ≤6 clock-hours (i.e., ≤50% involvement) versus >6 clock-hours (>50% involvement). We found that after subdividing study eyes into these subgroups (i.e., control versus ≤6 clock-hour detachment versus >6 clock-hour detachment), there was indeed a significant difference in the a- and b-wave amplitudes between these three groups at the 0.01 cd·s/m2 (χ2= 7.301, P = 0.026; χ2= 10.517, P = 0.005; respectively) [Figure 5a and b], the 0.50 cd·s/m2 (χ2= 10.125, P = 0.006; χ2= 10.434, P = 0.005; respectively) [Figure 5c and d], and the 3.0 cd·s/m2 flash intensities (χ2= 10.472, P = 0.005; χ2= 11.713, P = 0.003; respectively) [Figure 5e and f]. Likewise, we observed a significant difference in the a- and b-wave amplitudes between the three groups at the 3.0 cd·s/m2 flash intensity under 30 cd/m2 background illumination (χ2= 7.075, P = 0.029; χ2= 10.125, P = 0.006; respectively) [Figure 6a and b] and in their 30 Hz flicker peak amplitudes (χ2= 11.713, P = 0.003) [Figure 6c]. Post-hoc testing by Dunns multiple comparison test revealed that, in all above cases, the significant difference was between the >6 clock-hour detachment and control groups (P < 0.05 or P < 0.01). Although not statistically significant, the ≤6 clock-hour detachment group exhibited intermediate ERG testing results, generally falling between the >6 clock-hour and the control groups.
Figure 5.
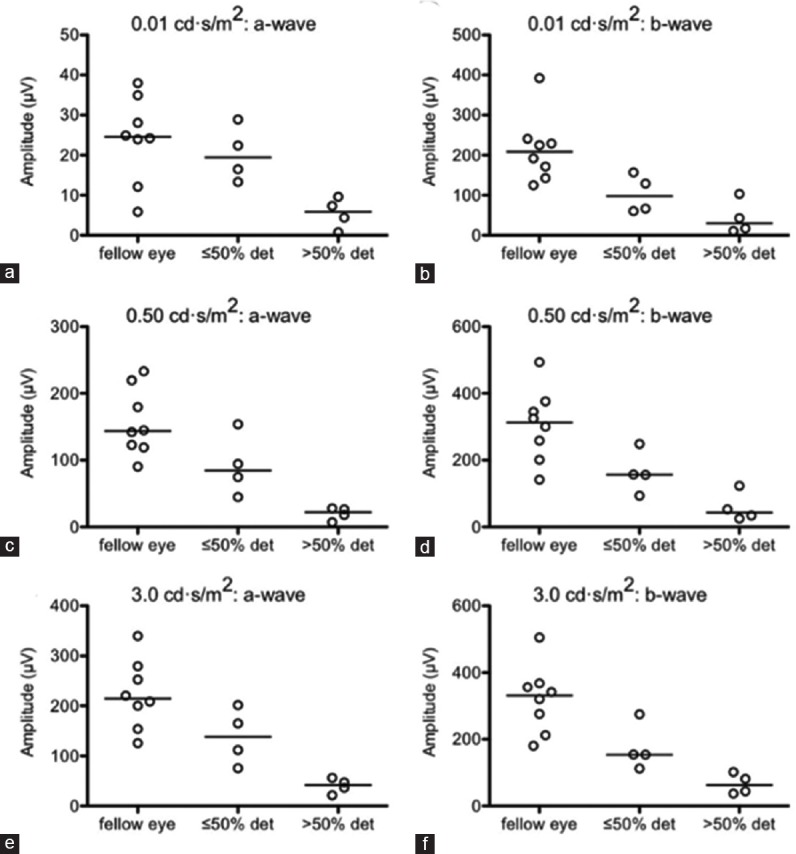
Dichotomizing study eyes based on the extent of retinal detachment (i.e., ≤6 clock-hours or ≤50% detached (det) versus >6 clock-hours or >50% det) revealed a pattern of step-wise decline in preoperative a- and b-wave amplitudes corresponding with more extensive detachments at 0.01 cd·s/m2 (a and b), 0.50 cd·s/m2 (c and d), and 3.0 cd·s/m2 (e and f) flash intensities. Open circles depict individual data points; horizontal lines depict medians (a–f).
Figure 6.
Dichotomizing study eyes based on the extent of retinal detachment (i.e., ≤6 clock-hours or ≤50% detached (det) versus >6 clock-hours or >50% det) revealed a pattern of step-wise decline corresponding with more extensive detachments in the preoperative a- and b-wave amplitudes at 3.0 cd·s/m2 flash intensity under 30 cd/m2 background (bkgd) illumination (a and b) and in 30 Hz flicker responses (c). Open circles depict individual data points; horizontal lines depict medians (a–c).
DISCUSSION
In this study, we performed preoperative characterization of the retinal dysfunction in patients with primary macula-involving RRD by full-field ERG. Consistent with past reports,[8,9,10,11,12] we observed significant deficits in both rod and cone photoreceptor-driven pathways in the eyes with detached retinas compared to fellow eyes. Previous studies have suggested that retinal detachment may have different magnitudes of effect on the scotopic versus photopic ERG responses.[14,15] However, in this study, we did not observe a difference in the magnitude of dysfunction between the rod- and cone-driven pathways, suggesting that both subsets of photoreceptors are similarly affected.
Additionally, in this study, we sought to determine whether there is a differential effect of RRD on the outer versus inner retina. When the neurosensory retina detaches from the RPE, the outer retina develops ischemia from lack of oxygen from the choriocapillaris, leading to rod and cone dysfunction. On the other hand, the inner retina is supplied by the retinal artery and may retain some oxygen supply in the context of detachment. Of interest, we did not observe any changes in latencies or b/a-wave ratios, which would suggest inner retina dysfunction. Taken together, our findings suggest that the retinal dysfunction associated with primary macula-involving RRD occurs primarily in the outer retina without any significant early effects on the inner retina and signal transmission in the short term.
In contrast with these findings, other studies have reported inner retinal defects associated with RRD, both preoperatively and postoperatively.[9,11] We speculate that this discrepancy may be related to the duration of detachment prior to preoperative electrophysiological characterization. It is still possible that inner retinal damage may occur progressively following outer retinal dysfunction and may be detectable preoperatively in the absence of timely surgical intervention. The numerous studies reporting that prompt surgical intervention is associated with improved outcomes in macula-involving RRD[6,7] support this hypothesis. Further studies are necessary to more precisely characterize the temporal progression of ERG findings in RRD; however, these studies may be challenging since they require patients to recall the onset of symptoms to determine the duration of retinal detachment.
Moreover, we found that the ERG measurements served as a good predictor of the extent of detachment (i.e., dichotomized as ≤6 clock-hours versus >6 clock-hours). Although not statistically significant, there was a clear pattern of decreasing ERG amplitudes when comparing healthy eyes to eyes with ≤6 clock-hour detachments to eyes with >6 clock-hour detachments. Cumulatively, this study is relevant since a recent study has reported that functional improvements in retinal function on ERG are correlated with microstructural changes in photoreceptors as measured by spectral-domain OCT.[16] ERG may therefore be helpful for determining the extent of functional damage of the neurosensory retina after RRD. Limitations of this study include small sample size and the unavailability of ERG data after surgical repair. Importantly, post-operative data could provide further details regarding the extent to which surgical repair restored normal retinal function. Larger prospective studies are therefore necessary to gain a more definitive answer about the extent of outer retinal recovery after RD repair. It may also be possible to incorporate multifocal ERG into future studies to provide additional spatial information that may facilitate a more complete understanding of the pathophysiology of RRD. Nonetheless, this study highlights that ERG is a useful clinical tool to assess retinal function and perhaps to monitor recovery after surgery.
Financial Support and Sponsorship
J.B.L. was supported by the Washington University in St. Louis Medical Scientist Training Program (NIH Grant T32GM07200) and the Washington University in St. Louis Institute of Clinical and Translational Sciences (NIH Grants UL1TR002345, TL1TR002344). Additional funding comes from an unrestricted grant to the Department of Ophthalmology and Visual Sciences of Washington University School of Medicine from Research to Prevent Blindness.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.D'Amico DJ. Clinical practice. Primary retinal detachment. N Engl J Med. 2008;359:2346–2354. doi: 10.1056/NEJMcp0804591. [DOI] [PubMed] [Google Scholar]
- 2.Halberstadt M, Chatterjee-Sanz N, Brandenberg L, Koerner-Stiefbold U, Koerner F, Garweg JG. Primary retinal reattachment surgery: Anatomical and functional outcome in phakic and pseudophakic eyes. Eye (Lond) 2005;19:891–898. doi: 10.1038/sj.eye.6701687. [DOI] [PubMed] [Google Scholar]
- 3.Salicone A, Smiddy WE, Venkatraman A, Feuer W. Visual recovery after scleral buckling procedure for retinal detachment. Ophthalmology. 2006;113:1734–1742. doi: 10.1016/j.ophtha.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 4.Mendrinos E, Dang-Burgener NP, Stangos AN, Sommerhalder J, Pournaras CJ. Primary vitrectomy without scleral buckling for pseudophakic rhegmatogenous retinal detachment. Am J Ophthalmol. 2008;145:1063–1070. doi: 10.1016/j.ajo.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni KM, Roth DB, Prenner JL. Current visual and anatomic outcomes of pneumatic retinopexy. Retina. 2007;27:1065–1070. doi: 10.1097/IAE.0b013e3180546928. [DOI] [PubMed] [Google Scholar]
- 6.Hassan TS, Sarrafizadeh R, Ruby AJ, Garretson BR, Kuczynski B, Williams GA. The effect of duration of macular detachment on results after the scleral buckle repair of primary, macula-off retinal detachments. Ophthalmology. 2002;109:146–152. doi: 10.1016/s0161-6420(01)00886-7. [DOI] [PubMed] [Google Scholar]
- 7.Diederen RM, La Heij EC, Kessels AG, Goezinne F, Liem AT, Hendrikse F. Scleral buckling surgery after macula-off retinal detachment: Worse visual outcome after more than 6 days. Ophthalmology. 2007;114:705–709. doi: 10.1016/j.ophtha.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Gong Y, Wu X, Sun X, Zhang X, Zhu P. Electroretinogram changes after scleral buckling surgery of retinal detachment. Doc Ophthalmol. 2008;117:103–109. doi: 10.1007/s10633-007-9109-2. [DOI] [PubMed] [Google Scholar]
- 9.Schatz P, Andreasson S. Recovery of retinal function after recent-onset rhegmatogenous retinal detachment in relation to type of surgery. Retina. 2010;30:152–159. doi: 10.1097/IAE.0b013e3181b32ed4. [DOI] [PubMed] [Google Scholar]
- 10.Azarmina M, Moradian S, Azarmina H. Electroretinographic changes following retinal reattachment surgery. J Ophthalmic Vis Res. 2013;8:321–329. [PMC free article] [PubMed] [Google Scholar]
- 11.Kim IT, Ha SM, Yoon KC. Electroretinographic studies in rhegmatogenous retinal detachment before and after reattachment surgery. Korean J Ophthalmol. 2001;15:118–127. doi: 10.3341/kjo.2001.15.2.118. [DOI] [PubMed] [Google Scholar]
- 12.Schatz P, Holm K, Andreasson S. Retinal function after scleral buckling for recent onset rhegmatogenous retinal detachment: Assessment with electroretinography and optical coherence tomography. Retina. 2007;27:30–36. doi: 10.1097/01.iae.0000256659.71864.83. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, et al. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 14.van Lith GH, van der Torren K, Vijfvinkel-Bruinenga S. ERG and VECPs in retinal detachments. Doc Ophthalmol. 1981;50:291–297. doi: 10.1007/BF00158012. [DOI] [PubMed] [Google Scholar]
- 15.Montrone L, Ziccardi L, Stifano G, Piccardi M, Molle F, Focosi F, et al. Regional assessment of cone system function following uncomplicated retinal detachment surgery. Doc Ophthalmol. 2005;110:103–110. doi: 10.1007/s10633-005-7554-3. [DOI] [PubMed] [Google Scholar]
- 16.Kominami A, Ueno S, Kominami T, Nakanishi A, Piao CH, Ra E, et al. Restoration of cone interdigitation zone associated with improvement of focal macular ERG after fovea-off rhegmatogenous retinal reattachment. Investig Ophthalmol Vis Sci. 2016;57:1604–1611. doi: 10.1167/iovs.15-19030. [DOI] [PubMed] [Google Scholar]




