Abstract
Background
Suboptimal glycaemic control in type 2 diabetes (T2D) is common and associated with psychological barriers.
Aim
To investigate whether it was possible to train practice nurses in six psychological skills (Diabetes-6 [D6]) based on motivational interviewing (MI) and basic cognitive behaviour therapy (CBT), and whether integrating these with diabetes care was associated with improved glycaemic control over 18 months compared with standard care.
Design and setting
Two-arm, single-blind, parallel cluster randomised controlled trial in primary care.
Method
Adult participants (n = 334) with T2D and persistent HbA1c ≥69.4 mmol/mol were randomised to receive 12 sessions of either the D6 intervention or standard care over 12 months. Practice nurses were trained in the six psychological skills and their competencies were measured by standardised rating scales. Primary outcome was a change in HbA1c level at 18 months from randomisation. Secondary outcomes were changes in systolic and diastolic blood pressure, body mass index, waist circumference, depressive symptoms, harmful alcohol intake, diabetes-specific distress, and cost-effectiveness.
Results
Using intention-to-treat analysis, there was no significant difference between D6 intervention and standard care in HbA1c (mean difference −0.79 mmol/mol, 95% confidence interval [CI] = −5.75 to 4.18) or for any of the secondary outcomes. The competency level of D6 nurses was below the beginner proficiency level and similar to the standard-care nurses.
Conclusion
Training nurses in MI and basic CBT to support self-management did not lead to improvements in glycaemic control or other secondary outcomes in people with T2D at 18 months. It was also unlikely to be cost-effective. Furthermore, the increased contact with standard-care nurses did not improve glycaemic control.
Keywords: cognitive therapy, hyperglycaemia, motivational interviewing, self-management, type 2 diabetes mellitus
INTRODUCTION
Around half of individuals with type 2 diabetes (T2D) have persistent suboptimal glycaemic control despite evidence-based pathways based on national guidance.1–3 Psychological factors, such as depressive symptoms and diabetes-specific fears, are common in T2D and associated with reduced self-management.4,5 Addressing these psychological barriers could lead to improvement in glycaemic control.
Common psychological interventions include motivational interviewing (MI)6 and cognitive behaviour therapy (CBT).7,8 Recent randomised controlled trials (RCTs) suggest that the effect of low-intensity psychological interventions on glycaemic control is lower than reported in systematic reviews.9–11
One of the roles of the practice nurse is to support diabetes self-management. Hospital diabetes specialist nurses can be trained to competently deliver MI and basic CBT skills with improvement in glycaemic control in type 1 diabetes,12 and psychological interventions could be delivered by nurses in research settings.13 In this study a package of six psychological skill sets for T2D, Diabetes-6 (D6), were defined, of similar intensity to low-level psychological treatments for common mental disorders in the NHS.14 This study investigated whether training practice nurses in D6 skills was associated with increased competency when compared with nurses who had not received the training in a cluster RCT. The study further investigated whether the D6 intervention was more effective than standard care in improving suboptimal glycaemic control in people with T2D over a period of 18 months; in improving secondary outcomes (such as lipids, depressive symptoms); and if it was cost-effective.
METHOD
Trial design
Diabetes-6 was a pragmatic parallel two-arm cluster RCT with an 18-month follow-up. GP practices with ≥6000 patients registered in the Lambeth, Southwark, Lewisham, Wandsworth, and Bexley Clinical Commissioning Groups (representing a resident population of 1.43 million), were invited to participate if they had a practice nurse delivering diabetes care. Recruitment of patients began after each practice consented to randomisation. Randomisation of clusters was conducted in two phases as recruitment of practices and patients had slowed down following the organisational uncertainties preceding the implementation of the Health and Social Care Act 2012. This act reorganised the NHS in the UK, dismantling current organisational structures and creating new ones for funding, management, accountability, and regulation.
How this fits in
The evidence that low-intensity psychological interventions to support self-management in people with poorly controlled type 2 diabetes (T2D) in the primary care setting is limited. It is not known whether practice nurses can be trained to deliver low-intensity psychological treatments to support self-management in T2D. Training on low-intensity psychological interventions based on motivational interviewing and basic cognitive behaviour therapy led to basic proficiency in these skills but this was not maintained. Offering more sessions with practice nurses to support self-management in people with persistent hyperglycaemia does not lead to improvement in glycaemic control in T2D.
Patients
Inclusion criteria were: adults aged 18–79 years; a duration of T2D for ≥2 years; persistent suboptimal glycaemic control defined as International Federation of Clinical Chemistry (IFCC) HbA1c ≥69.4 mmol/mol (National Glycohemoglobin Standardization Program [NGSP] 8.5%) on two occasions, at least once in the preceding 18 months and the second one at recruitment, while on at least two oral diabetes medication (metformin and one other); and/or requiring insulin therapy to ensure that efforts to optimise medical care had been offered to the patient in line with national guidance.15 The IFCC HbA1c was lowered to ≥64.0 mmol/mol (NGSP 8.0%) in Phase 2 to increase recruitment.
Exclusion criteria were: severe mental disorders; terminal illnesses and end-stage diabetes complications; morbid obesity (body mass index (BMI) >40 kg/m2 in Phase 1 and >50 kg/m2 in Phase 2); non-ambulatory; no phone/internet access; non-English-speaking; and receiving psychological treatment elsewhere. Patients who had Patient Health Questionnaire-9 (PHQ-9) depressive scores >20 were excluded if they had psychotic depression or active suicidal ideation.16
Baseline measures
Baselines measurements before randomisation were: age, sex, self-reported ethnicity, occupation, employment status, and smoking status. Complication status included: neuropathic ulcer risk by perception of 10 g monofilament; retinopathy coding of the most recent annual standardised digital retinal photography; nephropathy using the urinary albumin:creatinine ratio (ACR); and history of macrovascular complications.
Randomisation
Randomisation of practices (unit of cluster) was conducted by an independent statistician using a random number generator to assign equal numbers of practices to each arm at each phase. For allocation concealment, an independent manager held the randomisation list in a password-locked computer.
Intervention
Group 1: standard care
The nurse delivered diabetes care in both groups as recommended by national guidance, which included diabetes self-management education, monitoring of biomedical status, and giving clinical information and advice.15 To control for attention, standard-care nurses offered the same number of sessions as D6. This consisted of 12 sessions, each 30 minutes in duration, over 12 months. The sessions were held in routine primary care clinics and audiorecorded.
Group 2: standard care plus Diabetes-6
The theory underlying MI is that the patient’s state of ambivalence (resistance versus willingness to make lifestyle changes) is the core psychological construct that requires addressing.6 MI is a directive, counselling style that encourages patients to change behaviours using collaborative, non-judgemental, and affirming communications. The theory underlying CBT is that barriers to diabetes self-management are maintained by; unhelpful thoughts, for example, ‘if I can’t cure diabetes, what’s the point?’; unhelpful behaviours, such as missing insulin doses; and distressing emotions, for example, low mood/anxiety when seeing a high blood glucose reading.17,18 Identifying and challenging these cognitive barriers are effective in changing behaviours.19
The D6 nurses were trained to integrate diabetes care with six skills drawn from MI and CBT, using a Diabetes-6 manual (see https://www.kcl.ac.uk/ioppn/depts/pm/people/acaprof/d6-supplementary-material-for-upload-to-kcl.pdf or contact authors for further information), as follows:
active listening;
managing resistance;
directing change;
supporting self-efficacy;
addressing health beliefs; and
shaping behaviours.
The nurses offered the same number of sessions for the D6 group as the standard care group. This consisted of 12 sessions, each 30 minutes in duration, over 12 months. The sessions were held in routine primary care clinics and audiorecorded.
The Motivational Interviewing Treatment Integrity (MITI) Scale (version 3.1.1)20 and Behaviour Change Counselling Index (BECCI)21 were used to compare competencies in both groups. The middle 20 minutes of sessions were rated by two independent psychologists trained in MITI, and BECCI was rated by a clinical psychologist, blind to treatment allocation.
Outcomes
The follow-up was reduced from 24 months to 18 months secondary to the delays in recruitment. The primary outcome was a change in HbA1c (mmol/mol) from cluster randomisation to 18 months measured centrally, at King’s College Hospital NHS Foundation Trust, by affinity chromatography (Primus Ultra2). If the study HbA1c were missing at 18 months, the 15-month HbA1c test results were included as this clinically overlaps with the 3-month window for 18-month HbA1c measurement. The secondary outcomes were changes in: systolic and diastolic blood pressure measured using an electronic sphygmomanometer; BMI, and waist circumference measurements; depressive symptoms using the PHQ-9;16 alcoholism using the Alcohol Use Disorders Identification Test (AUDIT);22 diabetes-specific psychological burden using the Diabetes Distress Scale;23 and cost-effectiveness.24,25 A fasting blood sample was used for HbA1c measurements, total cholesterol, and triglycerides.
Sample size
An IFCC HbA1c 10.9 mmol/mol (NGSP HbA1c 1%) difference in D6 compared with standard care was the minimal clinically significant reduction at 18 months, considering that standard care may produce a 2.2 mmol/mol (NGSP HbA1c 0.2%) reduction in HbA1c (equivalent to a moderate effect size of d = 0.55). Assuming 20% dropout, 360 patients were required to achieve 80% power at a two-sided α-level of 5%, with 20 practices with 18 patients each per arm. Two practices per arm were assumed to drop out, thus requiring 24 practices with a total patient sample of n = 432 (24 × 18) patients. After adjusting for clustering by practice (clustering intra-correlation coefficient [ICC] = 0.05) and an inflation factor of 1.7, the final required sample size was n = 138 (81 × 1.7) patients per arm.
Out of 334 patients recruited, 231 had at least one follow-up in 24 clusters. The average cluster size was therefore 10 patients per cluster, smaller than the assumed size of 15 patients per cluster with a post-hoc power of 77% at two-sided α-level of 5%.26
Statistical analysis
Data were analysed using Stata 13. The sample characteristics were described as means (standard deviation [SD]) or as proportions (percentage). A comparison of patient list size and Index of Multiple Deprivation (IMD) 2010 rank score by practices that participated versus those that did not was conducted using Student’s t-test. The IMD 2010 score is a composite index of relative deprivation at a small area level, based on seven domains of deprivation: income; employment; health deprivation and disability; education, skills, and training; barriers to housing and services; crime and disorder; and living environment.27 A linear mixed-effects model estimated group differences in HbA1c levels between D6 and standard-care groups at 18 months. ‘Nurse’ was included as a random effect as the unit of randomisation. Secondary outcomes were also analysed using linear mixed models to estimate group differences at 18 months.
Twenty-nine participants with an HbA1c <64 mmol/mol were mistakenly recruited because of coding errors by the research team during assessment of eligibility, and this mistake was only discovered after randomisation. Therefore, they were retained for the intention-to-treat (ITT) analysis A sensitivity analysis was performed by including a binary covariate of this protocol violation using maximum likelihood under the missing-at-random assumption. Sensitivity to missingness in HbA1c was assessed by investigating and including predictors of missingness in the model and by using multiple imputation for the missing values of HbA1c.
Further details of the protocol, including the economic evaluation, can be found at https://www.kcl.ac.uk/ioppn/depts/pm/people/acaprof/d6-supplementary-material-for-upload-to-kcl.pdf.
RESULTS
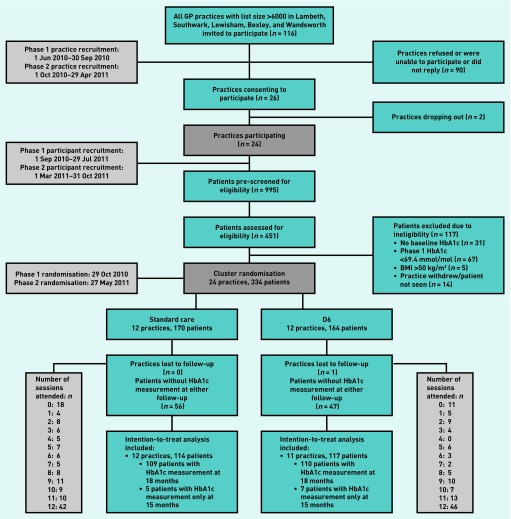
Out of 116 invited practices, 26 agreed to participate and two dropped out before randomisation (Figure 1), providing 995 potentially eligible participants. A breakdown of patients attending each practice and primary outcome follow-up rates by group is available from the authors. Of the 451 who consented for eligibility, 334 were recruited. Twelve practice clusters were randomly assigned to standard care (n = 170 participants) and 12 to standard care plus D6 (n = 164). One D6 practice dropped out after randomisation, before the nurse received the training, and before all patients were recruited (those who consented remained in the ITT analysis). Invited practices that participated (n = 24) compared with those that did not (n = 89) had higher mean patient list sizes (12 180 [SD 5099] versus 10 091 [SD 3894] respectively, P = 0.03) but no difference in IMD score (10 049 [SD 6910] versus 12 441 [SD 7785], P = 0.17). Table 1 presents the baseline characteristics of the sample.
Figure 1.
Diabetes-6 study flow chart.
Table 1.
Baseline characteristics of participants randomly assigned to receive D6 or standard care, N = 334
| Variablea | D6 (N= 164) | Standard care (N= 170) | Total | |
|---|---|---|---|---|
| Age, mean, years (SD) | 59.0 (11.1) | 58.9 (11.4) | 58.9 (11.2) | |
|
| ||||
| Sex, n (%) | Male | 82 (50.0) | 81 (47.7) | 163 (48.8) |
| Female | 82 (50.0) | 89 (52.4) | 171 (51.2) | |
|
| ||||
| Ethnicity, n (%) | White | 60 (36.8) | 74 (43.8) | 134 (40.4) |
| African/Caribbean | 81 (49.7) | 62 (36.7) | 143 (43.1) | |
| Asian/other | 22 (13.5) | 33 (19.5) | 55 (16.6) | |
|
| ||||
| Relationship status, n (%) | Married or cohabiting | 82 (50.3) | 89 (52.7) | 171 (51.5) |
| Separated/divorced/widowed | 52 (31.9) | 45 (26.6) | 97 (29.2) | |
| Single | 29 (17.8) | 35 (20.7) | 64 (19.3) | |
|
| ||||
| Education level, n (%) | A-level or higher | 47 (29.2) | 43 (25.8) | 90 (27.4) |
| O-level or GCSE equivalent | 68 (42.2) | 48 (28.7) | 116 (35.4) | |
| No formal qualifications | 46 (28.6) | 76 (45.5) | 122 (37.2) | |
|
| ||||
| Employment, n (%) | Yesb | 69 (42.1) | 70 (41.2) | 139 (41.6) |
| Noc | 95 (57.9) | 100 (58.8) | 195 (58.4) | |
|
| ||||
| Borough, n (%) | Lambeth | 83 (50.6) | 42 (24.7) | 125 (37.4) |
| Southwark | 25 (15.2) | 40 (23.5) | 65 (19.5) | |
| Lewisham | 19 (11.6) | 52 (30.6) | 71 (21.3) | |
| Wandsworth | 37 (22.6) | 24 (14.1) | 61 (18.3) | |
| Bexley | 0 (0.0) | 12 (7.1) | 12 (3.6) | |
|
| ||||
| Diabetes duration, years (IQR) | 10 (7–13) | 9 (5–12) | 9 (6–12) | |
|
| ||||
| HbA1c, mmol/mol (SD) | 81.0 (17.1) | 80.1 (19.1) | 80.5 (18.1) | |
|
| ||||
| Body mass index, kg/m2 (SD) | 32.0 (5.6) | 31.9 (6.6) | 31.9 (6.1) | |
|
| ||||
| Systolic blood pressure, mm/Hg (SD) | 135.2 (16.9) | 133.2 (17.3) | 134.2 (17.1) | |
|
| ||||
| Diastolic blood pressure, mm/Hg (SD) | 79.5 (9.8) | 79.0 (10.3) | 79.2 (10.1) | |
|
| ||||
| Total cholesterol, mmol/L (SD) | 4.3 (1.1) | 4.2 (1.2) | 4.2 (1.2) | |
|
| ||||
| Fasting triglycerides, mmol/L (SD) | 1.7 (1.2) | 1.7 (1.3) | 1.7 (1.3) | |
|
| ||||
| Taking insulin, n (%) | Yes | 75 (46.3) | 66 (39.8) | 141 (43.0) |
| No | 87 (53.7) | 100 (60.3) | 187 (57.0) | |
|
| ||||
| Any retinopathy, n (%) | Yes | 59 (35.9) | 65 (38.2) | 124 (37.1) |
| No | 105 (64.0) | 105 (61.8) | 210 (62.9) | |
|
| ||||
| Albumin:creatinine ratio, n (%) | Negative | 65 (59.1) | 83 (69.8) | 148 (64.6) |
| Positive | 45 (40.9) | 36 (30.3) | 81 (35.4) | |
|
| ||||
| Protein:creatinine ratio, n (%) | Negative | 33 (76.7) | 17 (77.3) | 50 (76.9) |
| Positive | 10 (23.3) | 5 (22.7) | 15 (23.1) | |
|
| ||||
| Foot ulcers, n (%) | Yes | 9 (5.6) | 12 (7.1) | 21 (6.4) |
| No | 152 (94.4) | 157 (92.9) | 309 (93.6) | |
|
| ||||
| Macrovascular disease, n (%) | Yes | 61 (37.2) | 55 (32.4) | 116 (34.7) |
| No | 103 (62.8) | 115 (67.7) | 218 (65.3) | |
|
| ||||
| Patient Health Questionnaire-9 score, n (%) | ||||
| ≥10 | 31 (20.4) | 35 (22.4) | 66 (21.4) | |
| <10 | 121 (79.6) | 121 (77.6) | 242 (78.6) | |
|
| ||||
| Diabetes Distress Scale (mean item score) | 2.1 (1.7–2.7) | 2.0 (1.6–2.7) | 2.1 (1.6–2.7) | |
Values missing for age (n =1), ethnicity (n =2), relationship status (n =2), education level (n =6), diabetes duration (n =20), body mass index (n =5), systolic blood pressure (n =25), diastolic blood pressure (n =26), HbA1c (n =1), total cholesterol (n =53), fasting triglycerides (n =58), insulin (n =6), albumin:creatinine ratio (n =105), protein:creatinine ratio (n =269), foot ulcers (n =4), Patient Health Questionnaire-9 (n =26), Diabetes Distress Scale (n =27).
Yes =full-time, part-time, student, or self-employed;
No =retired/unemployed/not seeking employment. D6 =Diabetes-6. IQR =interquartile range. SD =standard deviation.
The mean number of sessions attended was 7.42 (SD 4.4) and 8.20 (SD 4.4) in the standard-care and D6 groups, respectively.
Primary outcome data at 18-month follow-up were collected for 219 (65.6%) participants and a further 12 had 15-month HbA1c data, providing results for 231 participants. There was a non-significantly larger proportion with missing HbA1c at 18 months in the standard-care group compared with D6 group (35.9%, n = 616, versus 32.9% n = 54, respectively) and more likely to be African/Caribbean or Asian/other ethnicity. A comparison of missingness of HbA1c results at 18 months is available at https://www.kcl.ac.uk/ioppn/depts/pm/people/acaprof/d6-supplementary-material-for-upload-to-kcl.pdf. In the ITT analysis, there was no significant difference in mean HbA1c at follow-up in the D6 group compared with the standard-care group (mean difference −0.79 mmol/mol, 95% CI = −5.75 to 4.18 (Table 2). The ICC for the clustering effect of ‘nurse’ was 0.02 (95% CI = 0.001 to 0.37). Linear mixed models showed no significant effects of the intervention on the secondary outcomes including BMI, blood pressure, fasting triglyceride, or psychological distress (Table 2).
Table 2.
Results from primary and secondary outcomes: intention-to-treat analysis
| Measured variable for outcome at 18 months | Participants with baseline measurements, n | Participants with measurements at 18 months, n | Mean difference: D6 versus standard care (95% CI) |
|---|---|---|---|
| Primary | |||
| HbA1ca (mmol/mol) | 332 | 231 | −0.79 (−5.75 to 4.18) |
|
| |||
| Secondary | |||
| Body mass indexa (kg/m2) | 329 | 152 | −0.08 (−1.12 to 0.97) |
| Total cholesterola (mmol/L) | 281 | 140 | −0.08 (−0.42 to 0.27) |
| Systolic blood pressurea (mm/Hg) | 309 | 198 | −1.35 (−6.85 to 4.14) |
| Diastolic blood pressurea (mm/Hg) | 308 | 198 | 1.22 (−1.87 to 4.32) |
| Fasting triglyceridesb (mmol/L) | 276 | 135 | 0.02 (−0.22 to 0.26) |
| Patient Health Questionnaire-9 | 308 | 114 | −0.18 (−1.30 to 0.94) |
| Scorec | |||
Estimates based on linear combination from linear mixed-effects model with fixed effects of time (15 or 18 months), an interaction between time and randomisation group, randomisation phase, borough and baseline values of the outcome, a random effect for GP practice nurse clustering and with unstructured covariance matrix to account for dependency of repeated observations.
Estimates based on linear combination from linear mixed-effects model with fixed effects of time (15 months or 18 months), an interaction between time and randomisation group, randomisation phase, borough and baseline values of the outcome, a random effect for GP practice nurse clustering and with independent covariance structure due to convergence issues when estimating non-zero covariances.
Collected at 18 months only. Estimates based on linear combination from linear mixed model with fixed effects of randomisation phase, borough, baseline value, and random within-cluster effect of nurse with unstructured covariance matrix to account for dependency of repeated observations. CI = confidence interval. D6 = Diabetes-6.
Results were similar for the sensitivity analyses when: using practice as the clustering variable in place of ‘nurse’ as cluster; including a binary covariate for the 29 participants with baseline HbA1c <64 mmol/mol; including ethnicity and history of stroke as predictor of missingness at follow-up; or using multiple imputation to account for missingness in HbA1c. There was no evidence of an association between the number of D6 sessions attended and HbA1c at 18 months within the D6 group (−0.44 mmol/mol per additional session attended, 95% CI = −1.28 to 0.41).
Intervention costs were higher in the D6 group (mean difference £276, 95% CI = 225 to 327) (Table 3) due to greater training costs but there were no differences in mean total health and social care costs, including intervention costs, with discounting for non-intervention costs (adjusted mean difference £150, 95% CI = −34 to 333) or quality-adjusted life year (QALY) gains at 18 months. Supplementary data from the economic evaluation can be found at https://www.kcl.ac.uk/ioppn/depts/pm/people/acaprof/d6-supplementary-material-for-upload-to-kcl.pdf.
Table 3.
Mean costs (for the previous 6 months, £ sterling, 2011/2012 prices), SF-12-based utility scores and QALY gains at baseline and/or 18 months
| Costs at baseline | D6 | Standard care | UMDa | 95% CI | AMDb | 95% CIa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Valid, n | Mean, £ | SD | Valid, n | Mean, £ | SD | |||||
| Health and social care costs | 157 | 847 | 847 | 161 | 976 | 760 | −129 | −301 to 44 | −96 | −293 to 101 |
|
| ||||||||||
| Costs at 18 months | ||||||||||
| Health and social care costs, excluding intervention, without discounting | 133 | 707 | 579 | 137 | 793 | 558 | −85 | −252 to 81 | −71 | −242 to 100 |
| Health and social costs, excluding intervention, with discounting | 133 | 684 | 560 | 137 | 766 | 540 | −82 | −243 to 78 | −69 | −234 to96 |
| Intervention costs | 121 | 451 | 99 | 139 | 167 | 100 | 285 | 240 to 329 | 276 | 225 to 327 |
| Health and social care costs, including intervention costs, with discounting for non-intervention costs | 92 | 1184 | 572 | 107 | 1025 | 573 | 159 | −39 to 357 | 150 | −34 to 333 |
|
| ||||||||||
| SF-12-based utility scores at baseline | ||||||||||
| Utility | 157 | 0.75 | 0.16 | 159 | 0.74 | 0.16 | 0.01 | −0.03 to 0.04 | 0.01 | −0.03 to 0.05 |
|
| ||||||||||
| SF-12-based utility scores and QALY gains at 18 months | ||||||||||
| Utility | 60 | 0.79 | 0.13 | 53 | 0.75 | 0.13 | 0.04 | −0.01 to 0.08 | 0.01 | −0.03 to 0.06 |
| QALY gain since baseline, without discounting | 58 | 1.15 | 0.20 | 48 | 1.11 | 0.18 | 0.03 | −0.04 to 0.10 | 0.01 | 0.03 to 0.05 |
| QALY gain since baseline, with discounting and interpolation to match 6-month period for cost data | 58 | 0.37 | 0.06 | 48 | 0.36 | 0.06 | 0.01 | −0.01 to 0.03 | 0.00 | −0.01 to 0.02 |
Intervention minus control. Comparisons include clustering for nurse.
Intervention minus control. Cost comparisons account for clustering for nurse plus covariates for baseline cost, age, sex, marital status, ethnicity, duration of diabetes, and baseline utility. QALY comparisons account for clustering for nurse plus covariates for age, sex, marital status, ethnicity, duration of diabetes, and baseline utility. SF-12 = Short Form-12. QALY = quality-adjusted life year. D6 = Diabetes-6. AMD = adjusted mean difference. CI = confidence interval. SD = standard deviation. UMD = unadjusted mean difference.
The inter-rater reliability for the MITI global domains of spirit and empathy was 0.87 and 0.91 respectively so both sets of ratings were combined and the mean scores for each domain were derived. The researchers rated 69 sessions (4.0% of all available recordings) for fidelity from 33 out of 164 and 36 out of 170 patients from the D6 and standard-care groups respectively (Table 4). The level of competency in the D6 group was below the beginner proficiency level in all the scales for MI and BECCI. Except for a slightly higher proportion of open questions in D6, and a slightly larger reflection/question ratio in standard care, there were no statistically significant differences in the remaining mean MI domain scores or BECCI scores.
Table 4.
Group comparison for fidelity to MI and CBT
| Variable | D6 | Standard care | P-valuea |
|---|---|---|---|
| MI domainb | |||
| Global spirit, mean (SD) | 3.23 (1.13) | 2.87 (0.87) | 0.14 |
| Global empathy, median (IQR) | 3.00 (2.00–4.00) | 2.50 (2.00–3.00) | 0.19 |
| Proportion complex reflections, mean (SD) | 0.35 (0.20) | 0.40 (0.17) | 0.25 |
| Proportion open questions, mean (SD) | 0.36 (0.17) | 0.25 (0.10) | <0.01 |
| Reflection/question ratio, median (IQR) | 0.57 (0.47–0.72) | 0.74 (0.53–1.19) | 0.03 |
| Proportion motivational interviewing adherent, mean (SD) | 0.58 (0.32) | 0.54 (0.28) | 0.51 |
|
| |||
| CBT skills, mean (SD) | |||
| BECCI score | 1.33 (0.56) | 1.12 (0.55) | 0.12 |
Based on result of either a t-test or Mann–Whitney U-test.
The MITI guidance indicates that, to reach proficiency, a practitioner must achieve an average global spirit rating of 3.5, a reflection to question ratio of ≥1, ≥0.5 open questions relative to all questions, ≥0.4 complex reflections relative to all reflections, and ≥0.9 MI adherent. BECCI = Behaviour Change Counselling Index. CBT = cognitive behaviour therapy. D6 = Diabetes-6. IQR = interquartile range. MI = motivational interviewing. MITI = Motivational Interviewing Treatment Integrity. SD = standard deviation.
There were 43 serious adverse events: cardiovascular (n = 13); injury (n = 5); cancer (n = 4); infection (n = 5); diabetes-related (n = 3); psychiatric (n = 2); and other (n = 11), reported after 18 months for 38 different participants (D6: n = 14; standard care: n = 24) and two deaths from cancer. There were no differences in total number of serious adverse events between the groups, or between each type of serious adverse event using a χ2 test, or Fisher’s exact test where counts were low.
DISCUSSION
Summary
Training nurses in MI and basic CBT to support self-management did not lead to improvements in glycaemic control, or any other secondary outcomes, in people with T2D and persistent hyperglycaemia compared with attention control at 18 months from randomisation. Further, it was unlikely to be cost-effective and the increased contact with standard-care nurses did not improve glycaemic control.
Strengths and limitations
This was a pragmatic design set in real-world, inner-city primary care, representing the ethnic and social diversity of people with T2D.28 Only a few other RCTs had achieved similar ethnicity distributions.29–35 This was a high-risk group for diabetes complications. A cluster design was selected to reduce contamination of the intervention in the control group. Contamination is the process whereby an intervention intended for members of the trial (intervention or treatment) arm of a study is received by members of another (control) arm leading to a risk of under-estimation of the effect.36 The researchers assessed contamination by comparing the competencies in the intervention and control group. The hypothesis was that the control group would have lower competencies than the D6 group. As both groups had similar and borderline beginner proficiency competencies, which is probably the pre-training level of competency, the study concluded that contamination was unlikely. The researchers developed a theoretically informed intervention and an evidence-based manual. Fidelity (which is the same measure as competency in this study) was measured to the intervention (further details available at https://www.kcl.ac.uk/ioppn/depts/pm/people/acaprof/d6-supplementary-material-for-upload-to-kcl.pdf. The authors controlled for the non-specific effect of receiving more attention by D6 by offering similar numbers of sessions to patients randomised to the control group and were only slightly underpowered at 77% power compared with the 80% originally proposed. The upper limit of the 95% confidence interval of the estimated treatment effect for HbA1c (4.8 mmol/mol) was less than estimated treatment reductions in meta-analyses.37 The comprehensive within-trial economic evaluation assessed all relevant health and social care costs.
The limitations of D6 included a 20% uptake of practice participation, despite the offer of generous backfill payments. The main reasons given by the practices when feedback was informally asked were the pressures to deliver current services with limited resources exacerbated by coincidental national restructuring of primary care services creating organisational uncertainty. Data missingness for the economic analyses was high; however, imputing missing data confirmed the lack of cost-effectiveness of D6. The study did not obtain sufficient repeated measures of HbA1c and also failed to achieve a minimum level of beginner proficiency in MI in the D6 group, and was, therefore, unable to conclude that MI is not effective in supporting self-management.
Comparison with existing literature
Though there have been over 40 RCTs in this field since the last review,37 only three had defined poor glycaemic control (HbA1c ≥64 mmol/mol) as an inclusion criterion and showed no benefit from psychological support, and only one of these was delivered by nurse care managers.38–40 Recent pragmatic RCTs of similar interventions included samples with near optimal glycaemic control with less room for improvement in the primary outcome.10,11,41 The sample in the present study had high sustained HbA1c levels so the researchers may have selected a more severe group, which was unsuitable for practitioners with lower levels of psychological skill competencies.29–35
This study is one of a handful of RCTs to include fidelity and competency (a complex, laborious, and expensive process evaluation).42,43 On average patients attended only 50% of sessions in either group. This is a common observation in psychological interventions.44 However, no dose–response relationship was observed.
Implications for research and practice
There are several potential nurse, patient, and methodological reasons for the non-significant effect of D6. The nurses did not self-select and may not have had the generic psychotherapist factors often attributed as the active ingredients in psychological treatments.45 D6 nurses had concerns about over-stepping their professional roles, lacked confidence, and/or resented the extra workload.46 The low competencies in most MI and CBT domains suggest that practice nurses may need longer periods of training or should self-select for generic psychotherapist skills in advance. The findings from this study may also reflect the difficulty of engaging this high-risk clinical group that has low levels of worry. Even offering more nurse support in the form of more frequent sessions did not lead to improved glycaemic control. In exit interviews, patients stated they lacked time (though the majority were not employed) and difficulties in establishing a rapport with the nurses as reasons for dropout (unpublished observations). One methodological explanation is that the researchers selected HbA1c, strongly associated to the levels of glycaemia, as a surrogate outcome for diabetes complications. However, a landmark RCT47 and a meta-analysis of RCTs48 aimed at intensive glycaemic control have failed to consistently observe a positive effect on reduction of complications of diabetes or global mortality, and there may even be a negative effect of increased mortality when tight glycaemic control is the aim. Perhaps these negative findings represent an opportunity to focus on psychological interventions to improve other outcomes such as blood pressure, lipids, or a composite outcome. Another methodological implication is whether the duration of the intervention and the follow-up was too short. Brief psychological interventions are designed to be exactly that, with the added advantage of being cheap and not too demanding on the patient. However, patients in this study sample had a long history of poor self-management and may have needed a longer duration of therapy. Whether longer therapy would be pragmatic for funding as an RCT or in the NHS is to be debated but is showing promise for chronic depression.49
The implication for clinical practice is that low-intensity psychological interventions delivered at low levels of competencies may not be as effective in supporting self-management in individuals with T2D and longstanding suboptimal glycaemic control as previously thought.
A conceptual dilemma is that theoretical frameworks for MI and CBT assume that mental health conditions remit (alcohol problems, smoking, depression) and this assumption does not apply to T2D, which progressively worsens.50
The authors of this study suggest an urgent need to reconsider which skills, competencies, and workforce are the most effective in delivering psychological interventions to improve glycaemic control in people with T2D 51 before investing sparse funds into low-intensity psychological treatments for improving glycaemic control in T2D.52
Acknowledgments
The authors thank the practices, their nurses who gave so much of their time during a period of organisational insecurity, and all the patients. They also thank: the independent raters of the audio recordings, Pam Macdonald, Emma Shuttlewood, and Amy Harrison; the members of the Independent Trial Steering Committee, Sheila Burston, Tim Clayton, Simon Gilbody (Chair), Simon Heller, and Lynne Priest; Data Monitoring Ethics Committee, Rury Holman (Chair), Michael King, and Jonathan Bartlett; Nicholas Magill, NIHR Doctoral Fellow, for help with the fidelity analysis; Rebecca Upsher, PhD student, for conducting the meta-analysis (available from authors on request); the research assistants, Kurtis Stewart for administrative support, and to all researchers who helped with recruitment and rating.
Funding
This research was part-funded by the National Institute for Health Research (NIHR; Programme Grant for Applied Research: RP-PG-0606-1142). Khalida Ismail, Daniel Stahl, and Dominic Stringer were part-funded by the NIHR Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Ethical approval
Ethical approval was granted by the King’s College Hospital Research Ethics Committee (reference: 09/H0808/97) and Primary Care Trusts (references: RDLSLBex 534 and 2010/403/W). Changes to the protocol were approved by the Trial Steering Committee and the Research Ethics Committee. All participants gave written, informed consent and the trial was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Kalida Ismail has received honorarium from Eli Lilly, Sanofi, Janssen, and Sunovion for lectures at educational events. Kirsty Winkley received honorarium from Atlantis Healthcare and NIHR grants. Nicole de Zoysa has received honorarium paid to employer by Eli Lilly for educational lecture.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 2.Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353(9153):617–622. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AM, Krinsky S, Kontopantelis E, Doran T. Long-term evidence for the effect of pay-for-performance in primary care on mortality in the UK: a population study. Lancet. 2016;388(10041):268–274. doi: 10.1016/S0140-6736(16)00276-2. [DOI] [PubMed] [Google Scholar]
- 4.Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. 2015;3(6):450–460. doi: 10.1016/S2213-8587(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160(21):3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 6.Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd edn. New York, NY: Guilford Press; 2013. [Google Scholar]
- 7.Beck AT. Thinking and depression: I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9(4):324–333. doi: 10.1001/archpsyc.1963.01720160014002. [DOI] [PubMed] [Google Scholar]
- 8.Beck AT. Cognitive therapy and the emotional disorders. New York, NY: Meridian; 1976. [Google Scholar]
- 9.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363(9421):1589–1597. doi: 10.1016/S0140-6736(04)16202-8. [DOI] [PubMed] [Google Scholar]
- 10.Juul L, Maindal HT, Zoffmann V, et al. Effectiveness of a training course for general practice nurses in motivation support in type 2 diabetes care: a cluster-randomised trial. PLoS One. 2014;9(5):e96683. doi: 10.1371/journal.pone.0096683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich E, Candel MJ, Schaper NC, de Vries NK. Effect evaluation of a motivational interviewing based counselling strategy in diabetes care. Diabetes Res Clin Pract. 2010;90(3):270–278. doi: 10.1016/j.diabres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Ismail K, Thomas SM, Maissi E, et al. Motivational enhancement therapy with and without cognitive behavior therapy to treat type 1 diabetes: a randomized trial. Ann Intern Med. 2008;149(10):708–719. doi: 10.7326/0003-4819-149-10-200811180-00005. [DOI] [PubMed] [Google Scholar]
- 13.Alam R, Sturt J, Lall R, Winkley K. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialists and generalist clinicians on glycaemic control and on psychological status. Patient Educ Couns. 2009;75(1):25–36. doi: 10.1016/j.pec.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 14.NHS England Adult Improving Access to Psychological Therapies programme. https://www.england.nhs.uk/mental-health/adults/iapt/ (accessed 13 Jun 2018)
- 15.National Institute for Health and Care Excellence. Type 2 diabetes. CG66. London: NICE; 2008. [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 17.Beck J. Cognitive therapy: basics and beyond. New York, NY: Guilford Press; 1995. [Google Scholar]
- 18.Hawton K, Salkovskis PM, Kirk J, Clark DM, editors. Cognitive behaviour therapy for psychiatric problems: a practical guide. Oxford: Oxford University Press; 1989. [Google Scholar]
- 19.Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, et al. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res. 2012;36(5):427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyers T, Martin T, Manuel J, et al. Revised global scales: motivational interviewing treatment integrity 3.1.1 (MITI 3.1.1) Albuquerque, NM: University of New Mexico; 2010. [Google Scholar]
- 21.Lane C, Huws-Thomas M, Hood K, et al. Measuring adaptations of motivational interviewing: the development and validation of the behavior change counseling index (BECCI) Patient Educ Couns. 2005;56(2):166–173. doi: 10.1016/j.pec.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 23.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 24.Ware J, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 26.Batistatou E, Roberts C, Roberts S. Sample size and power calculations for trials and quasi-experimental studies with clustering. Stata J. 2014;14(1):159–174. [Google Scholar]
- 27.Department for Communities and Local Government. Indices of Multiple Deprivation, borough. Ministry of Housing and Local Government; 2010. https://data.london.gov.uk/dataset/indices-multiple-deprivation-borough (accesssed 14 Jun 2018) [Google Scholar]
- 28.Gulliford MC, Naithani S, Morgan M. Continuity of care and intermediate outcomes of type 2 diabetes mellitus. Fam Pract. 2007;24(3):245–251. doi: 10.1093/fampra/cmm014. [DOI] [PubMed] [Google Scholar]
- 29.Penckofer SM, Ferrans C, Mumby P, et al. A psychoeducational intervention (SWEEP) for depressed women with diabetes. Ann Behav Med. 2012;44(2):192–206. doi: 10.1007/s12160-012-9377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piette JD, Richardson C, Himle J, et al. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care. 2011;49(7):641–648. doi: 10.1097/MLR.0b013e318215d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pladevall M, Divine G, Wells KE, et al. A randomized controlled trial to provide adherence information and motivational interviewing to improve diabetes and lipid control. Diabetes Educ. 2015;41(1):136–146. doi: 10.1177/0145721714561031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plotnikoff RC, Karunamuni N, Courneya KS, et al. The Alberta diabetes and physical activity trial (ADAPT): a randomized trial evaluating theory-based interventions to increase physical activity in adults with type 2 diabetes. Ann Behav Med. 2013;45(1):45–56. doi: 10.1007/s12160-012-9405-2. [DOI] [PubMed] [Google Scholar]
- 33.Sacco WP, Malone JI, Morrison AD, et al. Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. J Behav Med. 2009;32(4):349–359. doi: 10.1007/s10865-009-9209-4. [DOI] [PubMed] [Google Scholar]
- 34.Safren SA, Gonzalez JS, Wexler DJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2014;37(3):625–633. doi: 10.2337/dc13-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolever RQ, Dreusicke M, Fikkan J, et al. Integrative health coaching for patients with type 2 diabetes. Diabetes Educ. 2010;36(4):629–639. doi: 10.1177/0145721710371523. [DOI] [PubMed] [Google Scholar]
- 36.Keogh-Brown MR, Bachmann MO, Shepstone L, et al. Contamination in trials of educational interventions. Health Technol Assess. 2007;11(43):iii, ix–107. doi: 10.3310/hta11430. [DOI] [PubMed] [Google Scholar]
- 37.Ismail K, Maissi E, Thomas S, et al. A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for people with type 1 diabetes mellitus with persistent sub-optimal glycaemic control: A Diabetes and Psychological Therapies (ADaPT) study. Health Technol Assess. 2010;14(22):1–101. iii–iv. doi: 10.3310/hta14220. [DOI] [PubMed] [Google Scholar]
- 38.Keogh KM, Smith SM, White P, et al. Psychological family intervention for poorly controlled type 2 diabetes. Am J Manag Care. 2011;17(2):105–113. [PubMed] [Google Scholar]
- 39.Li M, Li T, Shi BY, Gao CX. Impact of motivational interviewing on the quality of life and its related factors in type 2 diabetes mellitus patients with poor long-term glycemic control. Int J Nurs Sci. 2014;1(3):250–254. [Google Scholar]
- 40.Gabbay RA, Añel-Tiangco RM, Dellasega C, et al. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): results of a 2-year randomized controlled pragmatic trial. J Diabetes. 2013;5(3):349–357. doi: 10.1111/1753-0407.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jansink R, Braspenning J, Keizer E, et al. No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial. Scand J Prim Health Care. 2013;31(2):119–127. doi: 10.3109/02813432.2013.797178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waker CL. Effects of motivational interviewing on diabetes self-management behaviors and glycemic control in type 2 diabetes: a translational study. University of Cincinnati. 2012 https://etd.ohiolink.edu/ap/10?0::NO:10:P10_ACCESSION_NUM:ucin1342728810 (accessed 14 Jun 2018) [Google Scholar]
- 43.Welch G, Zagarins SE, Feinberg RG, Garb JL. Motivational interviewing delivered by diabetes educators: does it improve blood glucose control among poorly controlled type 2 diabetes patients? Diabetes Res Clin Pract. 2011;91(1):54–60. doi: 10.1016/j.diabres.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Community and Mental Health Team. Psychological therapies: annual report on the use of IAPT services: England 2014/15. 2015. http://content.digital.nhs.uk/catalogue/PUB19098/psyc-ther-ann-rep-2014-15.pdf (accessed 14 Jun 2018)
- 45.Baskin TW, Tierney SC, Minami T, Wampold BE. Establishing specificity in psychotherapy: a meta-analysis of structural equivalence of placebo controls. J Consult Clin Psychol. 2003;71(6):973–979. doi: 10.1037/0022-006X.71.6.973. [DOI] [PubMed] [Google Scholar]
- 46.Graves H, Garrett C, Amiel SA, et al. Psychological skills training to support diabetes self-management: qualitative assessment of nurses’ experiences. Prim Care Diabetes. 2016;10(5):376–382. doi: 10.1016/j.pcd.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;(11):CD008143. doi: 10.1002/14651858.CD008143.pub3. [DOI] [PubMed] [Google Scholar]
- 49.Fonagy P, Rost F, Carlyle J, et al. Pragmatic randomized controlled trial of long-term psychoanalytic psychotherapy for treatment-resistant depression: the Tavistock Adult Depression Study (TADS). World Psychiatry. 2015;14(3):312–321. doi: 10.1002/wps.20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulder BC, Lokhorst AM, Rutten GEHM, van Woerkum CMJ. Effective nurse communication with type 2 diabetes patients. West J Nurs Res. 2015;37(8):1100–1131. doi: 10.1177/0193945914531077. [DOI] [PubMed] [Google Scholar]
- 51.Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126–2140. doi: 10.2337/dc16-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donovan HS, Kwekkeboom KL, Rosenzweig MQ, Ward SE. Non-specific effects in psychoeducational intervention research. West J Nurs Res. 2009;31(8):983–998. doi: 10.1177/0193945909338488. [DOI] [PMC free article] [PubMed] [Google Scholar]