Abstract
Background
Those living in rural areas have poorer cancer outcomes, but current evidence on how rurality impacts melanoma care and survival is contradictory.
Aim
To investigate the impact of rurality on setting of melanoma excision and mortality in a whole-nation cohort.
Design and setting
Analysis of linked routine healthcare data comprising every individual in Scotland diagnosed with melanoma, January 2005–December 2013, in primary and secondary care.
Method
Multivariate binary logistic regression was used to explore the relationship between rurality and setting of melanoma excision; Cox proportional hazards regression between rurality and mortality was used, with adjustments for key confounders.
Results
In total 9519 patients were included (54.3% [n = 5167] female, mean age 60.2 years [SD 17.5]). Of melanomas where setting of excision was known, 90.3% (n = 8598) were in secondary care and 8.1% (n = 771) in primary care. Odds of primary care excision increased with increasing rurality/remoteness. Compared with those in urban areas, those in the most remote rural locations had almost twice the odds of melanoma excision in primary care (adjusted odds ratio [aOR] 1.92; 95% confidence interval [CI] = 1.33 to 2.77). No significant association was found between urban or rural residency and all-cause mortality. Melanoma-specific mortality was significantly lower in individuals residing in accessible small towns than in large urban areas (adjusted hazards ratio [HR] 0.53; 95% CI = 0.33 to 0.87) with no trend towards poorer survival with increasing rurality.
Conclusion
Patients in Scottish rural locations were more likely to have a melanoma excised in primary care. However, those in rural areas did not have significantly increased mortality from melanoma. Together these findings suggest that current UK melanoma management guidelines could be revised to be more realistic by recognising the role of primary care in the prompt diagnosis and treatment of those in rural locations.
Keywords: cancer, geography, melanoma, primary health care, rurality
INTRODUCTION
Rural patients appear to have a survival disadvantage following a cancer diagnosis compared with their urban counterparts.1 Melanoma skin cancer is an important cause of mortality and morbidity in the UK, and the incidence of melanoma is rising.2 Mortality from this visible cancer is strongly influenced by early detection and complete excision, with thin cancers that are fully excised having excellent rates of cure.3 Patient factors including socioeconomic status and delayed presentation are known to contribute to inequities in survival from melanoma.4 It seems likely that geography and processes of care could also influence melanoma survival. However, evidence of geographical and treatment inequities for melanoma is under-studied and potential mechanisms for rural disadvantage after a cancer diagnosis remain obscure.1
Existing evidence on the influence of geography on melanoma treatment and survival is contradictory. A study conducted in Queensland, Australia, found that patients with melanoma from rural areas had an adjusted case-fatality rate 20% higher than urban counterparts. The authors concluded that differences in access to services and variation in management practices may partly account for the observation, but they did not adjust for socioeconomic status in their analysis.5 The authors of the present study have previously reported that people living in rural areas within Northeast Scotland are more likely to have their melanoma excised by a GP than their city-dwelling counterparts.6 This is contrary to UK guidelines that mandate that all skin lesions suspicious of melanoma should be referred to secondary care for diagnosis and treatment.7–9 Recently, however, reassuring evidence was found in a whole-Scotland sample of 9519 people diagnosed and treated for melanoma from 2005 to 2013, which showed that primary care excision of melanoma does not result in increased mortality and morbidity.10
In the earlier work by the authors of this study, despite observing higher rates of initial excision of melanoma by GPs,. no evidence was found of rural patients in Northeast Scotland having higher rates of incomplete excision, nor did they have increased rates of morbidity or mortality.6,11,12 An acknowledged limitation was that the authors only studied patients from a single health board (Grampian) in Northeast Scotland.6,11,12 Grampian’s relative affluence could potentially have masked a rural disadvantage compared with other areas, because lower socioeconomic status is associated with later diagnosis of melanoma and poorer survival.13 The limitation is addressed in this study and reports the first ever investigation of the influence of rurality on the setting of melanoma excision and mortality in a whole-nation cohort.
How this fits in
Existing evidence of the impact of rural residence on melanoma management and outcomes is conflicting and drawn from small regional studies with limited external validity. This study was the first to investigate the impact of rurality on the processes and outcomes of melanoma treatment using a whole-nation cohort. Conducted in Scotland, and based upon all diagnoses of melanoma from 2007 to 2013, it found that those living in rural areas are significantly more likely to have their melanoma excised in primary care but that this did not confer increased all-cause or melanoma-specific mortality. These results are reassuring for rural patients in the UK and their GPs.
METHOD
Study design and population
This was a data-linkage study comprising a population-based cohort containing every individual in Scotland who received a pathological diagnosis of cutaneous invasive melanoma from January 2005 to December 2013. The primary outcome of interest was melanoma-specific survival based upon urban or rural residence, controlling for important confounders.
Data sources
The Scottish Cancer Registry (with underlying pathology records supplied electronically at regular intervals by all NHS pathology laboratories in Scotland); the National Records of Scotland (NRS) death registry; the Scottish Morbidity Record Acute Inpatient and Day Case Admission dataset (SMR01); and the Hospital Outpatient Attendance dataset (SMR00) were linked using the Community Health Index (CHI) number14 for all patients diagnosed with cutaneous melanoma in Scotland between 1 January 2005 and 3 December 2013. (The data used for this study are archived within the NHS Scotland National Statistics Service National Safe Haven and are not freely available. Other bona fide researchers wishing to access the data should apply to the authors in the first instance. Subsequent access to the data would be subject to application to, and approval by, the Public Benefit and Privacy Panel for Health and Social Care of NHS Scotland.)
The Scottish Cancer Registry (SMR06) and underlying pathology records provided data including: date of diagnosis, setting of melanoma excision (primary or secondary care), age, sex, deprivation measured by the Scottish Index of Multiple Deprivation (SIMD) quintile,15 health board of residence, melanoma type, anatomical site, Breslow thickness (depth in millimetres by which a melanoma has invaded the dermis),9 and presence of metastatic disease (from linked hospitalisation records (SMR01)). The NRS death registry provided date of death and primary underlying cause of death as detailed on the death certificate for individuals who had subsequently died. A Charlson comorbidity score was calculated for each cohort member using SMR01 information, following established methods.16 Patients diagnosed following their initial diagnostic excision biopsy in either primary or secondary care setting were followed until death, date of emigration, or end of follow-up which was 31 December 2015, whichever occurred first. Those patients who were alive at the end of follow-up or recorded as emigrated were considered censored.
Exposure
The exposure of interest was rurality. The Scottish Government 6 fold Urban Rural Classification provided a standard definition of rural areas in Scotland.17 The six-fold classification categorises Royal Mail postcodes into:
large urban areas of ≥125 000 people;
other urban areas of 10 000–124 999 people;
accessible small towns of 3000–9999 people within a 30-minute drive of a settlement of 10 000 people;
remote small towns with settlements of 3000–9999 people, with a 30-minute drive from a settlement of 10 000 people;
accessible rural areas with a population of <3000 people and within a 30-minute drive of a settlement of ≥10 000; and
remote rural areas with a population of <3000 people and a drive time of >30 minutes to a settlement of ≥10 000 people.
Statistical analyses
Demographics, clinical variables, and outcomes were described and compared using tests appropriate to continuous or categorical variables. Associations between the six-fold urban–rural classification and other categorical variables were examined using the χ2 test for trend. The association between the six-fold urban–rural classification and age, and Breslow thickness was examined using one-way ANOVA and the Kruskal–Wallis test respectively.
Multivariate binary logistic regression was used to explore the influence of rurality on the location of the initial diagnostic excision biopsy. The dependent variable was location of excision (primary versus secondary care) with the Scottish six-fold rural–urban classification as the indicator variable (reference category = large urban area). The unadjusted odds ratio (OR) and its 95% confidence interval (CI) for excision in primary (reference group) versus secondary care was calculated. The odds ratio was then adjusted for: sex, age, deprivation, anatomical site, melanoma type, Breslow thickness, the presence of metastatic disease at diagnosis, and Charlson score.
To explore the influence of rurality on survival, Kaplan–Meier curves were plotted for both cumulative observed survival and cumulative melanoma-specific survival from date of melanoma diagnosis for each of the six-fold urban–rural categories. Cox proportional hazards modelling was then used with adjustment for estimating the hazard ratio (HR) and associated 95% CI of all-cause and melanoma-specific survival for each of the six-fold urban–rural categories with adjustment for: sex, age, deprivation, anatomical site, melanoma type, setting of melanoma excision, Breslow thickness, metastatic disease at diagnosis, and Charlson score. The proportional hazard (PH) assumption is based on Schoenfeld residuals.18,19 There were no violations of PH assumption detected in the current analysis. The interaction effect between setting of excision and six-fold urban–rural classification was examined for all-cause and melanoma-specific mortality outcomes.
In both the binary logistic regression and Cox proportional hazards analysis robust variance and standard error estimates of the regression coefficients were computed to account for the correlation of observations within health boards.20 All analyses were conducted using SPSS version 24 and Stata/MP version 14. A two-sided P-value <0.05 was considered statistically significant throughout.
RESULTS
Comparisons of key demographic and clinical characteristics within the Scottish 6 fold Urban Rural Classification categories
A total of 9519 patients had a melanoma diagnosis recorded in Scotland, 2005–2013. Median follow-up was 71 months (interquartile range [IQR] 45–101 months). Over half the cohort (n = 5167, 54.3%) were female, and the mean age was 60.2 years (standard deviation [SD] 17.5) (Table 1). Around two-thirds of the cohort lived in large urban or suburban settings (n = 6349, 66.7%). Patients in remote rural areas were older compared with patients living in large urban areas (mean age = 62.8 years [SD 15.1] versus 59.5 years [SD 18.2]) (Table 2), P<0.001 for trend.
Table 1.
Characteristics of patients with melanoma excised in Scotland 2005–2013, N = 9519
| Characteristic | n (%)a |
|---|---|
| Sex | |
| Male | 4352 (45.7) |
| Female | 5167 (54.3) |
|
| |
| Age, years, | |
| Mean (SD) | 60.2 (17.5) |
|
| |
| Setting of melanoma excision | |
| Primary care | 771 (8.1) |
| Secondary care | 8598 (90.3) |
| Unknown | 150 (1.6) |
|
| |
| Urban–rural (six-fold) | |
| 1 = Large urban area | 3549 (37.3) |
| 2 = Suburban | 2800 (29.4) |
| 3 = Accessible small town | 886 (9.3) |
| 4 = Remote small town | 398 (4.2) |
| 5 = Accessible rural | 1177 (12.4) |
| 6 = Remote rural | 689 (7.2) |
| Missing | 20 (0.2) |
|
| |
| Deprivation — SIMD quintiles | |
| 1 = most deprived | 1292 (13.6) |
| 2 | 1652 (17.4) |
| 3 | 1923 (20.2) |
| 4 | 2124 (22.3) |
| 5 = least deprived | 2523 (26.5) |
| Missing | 5 (<0.1) |
|
| |
| Anatomical site of melanoma | |
| Head and neck | 2201 (23.1) |
| Body | 2596 (27.2) |
| Upper limb | 1958 (20.5) |
| Groin and lower limb | 2597 (27.3) |
| Missing | 167 (1.8) |
|
| |
| Melanoma subtype | |
| Superficial spreading | 4871 (51.2) |
| Nodular | 882 (9.3) |
| Lentigo | 1169 (12.3) |
| Acral | 236 (2.5) |
| Others | 1553 (16.3) |
| Missing | 808 (8.5) |
|
| |
| Metastases at presentation | |
| No | 9057 (95.1) |
| Yes | 462 (4.9) |
|
| |
| Vital status at end of follow-up | |
| Alive | 7411 (77.9) |
| Non-melanoma death | 1156 (12.1) |
| Died due to melanoma | 952 (10.0) |
|
| |
| Charlson comorbidity index | |
| 0 | 8677 (91.2) |
| 1–2 | 765 (8.1) |
| 3–4 | 53 (0.6) |
| ≥5 | 24 (0.3) |
|
| |
| Breslow thickness, mm | |
| Median (IQR) | 0.9 (0.5–2) |
Unless otherwise stated. IQR = interquartile range. SD = standard deviation. SIMD = Scottish Index of Multiple Deprivation.
Table 2.
Characteristics of individuals with melanoma excised in Scotland 2005–2013 by geographical location of residencea
| Characteristic | Large urban area (N = 3549) | Suburban (N = 2800) | Accessible small town (N = 886) | Remote small town (N = 398) | Accessible rural (N = 1177) | Remote rural (N= 689) | P-value for trend |
|---|---|---|---|---|---|---|---|
| Setting of excision, n(%) | |||||||
| Primary care | 145 (4.1) | 253 (9.2) | 73 (8.4) | 50 (12.7) | 131 (11.3) | 117 (17.3) | <0.001 |
| Secondary care | 3339 (95.8) | 2509 (90.8) | 797 (91.6) | 345 (87.3) | 1030 (88.7) | 561 (82.7) | |
|
| |||||||
| Age, years | |||||||
| Mean (SD) | 59.5 (18.2) | 59.9 (17.6) | 61.4 (17.5) | 64.0 (16.9) | 60.3 (16.1) | 62.8 (15.1) | <0.001 |
|
| |||||||
| Sex | |||||||
| Male | 1587 (44.7) | 1248 (44.6) | 424 (47.9) | 175 (44.0) | 556 (47.2) | 354 (51.4) | 0.002 |
| Female | 1962 (55.3) | 1552 (55.4) | 462(52.1) | 223 (56.0) | 621 (52.8) | 335 (48.6) | |
|
| |||||||
| Deprivation — SIMD quintiles, n | |||||||
| 1 = most deprived | 749 (21.1) | 370 (13.2) | 91 (10.3) | 24 (6.0) | 39 (3.3) | 17 (2.5) | 0.012 |
| 2 | 568 (16.0) | 649 (23.2) | 147 (16.6) | 95 (23.9) | 113 (9.6) | 76 (11.0) | |
| 3 | 481 (13.6) | 506 (18.1) | 167 (18.8) | 113 (28.4) | 308 (26.2) | 342 (49.6) | |
| 4 | 517 (14.6) | 551 (19.7) | 205 (23.1) | 101 (25.4) | 526 (44.7) | 223 (32.4) | |
| 5 = least deprived | 1234 (34.8) | 723 (25.8) | 276 (31.2) | 65 (16.3) | 191 (16.2) | 31 (4.5) | |
|
| |||||||
| Anatomical site, n | |||||||
| Head and neck | 787 (22.6) | 634 (23.0) | 223 (25.5) | 111 (28.4) | 274 (23.7) | 170 (25.4) | 0.066 |
| Body | 1000 (28.7) | 759 (27.6) | 241 (27.5) | 76 (19.4) | 302 (26.1) | 208 (31.0) | |
| Upper limb | 703 (20.2) | 594 (21.6) | 182 (20.8) | 93 (23.8) | 262 (22.6) | 123 (18.4) | |
| Groin and lower limb | 995 (28.6) | 767 (27.9) | 229 (26.2) | 111 (28.4) | 319 (27.6) | 169 (25.2) | |
|
| |||||||
| Melanoma subtype, n | |||||||
| Superficial spreading | 1857 (52.3) | 1441 (51.5) | 438 (49.4) | 177 (44.5) | 599 (50.9) | 347 (50.4) | 0.035 |
| Nodular | 291 (8.2) | 316 (11.3) | 55 (6.2) | 50 (12.6) | 115 (9.8) | 55 (8.0) | |
| Lentigo | 431 (12.1) | 322 (11.5) | 120 (13.5) | 57 (14.3) | 154 (13.1) | 83 (12.0) | |
| Acral | 100 (2.8) | 60 (2.1) | 21 (2.4) | 13 (3.3) | 27 (2.3) | 15 (2.2) | |
| Others | 565 (15.9) | 430 (15.4) | 155 (17.5) | 75 (18.8) | 201 (17.1) | 122 (17.7) | |
| Missing | 305 (8.6) | 231 (8.3) | 97 (10.9) | 26 (6.5) | 81 (6.9) | 67 (9.7) | |
|
| |||||||
| Metastases at presentation, n | |||||||
| No | 3386 (95.4) | 2654 (94.8) | 857 (96.7) | 364 (91.5) | 1123 (95.4) | 654 (94.9) | 0.532 |
| Yes | 163 (4.6) | 146 (5.2) | 29 (3.3) | 34 (8.5) | 54 (4.6) | 35 (5.1) | |
|
| |||||||
| Mortality, n | |||||||
| Non-melanoma death | 422 (11.9) | 323 (11.5) | 119 (13.4) | 54 (13.6) | 141 (12.0) | 97 (14.1) | |
| Melanoma death | 350 (9.9) | 298 (10.6) | 71 (8.0) | 51 (12.8) | 109 (9.3) | 72 (10.4) | |
|
| |||||||
| Breslow thickness, mm | |||||||
| Median (IQR) | 0.90 (0.5–1.9) | 0.90 (0.5–2.0) | 0.90 (0.5–2) | 1.0 (0.5–2.5) | 0.9 (0.5–1.9) | 0.90 (0.5–1.9) | 0.390 |
|
| |||||||
| Charlson comorbidity index, n | |||||||
| 0 | 3250 (91.6) | 2554 (91.2) | 812 (91.6) | 352 (88.4) | 1077 (91.5) | 613 (89.0) | 0.060 |
| 1 | 140 (3.9) | 121 (4.3) | 40 (4.5) | 24 (6.0) | 37 (3.1) | 26 (3.8) | |
| 2 | 136 (3.7) | 103 (3.7) | 31 (3.5) | 18 (4.5) | 49 (4.2) | 39 (5.7) | |
| ≥3 | 23 (0.6) | 22 (0.8) | 3 (0.3) | 4 (1.0) | 14 (1.2) | 11 (1.6) | |
All rows have missing data. The authors have not presented missing data for the purposes of conciseness. Percentages are calculated from the data presented here, not accounting for missing data. The numbers missing from each row and column can be calculated from the data presented. IQR = interquartile range. SD = standard deviation. SIMD = Scottish Index of Multiple Deprivation.
Almost 17% (n = 117 of 689) of patients residing in the most remote rural area had their excision in primary care compared with 4.1% (n = 145 of 3549) of patients residing in large urban settings. Rural patients were less likely to be in the least or most deprived quintiles than urban dwellers: 4.5% (n = 31) of remote rural dwellers were in the least deprived category compared with 34.8% (n = 1234) of dwellers from large urban areas, and 2.5% of remote rural dwellers were in the most deprived category compared with 21.1% of urban dwellers (P<0.012 for trend).
There was a significantly higher proportion of males in rural (51.4%, n = 354) than urban areas (44.7%, n = 1587, P = 0.002 for trend). There were no statistically significant differences in Breslow thickness of tumour at diagnosis, anatomical site of melanoma, death (any cause and melanoma-specific), Charlson comorbidity index, or metastases at presentation between urban and rural dwellers.
Setting of excision
All patients living outside of large urban areas had significantly greater odds of having their melanoma excised in primary care (Table 3). Those in the most remote rural areas (category 6) had nearly twice the odds of having their melanoma excised in primary care than those dwelling in large urban (category 1) areas, (adjusted odds ratio [OR] 1.92; 95% CI = 1.33 to 2.77). Those in accessible rural areas also had significantly greater odds of melanoma excision in primary care (aOR 1.75; 95% CI = 1.15 to 2.67). Those in accessible small towns (category 3) and other urban areas (category 2) also had significantly greater odds of having their melanoma excised in primary care than large urban areas, aOR 1.52; 95% CI = 1.02 to 2.27, and aOR 1.83; 95% CI = 1.17 to 2.88, respectively (Table 3). After adjusting for important confounders, there was no significant association between deprivation category and primary care melanoma excision.
Table 3.
Factors associated with primary care melanoma excision
| Patient characteristic | Setting: primary care (Na = 771)b | Unadjusted OR (95% CI) | Adjusted ORc (95% CI) |
|---|---|---|---|
| Sex | |||
| Female versus male | 416 | 1.04 (0.95 to 1.13) | 1.05 (0.93 to 1.19) |
|
| |||
| Age, years | |||
| Mean, + 1 year (SD) | 57.6 (16.8) | 0.99 (0.98 to 0.99) | 0.99 (0.98 to 0.99) |
|
| |||
| Urban–rural (six-fold) | |||
| 1 = Large urban area | 145 | 1 | 1 |
| 2 = Other urban area | 253 | 1.68 (1.06 to 2.67) | 1.83 (1.17 to 2.88) |
| 3 = Accessible small town | 73 | 1.35 (0.91 to 2.02) | 1.52 (1.02 to 2.27) |
| 4 = Remote small town | 50 | 1.21 (0.82 to 1.76) | 1.18 (0.76 to 1.83) |
| 5 = Accessible rural | 131 | 1.57 (1.00 to 2.46) | 1.75 (1.15 to 2.67) |
| 6 = Remote rural | 117 | 1.63 (1.17 to 2.28) | 1.92 (1.33 to 2.77) |
|
| |||
| Deprivation — SIMD quintiles | |||
| 1 = most deprived | 67 | 1 | 1 |
| 2 | 127 | 1.07 (0.89 to 1.28) | 1.13 (0.98 to 1.31) |
| 3 | 197 | 1.11 (0.72 to 1.73) | 1.05 (0.72 to 1.54) |
| 4 | 182 | 0.94 (0.64 to 1.39) | 0.84 (0.60 to 1.17) |
| 5 = least deprived | 197 | 1.05 (0.77 to 1.45) | 1.05 (0.75 to 1.48) |
|
| |||
| Anatomical site | |||
| Head and neck | 90 | 1 | 1 |
| Body | 272 | 3.13 (2.60 to 3.76) | 2.32 (1.77 to 3.00) |
| Upper limb | 201 | 2.92 (2.40 to 3.54) | 2.32 (1.77 to 3.04) |
| Groin and lower limb | 196 | 2.07 (1.63 to 2.62) | 1.59 (1.10 to 2.28) |
|
| |||
| Melanoma subtype | |||
| Superficial spreading | 388 | 1 | 1 |
| Nodular | 113 | 1.75 (1.39 to 2.20) | 2.39 (1.84 to 3.11) |
| Lentigo | 42 | 0.40 (0.32 to 0.50) | 0.69 (0.50 to 0.96) |
| Acral | 6 | 0.34 (0.16 to 0.72) | 0.46 (0.20 to 1.06) |
| Others | 151 | 1.21 (1.02 to 1.45) | 1.46 (1.25 to 1.70) |
|
| |||
| Metastasis at presentation | 32 | 0.75 (0.38 to 1.49) | 1.15 (0.63 to 2.08) |
|
| |||
| Breslow thickness, mm | |||
| Median, (IQR) | 0.95 (0.5 to 2.35) | 0.99 (0.96 to 1.01) | 0.96 (0.92 to 1.00) |
|
| |||
| Charlson comorbidity index | |||
| 0 | 730 | 1 | |
| 1 | 20 | 0.62 (0.30 to 1.25) | 0.93 (0.42 to 2.03) |
| 2 | 19 | 0.51 (0.34 to 0.78) | 0.53 (0.39 to 0.74) |
| ≥3 | 2 | 0.24 (0.05 to 1.12) | 0.31 (0.07 to 1.43) |
Unless otherwise stated.
Some rows have missing data. The number available for each analysis is indicated within individual rows.
Adjusted for sex, age, deprivation, anatomical site, melanoma subtype, Breslow thickness, metastasis at presentation, and Charlson comorbidity index, except where the variable itself is being considered. IQR = interquartile range. OR = odds ratio. SD = standard deviation. SIMD = Scottish Index of Multiple Deprivation.
Melanomas on the body and upper limbs had significantly greater odds of being excised in primary care than those on the head and neck: body aOR 2.32; 95% CI = 1.77 to 3.00, and upper limb aOR 2.32; 95% CI = 1.77 to 3.04. Nodular melanomas had significantly greater odds of being excised in primary care compared with superficial spreading melanomas, aOR 2.39; 95% CI = 1.84 to 3.11.
Mortality
There was no significant association between urban or rural residency and overall risk of death from any cause (Figure 1 and Table 4). However, there was a significantly reduced risk of mortality associated with primary care excision in the unadjusted analysis (31% reduction), but this was no longer significant following adjustment. On further investigation, age at diagnosis was the factor that was primarily responsible for the loss of statistical significance.
Figure 1.
Kaplan–Meier curve displaying cumulative all-cause survival proportions by six-fold urban–rural classification (in months) from the date of melanoma diagnosis.
Table 4.
Factors associated with hazard of death (any cause)
| Patient characteristic | Any-cause death (Na = 2108)b | Unadjusted HR (95% CI) | Adjustedc HR (95% CI) |
|---|---|---|---|
| Sex (N= 2108) | |||
| Female versus male | 886 | 0.57 (0.53 to 0.63) | 0.72 (0.64 to 0.81) |
|
| |||
| Age (N= 2108) | |||
| Mean, + 1 year (SD) | 72.8 (14.3) | 1.07 (1.06 to 1.07) | 1.06 (1.06 to 1.07) |
|
| |||
| Urban–rural (six-fold) (N= 2079) | |||
| 1 = Large urban area | 759 | 1 | 1 |
| 2 = Other urban area | 614 | 1.02 (0.92 to 1.13) | 0.95 (0.83 to 1.08) |
| 3 = Accessible small town | 187 | 0.98 (0.82 to 1.18) | 0.82 (0.64 to 1.04) |
| 4 = Remote small town | 104 | 1.27 (1.07 to 1.50) | 0.90 (0.79 to 1.02) |
| 5 = Accessible rural | 246 | 0.97 (0.82 to 1.15) | 1.02 (0.86 to 1.22) |
| 6 = Remote rural | 169 | 1.18 (0.99 to 1.39) | 1.03 (0.86 to 1.22) |
|
| |||
| Deprivation — SIMD quintiles (N= 2080) | |||
| 1 = most deprived | 336 | 1 | 1 |
| 2 | 410 | 0.95 (0.85 to 1.06) | 0.87 (0.77 to 0.98) |
| 3 | 451 | 0.89 (0.76 to 1.05) | 0.79 (0.67 to 0.92) |
| 4 | 428 | 0.76 (0.68 to 0.86) | 0.69 (0.63 to 0.77) |
| 5 = least deprived | 455 | 0.65 (0.57 to 0.75) | 0.56 (0.45 to 0.70) |
|
| |||
| Setting of excision (N= 2080) | |||
| Secondary care | 1950 | 1 | 1 |
| Primary care | 130 | 0.69 (0.55 to 0.86) | 0.90 (0.71 to 1.13) |
|
| |||
| Anatomical site (N= 2089) | |||
| Head and neck | 706 | 1 | 1 |
| Body | 598 | 0.55 (0.51 to 0.61) | 1.08 (0.92 to 1.28) |
| Upper limb | 324 | 0.47 (0.43 to 0.51) | 0.85 (0.69 to 1.05) |
| Groin and lower limb | 461 | 0.50 (0.46 to 0.54) | 1.02 (0.82 to 1.27) |
|
| |||
| Melanoma subtype (N= 1795) | |||
| Superficial spreading | 565 | 1 | 1 |
| Nodular | 375 | 4.61 (4.04 to 5.28) | 1.75 (1.46 to 2.10) |
| Lentigo | 309 | 2.43 (2.07 to 2.87) | 1.14 (0.91 to 1.42) |
| Acral | 76 | 3.29 (2.58 to 4.18) | 1.54 (1.33 to 1.79) |
| Others | 470 | 2.96 (2.59 to 3.38) | 1.43 (1.26 to 1.62) |
|
| |||
| Breslow thickness, mm (N= 2108) | |||
| Median (IQR) | 0.8 (0.5–1.4) | 1.13 (1.11 to 1.15) | 1.09 (1.06 to 1.12) |
|
| |||
| Metastasis at presentation (N= 2108) | |||
| Yes | 296 | 5.83 (4.52 to 7.51) | 3.50 (2.60 to 4.70) |
|
| |||
| Charlson comorbidity index (N= 2080) | |||
| 0 | 1678 | 1 | 1 |
| 1 | 192 | 3.30 (2.73 to 4.00) | 1.89 (1.61 to 2.2) |
| 2 | 157 | 2.63 (2.17 to 3.19) | 1.53 (1.32 to 1.79) |
| ≥3 | 53 | 6.40 (5.11 to 8.01) | 2.93 (2.33 to 3.68) |
Unless otherwise stated.
Some data are missing in each row. The number available for analysis is presented individually in each row.
Adjusted for sex, age, deprivation, setting of excision, anatomical site, melanoma subtype, Breslow thickness, metastasis at presentation, and Charlson index except where the variable itself is being examined. HR = hazards ratio. IQR = interquartile range. SD = standard deviation. SIMD = Scottish Index of Multiple Deprivation.
There were statistically significant associations with higher all-cause mortality and each of: lower socioeconomic status, increasing Breslow thickness, and nodular melanoma (compared with superficial spreading melanoma). Lower levels of deprivation were associated with lower hazard of all-cause mortality (SIMD category 5, least deprived, adjusted hazard ratio (HR) 0.56; 95% CI = 0.45 to 0.70, and SIMD category 4, adjusted HR 0.69; 95% CI = 0.63 to 0.77). Nodular melanoma was associated with increased hazard of death (any cause) compared with superficial spreading melanoma, adjusted HR 1.75; 95% CI = 1.46 to 2.10 (Table 4).
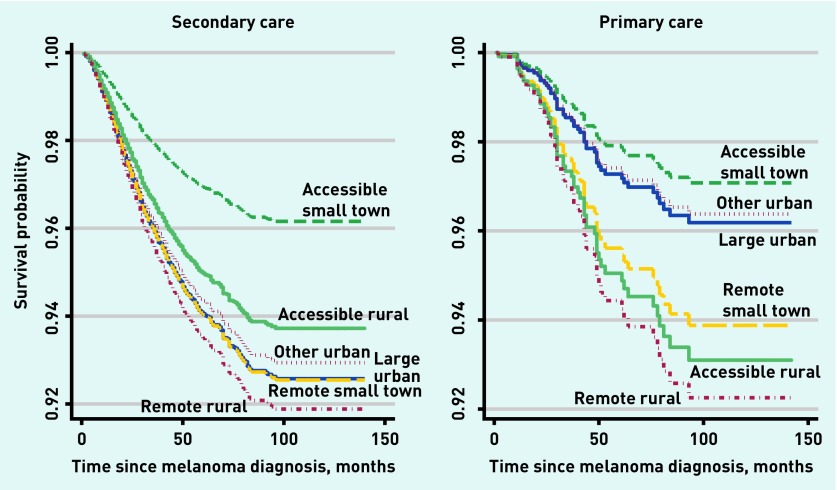
Melanoma-specific mortality (Figure 2 and Table 5) was significantly lower in individuals residing in accessible small towns than in large urban areas (adjusted HR 0.53; 95% CI = 0.33 to 0.87) but there were no other significant associations between urban/rural residency and risk of death from melanoma. Remote rural dwellers were no more likely to die from melanoma than those residing in large urban areas (adjusted HR 1.09; 95% CI = 0.87 to 1.37). Setting was significantly associated with melanoma-specific mortality in the unadjusted analysis, but this was lost on multiple adjustment, primarily due to the combined impact of several confounders such as age at diagnosis, rurality, SIMD, anatomical site and Breslow thickness. Further analysis revealed that the effect of urban–rural classification on hazard of death from melanoma was significantly different by setting of excision (P = 0.005). There was a clearer separation of survival curves between remote and accessible rural locations among those undergoing excision in primary care (Figure 3).
Figure 2.
Kaplan–Meier curve displaying cumulative melanoma to specific survival proportions by six-fold urban–rural classification (in months) from the date of melanoma diagnosis.
Table 5.
Factors associated with melanoma-specific death
| Patient characteristic | Melanoma-specific death (Na = 952)b | Unadjusted HR (95% CI) | Adjusted HRc (95% CI) |
|---|---|---|---|
| Sex | |||
| Female versus male (male n = 952) | 381 | 0.54 (0.47 to 0.62) | 0.68 (0.57 to 0.81) |
|
| |||
| Age (N = 952) | |||
| Mean, + 1 year (SD) | 66.4 (15.4) | 1.03 (1.02 to 1.03) | 1.02 (1.02 to 1.03) |
|
| |||
| Urban–rural (six-fold) (N = 937) | |||
| 1 = Large urban area | 344 | 1 | 1 |
| 2 = Other urban area | 295 | 1.08 (0.91 to 1.30) | 0.95 (0.76 to 1.18) |
| 3 = Accessible small town | 69 | 0.80 (0.64 to 1.01) | 0.53 (0.33 to 0.87) |
| 4 = Remote small town | 50 | 1.34 (1.03 to 1.75) | 1.03 (0.77 to 1.37) |
| 5 = Accessible rural | 107 | 0.93 (0.77 to 1.11) | 0.90 (0.70 to 1.17) |
| 6 = Remote rural | 72 | 1.11 (0.92 to 1.33) | 1.09 (0.87 to 1.37) |
|
| |||
| Deprivation (SIMD) (N = 938) | |||
| 1 = most deprived | 148 | 1 | 1 |
| 2 | 195 | 1.03 (0.83 to 1.27) | 1.03 (0.84 to 1.27) |
| 3 | 209 | 0.94 (0.74 to 1.20) | 0.74 (0.58 to 0.96) |
| 4 | 193 | 0.78 (0.61 to 0.99) | 0.79 (0.61 to 1.02) |
| 5 = least deprived | 193 | 0.63 (0.53 to 0.75) | 0.61 (0.45 to 0.81) |
|
| |||
| Setting of excision (N = 938) | |||
| Secondary care | 875 | 1 | 1 |
| Primary care | 63 | 0.76 (0.58 to 0.99) | 0.91 (0.65 to 1.29) |
|
| |||
| Anatomical site (N = 865) | |||
| Head and neck | 198 | 1 | 1 |
| Body | 286 | 1.16 (1.04 to 1.29) | 1.38 (1.10 to 1.74) |
| Upper limb | 145 | 0.76 (0.62 to 0.92) | 0.93 (0.71 to 1.21) |
| Groin and lower limb | 236 | 0.93 (0.81 to 1.05) | 1.24 (0.87 to 1.77) |
|
| |||
| Melanoma subtype (N = 805) | |||
| Superficial spreading | 226 | 1 | 1 |
| Nodular | 218 | 6.53 (5.47 to 7.81) | 2.71 (2.11 to 3.48) |
| Lentigo | 49 | 0.95 (0.65 to 1.40) | 0.82 (0.56 to 1.22) |
| Acral | 42 | 4.44 (3.25 to 6.05) | 2.32 (1.59 to 3.40) |
| Others | 270 | 4.25 (3.52 to 5.14) | 1.83 (1.54 to 2.19) |
|
| |||
| Breslow thickness (N = 952) | |||
| Median (IQR) | 3.9 (2.0–6.5) | 1.15 (1.12 to 1.18) | 1.13 (1.10 to 1.16) |
|
| |||
| Metastasis at presentation (N = 952) | |||
| Yes | 226 | 10.75 (8.89 to 12.99) | 4.35 (3.24 to 5.84) |
|
| |||
| Charlson comorbidity index (N = 938) | |||
| 0 | 809 | 1 | 1 |
| 1 | 51 | 1.76 (1.38 to 2.24) | 1.28 (0.96 to 1.70) |
| 2 | 54 | 1.82 (1.49 to 2.24) | 1.04 (0.70 to 1.54) |
| ≥3 | 24 | 5.56 (3.41 to 9.06) | 2.96 (1.65 to 5.28) |
Unless otherwise stated.
Some data are missing. The number available for analysis is presented invidually in each row.
Adjusted for sex, age, deprivation, setting of excision, anatomical site, melanoma subtype, Breslow thickness, metastasis at presentation, and Charlson index except where the variable itself is being examined. HR = hazards ratio. IQR = interquartile range. SD = standard deviation. SIMD = Scottish Index of Multiple Deprivation.
Figure 3:
Kaplan–Meier curve displaying cumulative melanoma-specific survival proportions by six-fold urban–rural classification (in months) from the date of melanoma diagnosis stratified by setting of excision.
Death from melanoma was significantly associated with increasing age (per year, adjusted HR 1.02; 95% CI = 1.02 to 1.03) and increasing Breslow thickness (adjusted HR 1.13; 95% CI = 1.10 to 1.16) (Table 5). Those in the least deprived SIMD category had lower hazard of melanoma-specific death than the most deprived (adjusted HR 0.61; 95% CI = 0.45 to 0.81). Nodular and acral melanomas had an increased hazard of melanoma-specific mortality compared with superficial spreading melanoma, adjusted HRs 2.71 (95% CI = 2.11 to 3.48) and 2.32 (95% CI = 1.59 to 3.40), respectively. A Charlson index of three or more was associated with a near three-fold increase in hazard of melanoma-specific death (adjusted HR 2.96; 95% CI = 1.65 to 5.28).
DISCUSSION
Summary
Rural residency did not confer significantly poorer all-cause or melanoma-specific survival for people living in Scotland diagnosed and treated with melanoma, January 2005–December 2013. Overall 8.1% of melanomas had been excised in primary care, but initial primary care excision of melanoma was significantly more likely for those living in rural areas. Those living in the most remote rural areas were almost twice as likely to have had an initial excision performed by a GP compared with city dwellers. Strikingly, in adjusted analysis, those living in accessible small towns had a near 50% reduction in melanoma-specific survival compared with other urban–rural categories. This may relate to a concentration of favourable sociodemographic and service characteristics, for example, relatively affluent patients living close to accessible, well-staffed, and slightly less pressured practices, an observation worthy of further study.
In Scotland, rural residence does not appear to confer poorer survival for cutaneous melanoma. This contradicts the balance of evidence on rural cancer outcomes and is therefore reassuring for rural residents with melanoma. These patients are, however, more likely to have their melanomas initially excised by a GP, contrary to prevailing UK guidelines. This finding perhaps suggests that, despite the guidelines, a pragmatic approach is being practised with respect to melanoma in rural healthcare settings and it is reassuring to note that this is occurring without adversely affecting the survival of patients with melanoma living in rural areas.
These data provide a basis for current UK melanoma guidelines to be reviewed and consideration given to making management recommendations, which consider a patient’s place of residence.
Strengths and limitations
The key strength of this study was the quality of the data. It was based on a large national sample of patients followed up for a median of 71 months. The data were comprehensive and largely complete. The Scottish Government 6 fold Urban Rural Classification is an established method of defining rurality and was available for all of the subjects contained in the dataset. Deprivation was also assigned to every subject, though it should be noted that the SIMD provides a measure based on small-area estimates of relative deprivation so there exists the potential for some individuals to be misclassified.
A further limitation is that, despite the Scotland-wide sample, numbers in some categories were small. The analysis accounted for clustering by health board, but not at GP level or by the clinician performing the excisions, where outcomes might be more strongly correlated. Additional data on, for example, diagnostic intervals, completeness of excision, and details of the diagnostic impression of the clinician submitting the sample may have enabled a more definitive analysis; obtaining these data should be considered by future researchers.
Though this is a large Scotland-wide sample, data may not apply internationally because international healthcare systems vary markedly with respect to the balance between primary care gate-keeping and direct patient access to secondary care specialists and treatment.21
In some countries the proportion of primary care excisions occurring in rural areas will be even higher and it would be very interesting to compare these findings with those settings. As they stand, however, data appear to support the notion of rural GPs excising suspicious skin lesions without detriment to their patients.
Comparison with existing literature
It is reassuring to note that rural residence did not lead to significantly poorer survival from cutaneous melanoma in this large Scotland-wide sample.
Previous work in Scotland has found evidence of poorer survival for rural patients with prostate and lung cancers, but rural versus urban melanoma outcomes have not previously been studied in Scotland, or in fact anywhere on the scale reported here.22
The current results also admit the possibility that rurality may impact cancer sites differentially. Because Australian researchers found evidence of poorer survival for rural dwellers with melanoma, it also seems plausible that there may be international differences in geographical impact on cancer outcomes.5
Implications for research and practice
The results from this study also cast further doubt on the evidential basis with which existing guidelines mandate that initial excision by GPs has no place in the management of melanoma.7–9
Policymakers, particularly in Scotland, are calling for ‘Realistic Medicine’, with more effective and efficient use of healthcare resources.23 Revising existing guidelines to take greater cognisance of the geographical location could result in more satisfying and effective care for patients, which at the same time utilises the wider skill set of many of Scotland’s rural GPs.24
The MiSTIC randomised trial supports this, reporting that GP minor surgery was more satisfying for patients without major difference in quality.25 Furthermore, primary care excision of melanoma may mean shorter diagnostic delays for patients.26 The current data suggest that it could be timely for clinical guidelines to consider geographical realities when making recommendations
Acknowledgments
The authors would like to acknowledge support received from Lizzie Nicholson at eDRIS, NHS Scotland, and Doug Kidd at the National Data Safe Haven of NHS Scotland.
Funding
The project was funded by a grant from the Friends of Anchor (grant reference RG12991-10). The funder had no role in writing the manuscript or deciding to submit for publication. No payment was received by any of the authors to write this article from any agency. The corresponding author had full access to all the data in the study and had final responsibility for deciding to submit this manuscript for publication. The University of Aberdeen sponsored the study and took responsibility for the initiation, management, and financing of the study. The sponsor did not have any direct involvement in the design, conduct, or reporting of this study.
Ethical approval
This study was approved by the Public Benefit and Privacy Panel for Health and Social Care of NHS Scotland on 8 July 2015 (reference number 1516-0154). It received ethical approval from NRES Committee South East Coast — Surrey on 4 August 2015 (REC reference: 15/LO/1385; Protocol: 2/031/15; IRAS project ID: 183757). No patients were directly involved in the design, conduct, or reporting of this study. This study has been registered with ClinicalTrials.gov ID NCT03169036 protocol ID 183757.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Turner M, Fielding S, Ong Y, et al. A cancer geography paradox? Poorer cancer outcomes with longer travelling times to healthcare facilities despite prompter diagnosis and treatment: a data-linkage study. Br J Cancer. 2017;117(3):439–449. doi: 10.1038/bjc.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Research UK Skin cancer incidence statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/skin-cancer/incidence (accessed 21 May 2018) [Google Scholar]
- 3.Saranga–Perry V, Ambe C, Zager JS, Kudchadkar RR. Recent developments in the medical and surgical treatment of melanoma. CA Cancer J Clin. 2014;64(3):171–185. doi: 10.3322/caac.21224. [DOI] [PubMed] [Google Scholar]
- 4.Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36(3):461–468. doi: 10.1007/s10900-010-9328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coory M, Smithers M, Aitken J, et al. Urban-rural differences in survival from cutaneous melanoma in Queensland. Aust NZ J Public Health. 2006;30(1):71–74. doi: 10.1111/j.1467-842x.2006.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 6.Green J, Murchie P, Lee AJ. Does place of residence affect the type of physician performing primary excision of cutaneous melanoma in Northern Scotland? J Rural Health. 2013;29(Suppl 1):s35–s42. doi: 10.1111/jrh.12011. [DOI] [PubMed] [Google Scholar]
- 7.Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. Br J Dermatol. 2010;163(2):238–256. doi: 10.1111/j.1365-2133.2010.09883.x. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Skin cancer. QS130. London: NICE; 2016. https://www.nice.org.uk/guidance/qs130 (accessed 21 May 2018) [Google Scholar]
- 9.Healthcare Improvement Scotland, Scottish Intercollegiate Guidelines Network. SIGN 146 Cutaneous melanoma A national clinical guideline. 2017 http://www.sign.ac.uk/assets/sign146.pdf (accessed 21 May 2018) [Google Scholar]
- 10.Murchie P, Raja EA, Brewster DH, et al. Is initial excision of cutaneous melanoma by General Practitioners (GPs) dangerous? Comparing patient outcomes following excision of melanoma by GPs or in hospital using national datasets and meta-analysis. Eur J Cancer. 2017;86:373–384. doi: 10.1016/j.ejca.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Murchie P, Sinclair E, Lee AJ. Primary excision of cutaneous melanoma: does the location of excision matter. Br J Gen Pract. 2011. . [DOI] [PMC free article] [PubMed]
- 12.Murchie P, Raja EA, Lee AJ, Campbell NC. Mortality and morbidity after primary excision of cutaneous melanoma in primary versus secondary care. Br J Gen Pract. 2013. . [DOI] [PMC free article] [PubMed]
- 13.Eriksson H, Lyth J, Månsson-Brahme E, et al. Low level of education is associated with later stage at diagnosis and reduced survival in cutaneous malignant melanoma: a nationwide population-based study in Sweden. Eur J Cancer. 2013;49(12):2705–2716. doi: 10.1016/j.ejca.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Scottish Government. A health and biomedical informatics research strategy for Scotland. 2015. http://www.gov.scot/Publications/2015/04/6687 (accessed 21 May 2018)
- 15.Scottish Government. The Scottish Index of Multiple Deprivation. 2016. http://www.gov.scot/Topics/Statistics/SIMD (accessed 21 May 2018)
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Scottish Government. Scottish Government urban rural classification 2013–2014. 2014. http://www.gov.scot/Publications/2014/11/2763 (accessed 21 May 2018)
- 18.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14(15):1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 20.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. JASA. 1989;84:1074–1078. [Google Scholar]
- 21.Rose PW, Rubin G, Perera-Salazar R, et al. Explaining variation in cancer survival between 11 jurisdictions in the International Cancer Benchmarking Partnership: a primary care vignette survey. BMJ Open. 2015;5(5):e007212. doi: 10.1136/bmjopen-2014-007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell NC, Elliott AM, Sharp L, et al. Rural factors and survival from cancer: analysis of Scottish cancer registrations. Br J Cancer. 2000;82(11):1863–1866. doi: 10.1054/bjoc.1999.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calderwood C. Chief Medical Officer’s annual report 2014–15: realistic medicine. Scottish Government, NHS Scotland; 2016. http://www.gov.scot/Resource/0049/00492520.pdf (accessed 21 May 2018) [Google Scholar]
- 24.Mack M, Maxwell H, Hogg D, Gillies J. Being rural: exploring sustainable solutions for remote and rural healthcare: RCGP Scotland Policy Paper written by the Rural Strategy Group Scotland. 2014 http://www.rcgp.org.uk/policy/rcgp-policy-areas/rural-general-practice.aspx (accessed 23 May 2018) [Google Scholar]
- 25.George S, Pockney P, Primrose J, et al. A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial. Health Technol Assess. 2008;12(23):iii–iv. ix–38. doi: 10.3310/hta12230. [DOI] [PubMed] [Google Scholar]
- 26.Murchie P, Campbell NC. Pigmented lesions, cutaneous melanoma, and future challenges for primary care. Eur J Gen Pract. 2007;13(3):151–154. doi: 10.1080/13814780701627354. [DOI] [PubMed] [Google Scholar]