Abstract
Purpose:
Latanoprost is known to have several ocular side effects, including redness of the eyelids, lengthening of the eyelashes, an increase in iris pigmentation, and dryness of the eyes that causes discomfort. There are also several rarer systemic side effects reported in the literature, including chest pain.
Case Report:
Here we report a rare case of a patient who developed a cutaneous blistering rash on both eyelids, both sides of the neck, and the dorsum of both hands after direct contact with latanoprost eye drops. The lesions cleared following withdrawal of the eye drops.
Conclusion:
Topical latanoprost can cause severe blistering of the skin that could undermine a patient's confidence in the treating medical team. Reporting of an adverse effect is difficult when a generic topical medication is used.
Keywords: Adverse Effects, Cutaneous, Latanoprost, Topical Prostaglandin Analogues
INTRODUCTION
It is estimated that 500,000 individuals in the UK have glaucoma, and this figure does not include those who have yet to be diagnosed.[1] The National Institute for Health and Care Excellence guidance[2] advocates use of a topical prostaglandin analog as first line treatment. Latanoprost is the topical prostaglandin analog most widely used in routine clinical practice. Latanoprost is known to have ocular side effects, including redness of the eyelids, lengthening of the eyelashes, increased pigmentation of the iris, and dryness of the eyes that causes discomfort.[3] There are also a number of rarer systemic side effects reported in the literature, including chest pain.[4] Here we report a rare case of a severe cutaneous reaction to latanoprost eye drops.
CASE REPORT
A retired 87-year-old ex-army officer and policewoman attended the clinic for follow-up after starting generic latanoprost 50-μg/ml eye drops 3 months earlier. The patient had been monitored regularly for 3 years as a glaucoma suspect and at her last appointment had been diagnosed with normal-tension glaucoma. At that time, her best-corrected vision was 6/24 in the right eye and 6/18 in the left eye. Her decreased vision was explained by the presence of significant nuclear sclerotic cataract bilaterally. Her intraocular pressure was 14 mmHg in both eyes. The central corneal thickness reading was 555 μm in the right eye and 559 μm in the left eye. The right eye had a cup disc ratio of 0.65 with an inferior hemorrhage and the left eye had a cup disc ratio of 0.8 with superior and temporal thinning. The patient recorded a normal visual field on Humphrey visual field testing (SITA Fast 24-2). The patient was presented with the options of starting latanoprost eye drops or undergoing micropulse diode laser trabeculoplasty and chose eye drops.
At the follow-up appointment, the patient informed us that she had stopped using the drops a month earlier when she developed a blistering rash around both eyes and on her hands. She reported that the pain was so severe initially that she had been unable to sleep. The patient sought advice from her general practitioner, who suggested that she stop using the drops. Thereafter, her rash and pain improved significantly but traces of the erythematous rash remained on her face, neck [Figures 1 and 2] and hands [Figures 3 and 4]. The lesions were still slightly tender on palpation. The patient's general health was excellent for her age and she was not taking any prescribed medications. She was independently mobile with no walking aids and was completely self-caring. She was well presented at clinic and was accompanied by her son. There was no history of allergy to medication and she was on no other treatment at the time.
Figure 1.
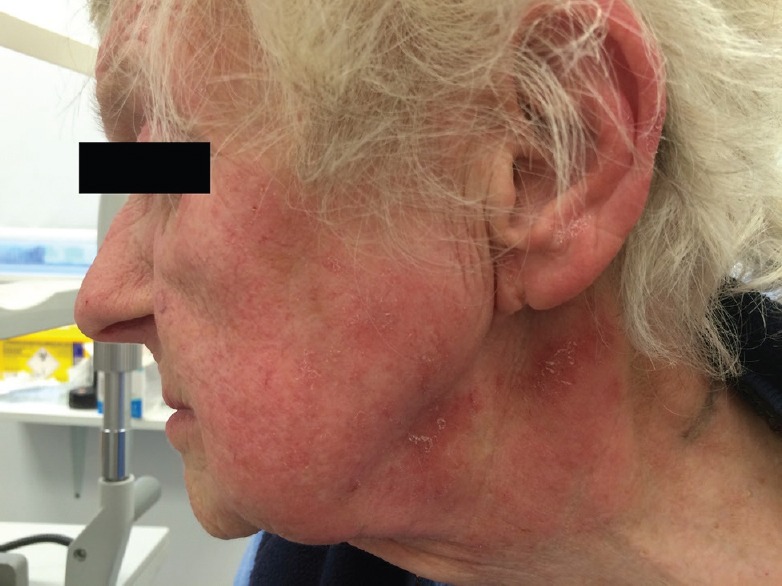
Blistering rash on face and neck.
Figure 2.
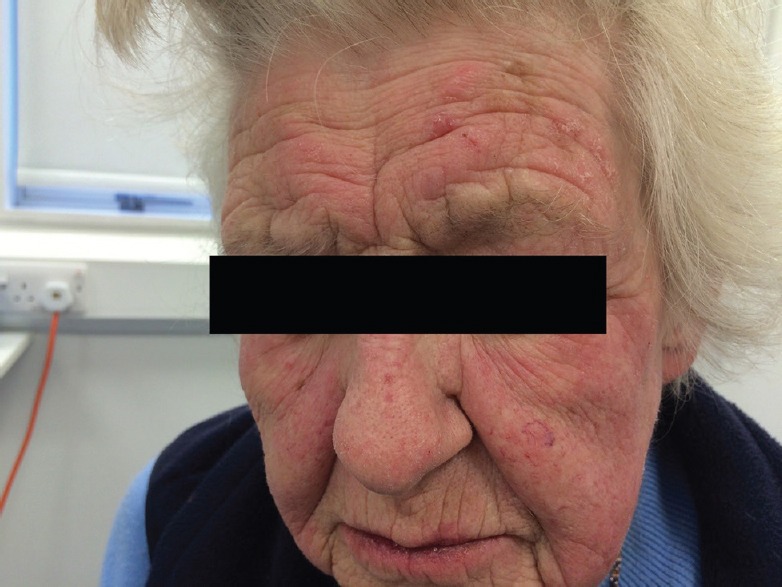
Blistering rash on brow and face.
Figure 3.
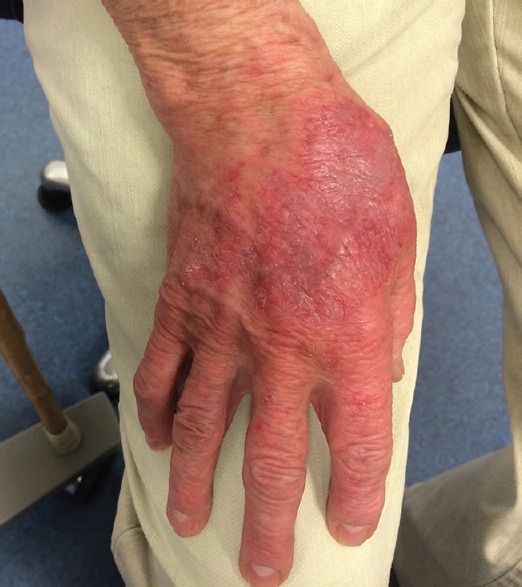
Blistering rash on the dorsum of the right hand.
Figure 4.
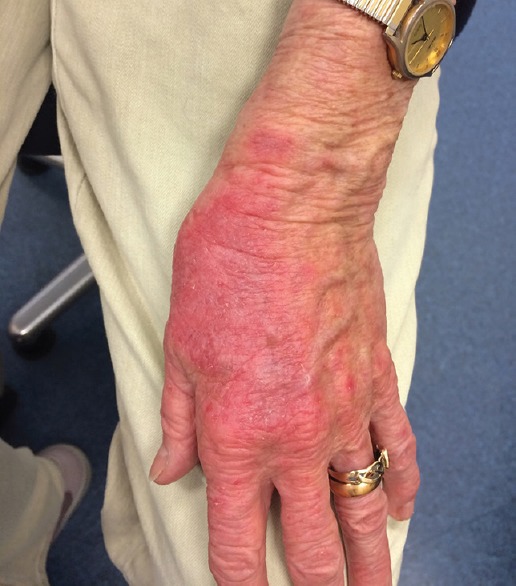
Blistering rash on the dorsum of the left hand.
Given the painful blistering rash, the patient declined further eye drops and did not want to undergo micropulse diode laser trabeculoplasty. There was no concern regarding the patient's competency. The patient's son, who was also present at the consultation, agreed with her decision. We respected her wishes and informed her fully regarding the possible impact of her choice. The patient was offered further follow-up with the department but did not attend the appointments. The authors subsequently contacted the patient via telephone. She informed us that the cutaneous lesions resolved following cessation of the offending latanoprost eye drops and that she remains very well.
DISCUSSION
This is the first reported case of a blistering cutaneous rash secondary to use of latanoprost eye drops. The patient and authors attribute the rash to the latanoprost eye drops because the distribution of the rash traced where the drops had been in direct contact with her skin. This belief is supported by the fact that when she stopped the drops, the rash spontaneously improved without the need for treatment. Furthermore, the patient was not taking any other medication when she developed the rash, ruling out a drug interaction. The patient also denied use of topical ointments or perfumes, or any new behaviors that could have accounted for the rash. The patient reported no previous allergies, sensitivities, or skin conditions. She was medically fit and healthy, ruling out a systemic cause for the rash. Although elderly, she retained excellent physical and mental function, as evidenced by her good appearance and demeanor in clinic. She was fully independent at home and was eloquent when discussing her care with us. We concluded that there was no suggestion of the cutaneous lesion being caused by poor self-hygiene, as might be the case with less independent elderly patients. We contacted the patient 15 months following the appearance of the rash and were pleased to learn that the rash had completely settled. The patient's cutaneous lesion has not recurred since, ruling out a seasonal cause. Therefore, we believe the cause of the rash to be the latanoprost eye drops.
The pathologic mechanism underlying the appearance of this patient's skin lesion is unknown. The lesion was distributed where the drops had come into direct contact with the skin, suggesting contact dermatitis caused by a type 4 hypersensitivity reaction. Type 4 hypersensitivity reactions are T-cell-mediated, whereby initial contact with an antigen sensitizes antigen-presenting cells, so that the T-cells are primed when the antigen is reintroduced, resulting in cell destruction and an associated inflammatory reaction. It would have taken time for the patient to become sensitized, which explains why the reaction occurred after a short period of using the drops.
Many side effects of latanoprost have been reported in the literature. Local effects such as lash lengthening, increase in iris pigmentation, and dry eye are well known. There have also been isolated case reports of rarer side effects, including central macular edema,[5] reactivation of herpes simplex,[6] and even chest pain.[4] However, reports of side effects involving the skin are rarer, with only one report of increased skin pigmentation following use of latanoprost.[7]
It is important for patients to bring the bottles of any eye drops to clinic when an adverse reaction has occurred because of the numerous generic versions and formulations now available. In this case, it was impossible for the authors to identify the generic latanoprost eye drops that the patient had used. It is also difficult to ascertain whether it was the active ingredient or one of the preservatives in the eye drop that caused the reaction. Unfortunately, the patient did not attend any further appointments with us, so we could not make a referral to a dermatologist or allergy specialist.
Side effects can cause serious harm to patients and this may have been the case in our patient if she had persisted with the drops. Luckily, there were no other medications or systemic problems that could have confounded the diagnosis. It is important for clinicians to recognize the side effects of treatment prescribed to avoid harming their patients. Only by withdrawing the offending agent will the symptoms cease. Clinicians are also reminded to report side effects to the relevant authorities in their respective countries. If patients are not warned regarding potential side effects; compliance with medication will cease and they may lose confidence in the treating clinician.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent form. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initial will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1. [Last accessed on 2016 Aug 09]. Available from: http://www.nhs.uk/conditions/glaucoma/pages/introduction.aspx .
- 2.Glaucoma: Diagnosis and management. NICE guidelines (CG85) [Last accessed on 2016 Aug 09]. Available from: https://www.nice.org.uk/guidance/scg85?unl id=7812309582016218213038 .
- 3.Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analogue therapy. Surd Ophthalmol. 2008;53(Suppl 1):S93–105. doi: 10.1016/j.survophthal.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Rajan M, Syam P, Liu C. Systemic side effects of topical latanoprost. Eye. 2003;17:442–444. doi: 10.1038/sj.eye.6700351. [DOI] [PubMed] [Google Scholar]
- 5.Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology. 1998;105:263–268. doi: 10.1016/s0161-6420(98)92977-3. [DOI] [PubMed] [Google Scholar]
- 6.Wand M, Gilbert CM, Liesegang TJ. Latanoprost and herpes simplex keratitis. Am J Ophthalmol. 1999;127:602–604. doi: 10.1016/s0002-9394(99)00050-1. [DOI] [PubMed] [Google Scholar]
- 7.Chine KH, Lu DW, Chen JT. Extensive facial skin pigmentation after latanoprost treatment. Cutan Ocul Toxicol. 2009;28:185–187. doi: 10.3109/15569520903232534. [DOI] [PubMed] [Google Scholar]


