Abstract
The bioactive alkaloids (e.g. vincristine, hydroxycamptothecin, ligustrazine, and so on) from traditional Chinese medicine (TCM) have exerted potent efficacies (e.g. anti-tumor, anti-inflammation, immunosuppression, etc.). However, a series of undesirable physicochemical properties (like low solubility and weak stability) and baneful pharmacokinetic (PK) profiles (e.g. low bioavailability, short half time, rapid clearance, etc.) have severely restricted their applications in clinic. In addition, some side effects (like cumulative toxicities caused by high-frequency administration and their own toxicities) have recently been reported and also confined their clinical uses. Therefore, developments in drug delivery of such alkaloids are of significance in improving their drug-like properties and, thus, treatment efficiencies in clinic. Strategies, including (i) specific delivery via liposomes; (ii) sustained delivery via nanoparticles, gels, and emulsions; and (iii) transdermal delivery via ethosomes, solid lipid nanoparticles, and penetrating enhancers, have been reported to improve the pharmacokinetic and physicochemical characters of problematic TCM alkaloids, decline their adverse effects, and thus, boost their curative efficacies. In this review, the recent reports in this field were comprehensively summarized with the aim of providing an informative reference for relevant readers.
Keywords: Bioactive alkaloids, traditional Chinese medicine, pharmacokinetics, toxicity, drug delivery
1. Introduction
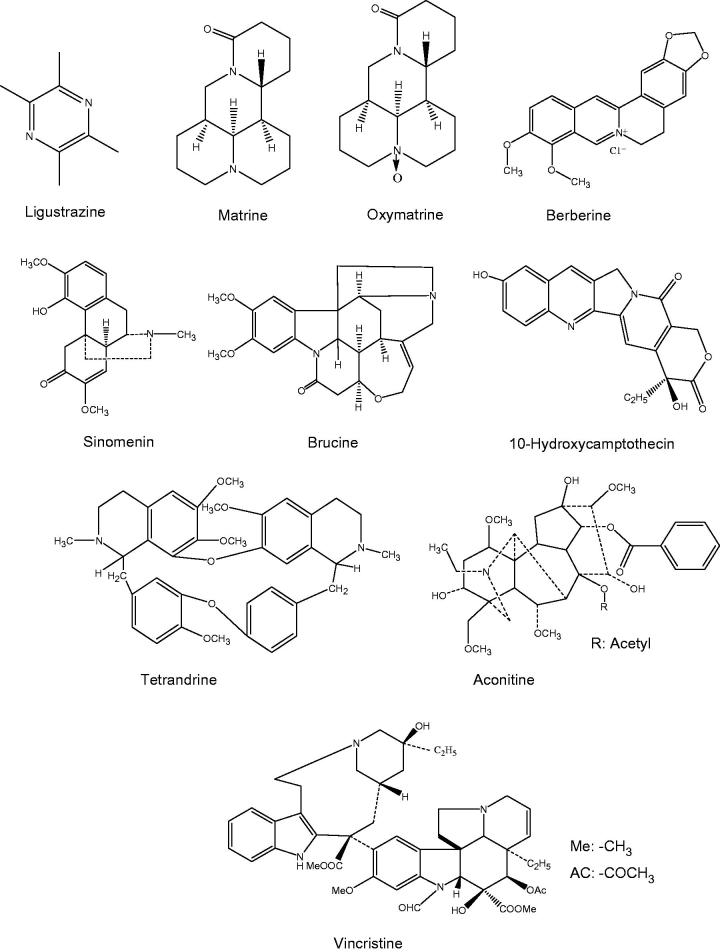
In recent years, a variety of bioactive ingredients have been extracted from traditional Chinese medicine (TCM), some of which are alkaloids (Table 1). The alkaloids are a type of nitrogen-containing organic compounds with the magnitude of molecular weight being generally less than 1 kDa (e.g. in this review being from 136 to 825 Da). They could be isolated from the root, stem, rhizome, fruit, and bark parts of the medicinal plants. According to their chemical structures, the alkaloids could be classified into the following types: quinoline alkaloids (e.g. 10-hydroxycamptothecin, berberine), quinolizidine alkaloids (e.g. matrine, oxymatrine), indole alkaloids (e.g. vincristine, brucine), and others like β-carboline, terpenoid alkaloids, and so on (Figure 1).
Table 1.
Basic information of TCM alkaloids involved in this review.
| Alkaloids | Chemical formula | Sources | Bioactivities | Existing problems | References |
|---|---|---|---|---|---|
| Vincristine | C46H56N4O10 | Catharanthi Rosei herba | Curing non-Hodgkin’s lymphoma, acute lymphoblastic leukemia, hematologic malignancies, and other solid tumors | Extensive distribution and long elimination; severe neurotoxicity | (Sethi et al., 1981; Silverman et al., 2013; Silverman & Deitcher, 2013; Said & Tsimberidou, 2014) |
| 10-Hydroxycamptothecin (10-HCPT) | C20H16N2O5 | Camptothecae Acuminatae fructus; Camptothecae Acuminatae folium; Camptotheca Acuminata decne; etc. | Treating colon cancer and other tumors; reducing epidural fibrosis | Poor solubility; short half-life | (Ping et al., 2006; Sun et al., 2008; Yang et al., 2011) |
| Brucine | C23H26N2O4 | Strychni semen; Strychni semen pulveratum; Strychnos ignatii; etc. | Inducing apoptosis of human cancer cell lines; decreasing peritoneal angiogenesis and microvessel density in vivo | Severe central nervous system toxicity; remarkable increase of blood pressure and even lethal poisoning | (Malone et al., 1992; Agrawal et al., 2011; Chen et al., 2012) |
| Berberine chloride | C20H18ClNO4 | Coptidis rhizoma; Phellodendri Chinensis cortex; Berberidis radix; etc. | Having the activities of anti-inflammation, vasorelaxation, and cardioprotection; inducing apoptosis of cancer cells | Low oral bioavailability; poor aqueous solubility; short half-life | (Pereira et al., 2007; Ma et al., 2013; Pirillo & Catapano, 2015; Allijn et al., 2017) |
| Matrine | C15H24N2O | Sophorae flavescentis radix; Sophorae tonkinensis radix et phizoma; Sophora alopecuroides L; etc. | Treating the infection of hepatitis B virus; inhibiting hepatic fibrosis | Low oral bioavailability | (Li et al., 2004; Wang et al., 2011; Chai et al., 2012) |
| Oxymatrine | C15H24N2O2 | Sophorae flavescentis radix; Sophorae tonkinensis radix et phizoma; Sophora alopecuroides L; etc. | Treating the infection of hepatitis B virus; inhibiting hepatic fibrosis | Low oral bioavailability | (Li et al., 2004; Wang et al., 2011; Chai et al., 2012) |
| Ligustrazine | C8H12N2 | Chuanxiong rhizoma; Curcumae rhizoma; Jatrophae rhizoma; etc. | Curing vascular illnesses and the corresponding complications | Short half-life, low oral bioavailability | (Chen & Chen, 1992; Mei et al., 2008; Li et al., 2012) |
| Tetrandrine | C38H42N2O6 | Stephaniae tetrandrae radix; Stephaniae Cepharanthae radix; Stephaniae Dielsianae radix; Stephaniae Epigaeae radix; etc. | Having the efficacies of anti-cancer and anti-inflammation; treating hypertension and pneumosilicosis | Poor solubility; low oral bioavailability | (Cai et al., 2011; Xu et al., 2014) |
| Aconitine | C34H47NO11 | Aconiti Lateralis radix praeparata; Aconiti radix; Aconiti Kusnezoffii radix; etc. | Having the efficacies of anti-inflammation and analgesia; treating neuronal disorders | High toxicity to the central nervous system and heart; limited oral administration; short elimination half-time; frequent dosing | (Wang & Xie, 1999; Rao & Sikdar, 2000; Zhao et al., 2011) |
| Sinomenine | C19H23NO4 | Sinomenii Caulis; Menispermum dauricum DC; Stephaniae Tetrandrae radix; Stephaniae Epigaeae radix; etc. | Having the efficacies of anti-inflammation and immunoregulation; treating allogeneic graft rejection and rheumatoid arthritis | Damage to the kidney and intestine; affecting the function of liver and heart | (Xu et al., 2008; Wang & Li, 2011) |
Figure 1.
The chemical structures of TCM alkaloids involved in this review.
So far, a wide spectrum of bioactivities has been found in the TCM-derived alkaloids. Particularly, most of them possess anti-tumor efficacies by virtue of inhibiting the formation of microtubule of tumor cell’s mitotic spindle (Douer, 2016), blocking DNA relegation through binding to topoisomerase (Shi et al., 2010b), inducing an arrest of cell cycle at the G1 phase (Pirillo & Catapano, 2015), and thus inhibiting proliferation and inducing cytotoxicity to tumor cells (Liu et al., 2010; Chen et al., 2012). Moreover, they also show other pharmacological effects, including anti-inflammation (Wang & Xie, 1999; Pereira et al., 2007; Cai et al., 2011), analgesia, anti-hepatic fibrosis, immunosuppression (Xu et al., 2008; Wang & Li, 2011), and the treatment of cerebrovascular and cardiovascular diseases (Xu & Shi, 2003), and rheumatic diseases (Liu et al., 2005).
However, some of undesirable physicochemical properties [like low solubility and weak stability (Yang et al., 2011)] have severely restricted their applications in clinic. For example, the active moiety of 10-hydroxycamptothecine is ease to be degraded in physiological pH (Chourpa et al., 1998), thus significantly limiting its clinical use. In addition, several baneful pharmacokinetic (PK) behaviors of bioactive alkaloids, such as low oral absorption/bioavailability (Li et al., 2004; Pirillo & Catapano, 2015), short half-life (Zhao et al., 2011), and rapid clearance, have been reported and significantly declined their efficacies. Besides the negative properties, side effects caused by the alkaloids play a vital role in impeding their use in clinic, as well. For example, in order to maintain the efficacies of some bioactive alkaloids, high-frequency administration is required, thereby resulting in low patient compliance and even producing cumulative toxicity in patients (Wei et al., 2012). What’s more, some alkaloids themselves could produce neurotoxicity, liver and kidney damage, and so forth in our bodies (Rao & Sikdar, 2000; Xu et al., 2008; Liu et al., 2010; Douer, 2016).
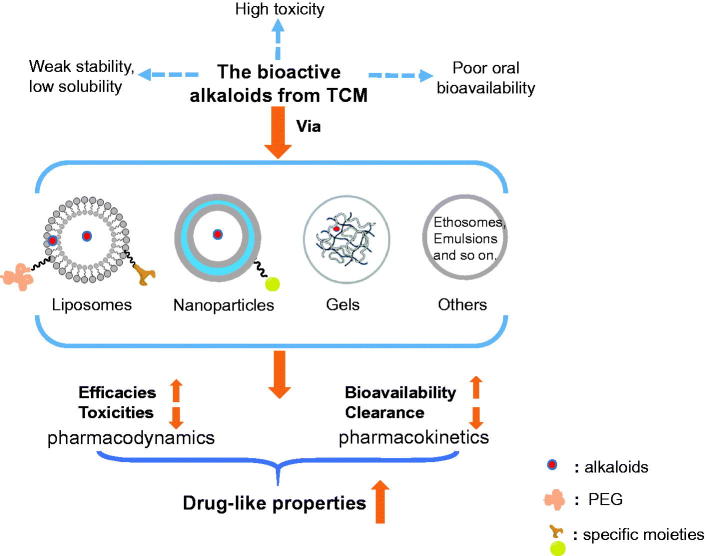
To improve the efficacies of alkaloids and reduce their adverse effects, strategies, including (i) specific delivery via liposomes; (ii) sustained delivery via nanoparticles, gels, and emulsions; and (iii) transdermal delivery via ethosomes, solid lipid nanoparticles, and penetrating enhancers, have been reported. In this review, the recent reports in this field were comprehensively summarized with the aim of providing an informative reference for relevant readers (Figure 2).
Figure 2.
Improvement of drug-like properties of TCM-derived alkaloids via specific drug delivery systems.
2. Specific delivery via various liposomes
Liposomes, having vesicular structure composed of lipid bilayers (Liu & Feng, 2015), have been widely used to deliver pharmacologically active alkaloids isolated from the effective parts of TCM (Table 2). According to the different purposes in application, these liposomes could be categorized into normal liposomes, long-circulating liposomes, active liposomes (containing components that provide a selective and controlled release of the liposome), and so on.
Table 2.
Delivery of alkaloids derived from traditional Chinese medicine by various liposomes.
| Liposome types | Alkaloids | Liposome composition | Preparation | Method of model | Method of dosing | Effects | References |
|---|---|---|---|---|---|---|---|
| Conventional liposomes | 10-HCPT | Lecithin: Chol; or EPC: Chol | Modified thin-film hydration method | Healthy rabbits | Iv. | The lactone: CL ↓ 52.6%, AUC0–∞ ↑ over one folds, Vd ↓ 50.5%; scar adhesion ↓ | (Shi et al., 2010b; Yang et al., 2011) |
| SN-38 | SPC:Chol (1:6) | Carrier-deposition method | New Zealand white adult rabbits | Iv. drip | AUC0–6 h of the liver ↑ 3.95-fold; AUC0–6 h of the spleen ↑ 6.7-fold; AUC0–6 h of the lung ↑ 4.7-fold; AUC0–6 h of the kidney ↓ 85.6%; AUC0–6 h of the heart ↓ 30.8% | (Li & Wang, 2016) | |
| DB-67 | DMPC: DMPG (7:3) or DSPC:m-PE G: DSPE (95:5) | – | SCID mice | Iv. | AUClactone of the spleen ↑ 1.07-fold; AUClactone of the lung ↑ 0.95-fold; t1/2 in PBS ↑ 5.66-fold | (Joguparthi et al., 2008; Zamboni et al., 2008) | |
| Vincristine | Sohingomyelin: Chol or HSPC: Chol: PEG5000-DSPE | The pH gradient method | Adults | Iv. | Therapeutic index ↑ ; target tumor ↑ ; individual dose ↑ over 2-fold; CL ↓ 40.4%; Cmax ↑ 52.3%, AUC ↑ 73.1%, MRT ↑ 56.6%, t1/2 ↑ 55.6% | (Thomas et al., 2009; Yan et al., 2012; O'Brien et al., 2013) | |
| Long-circulating liposomes | Ligustrazine | – | – | – | – | MRT ↑ , AUC ↑ , t1/2 ↑ , CL ↓ ; uptake of the drug by the phagocyte cells ↓ | (Yang et al., 2016) |
| Berberine | DPPC, DSPE-PEG2000, Chol (1: 0.08: 0.28); or HSPC: DSPC: DSPE-PEG2000 | Ethanol injection method or thin film hydration reverse phase evaporation method | Mice | Iv. | LVEF, FS ↓ ; EDV, ESV, HR ↓ ; t1/2 ↑ 5.13-fold, AST ↑ , ALT ↑ ; tumor weight ↓ 46.5% than the PBS; IC50 towards HepG2 cells ↓ 60.5% | (Lin et al., 2013; Allijn et al., 2017) | |
| Brucine | DSPE-PEG2000, Chol SPC/DPPC/HSPC/DSPC or SPC:HSPC:Chol:DSPE-mPEG | Ammonium sulfate gradient loading method | SD rats or Kun-Ming mice | Iv. | AUC0–∞ of the HSPC ↑ 4.7-fold than the SPC, CL/F of the HSPC ↓ 88.9% than the SPC; LD50 of the HSPC ↑ 37.2% than the SPC; The retention time of the mixture of HSPC and SPC ↑ 2.22-fold compared to the SPC; AUC of the mixture of HSPC and SPC in tumor ↑ 29.3% compared to the SPC | (Chen et al., 2012; Li et al., 2013) | |
| Vincristine | PEG-DSPE:HSPC:Chol (1:22:10) | The pH gradient method | SD rats | Iv. | t1/2 of PEG-DSPE formulation ↑ 0.4-fold compared to the market; 12 h of retardation in the clearance from blood | (Zhang et al., 2016) | |
| Targeted-release liposomes | Oxymatrine | Lecithin:Chol:RGD | – | SD rats | Iv. | MMP-2, TIMP-1 ↓ , type 1 procollagen ↓ , ALP ↓ | (Chai et al., 2012) |
| Berberine | EPC:Chol DQA-PEG2000-DSPE (57/38/4.35) | Film dispersion method | Female BALB/c nude mice | Iv. | Drug in MCF-7 cancer stem cells ↑ 0.73-fold; Caspase 3, 9 activity ↑ , Bax ↑ , Bcl-2 ↓ | (Ma et al., 2013) | |
| Matrine | HSPC:Chol:DSPE-mPEG2000:DSPE-PEG-MAL (2:1:0.1:0.01) | The pH-gradient method | – | – | Bcap-37, HT-29, A375 cells growth ↓ , apoptosis and anti-proliferation to cancer cells ↑ | (Liu et al., 2010) | |
| Vincristine | EPC:Chol:DSPE-PEG2000:TF:DSPE-PEG2000-NHS | Film dispersion method | SD rats | Iv. | t1/2α ↑ 0.93-fold , Vd ↓ 89.6% | (Song et al., 2017) | |
| Triggered-release liposomes | Berberine | Spc/P(NIPAM-co-MAA-co-ODA) | Lipid film hydration method | – | – | Thermo sensitivity | (Zhou et al., 2012) |
| Vincristine | EYPC:DSPE-PEG2000 (90:10 or DPPC:DSPE-PEG2000:MSPC (75:17:8) | Lipid film hydration method | Nude mice | Iv. | Anti-proliferation and apoptosis to Hela cells ↑ , tumor volume ↓ , temperature sensitivity | (Liu et al., 2015; Li et al., 2016) | |
| Others | Oxymatrine | SPC:PS:Chol:TO:TMC | Double emulsification method | Wistar rats | Orally | AUC ↑ 2.26-fold, Cmax ↑ 1.29- fold , Tmax ↑ 1.32-fold | (Cao et al., 2009) |
| Harmine | SPC:Chol:TMC (20:5:4) | Thin-film hydration method | SD rats | Ig. | AUC ↑ 1.26-fold than the free drug, AUC ↑ 0.53 fold than the normal liposome, bioavailability ↑ 0..52-fold than the normal liposome; degradation to cancer cells ↓ | (Chen et al., 2016) |
10-HCPT: 10-hydroxycamptothecin; Chol: cholesterol; EPC: egg phosphatidyl choline; Iv.: intravenously; CL: clearance; AUC: area under the concentration-time curve; Vd: the apparent volume of distribution; SN-38: 7-ethyl-10-hydroxy-camptothecin; SPC: phosphatidylcholine; DB-67: 7-silyl-modified camptothecin; DMPC: 1,2-dimyristoylsn-glycero-3-phosphocholine; DMPG: 1,2-dimyristoylsn-glycerol-3-phospho-sn-1-glyercol; DSPC: 1,2-distearoyl-3-sn-phosphatidylcholine; PEG: polyethylene glycol; DSPE: distearoylphosphatidylethanolamine; SCID mice: severe combined immunodeficient mice; t1/2: half-life; PBS: phosphate buffer saline; HSPC: hydrogenated soybean lecithin; Cmax: maximum plasma concentration; MRT: mean residence time; DPPC: dipalmitoyl phosphatidylcholine; LVEF: left ventricular ejection fraction; FS: fraction shortening; EDV: end diastolic volume; ESV: end systolic volume; HR: indicates heart rate; AST: glutamic oxaloacetic transaminase; ALT: glutamic pyruvic transaminase; IC50: the half maximal inhibitory concentration; HepG2 cells: human hepatoma cell lines; SD rats: male Sprague-Dawley rats; CL/F: apparent plasma clearance; LD50: the median lethal dose; RGD: Arg-Gly-Asp peptide; MMP-2: matrix metallopeptidase; TIMP-1: tissue inhibitor of metalloproteinase; ALP: alkaline phosphatase; DQA: dequlinium; MCF-7: Michigan Cancer Foundation-7 human breast cancer; Bax: pro-apoptotic protein; Bcl-2: anti-apoptotic protein; MAL: maleimide; A375: melanoma cell lines; Bcap-37: the breast cancer cell lines; HT-29: colon cancer cell lines; TF: transferrin; NHS: N-hydroxysuccinimidyl; P (NIPAM-co-MAA-co-ODA): Poly (Nisopropylacrylamide-co-methacrylic acid-co-octadecyl acrylate); EYPC: egg yolk lecithin; MSPC: 1-stearoyl-2-hydroxy-sn-glycero-3- phosphatidylcholine; PS: Phosphatidylserine; TO: Glycerol trioleate; TMC: N-trimethyl chitosan; Ig: intragastrically.
2.1. Normal liposomes
The lipid part of normal liposomes is often composed of cholesterol and soy phosphatidylcholine (SPC) in a certain proportion, which is not modified with other moieties. Some bioactive alkaloids, typically hydroxycamptothecin (HCPT) and vincristine, have been effectively delivered by such liposomes.
HCPT has been proven to possess potent antitumor effects such as anti-colon cancer (Ping et al., 2006) by virtue of inhibiting the DNA topoisomerase1 (Yang et al., 2011), particularly functioning in the process of DNA synthesis. In addition, HCPT has been found clinically to ameliorate the extent of the epidural fibrosis through inhibiting the fibroblast growth (Sun et al., 2008). To improve the treatment efficiency of HCPT, Yang et al. (2011) designed HCPT-encapsulated liposomes (L-HCPT) and administrated them to the laminectomy site of New Zealand white adult rabbits that suffered from Lumbar laminectomies via a L-HCPT-soaked cotton swab. The results showed that the laminectomy site incubated with L-HCPT was clean adequately without any obvious adhesion compared to a thin adhesion in the laminectomy sites dealt with HCPT, although both of L-HCPT and HCPT decreased the dura mater compared to the saline group. Namely, L-HCPT achieved a better curative efficacy to spine scarring after surgery. Besides, the clinical application of HCPT has also been limited by some other problems such as the rapid inactivation of its active lactone form (Shi et al., 2010b). With the aim of resolving this defect, Shi et al. (2010b) developed a kind of L-HCPT. The in vitro experiments indicated that the lactone form was favored at pH below 5. And, compared with the free HCPT, the pH of ring opening of the lactone loaded in the liposomes was significantly higher. Furthermore, the in vivo results showed that the AUC0–∞ of the lactone was increased by over one folds along with essentially declined clearance (CL) when it was administered in the form of L-HCPT to healthy rabbits. Namely, its stability and bioavailability were both boosted by virtue of the liposome carrier.
Meanwhile, HCPT has a lot of analogs. First, 7-ethyl-10-hydroxy-camptothecin (SN-38) is the bioactive metabolite of irinotecan hydrochloride (CPT-11). It has some similar pharmacological actions as HCPT. However, it also has some similar disadvantages like high toxicity and poor stability (Pal et al., 2005). To overcome them, Li & Wang (2016) prepared SN-38-loaded liposomes (L-SN38). The tissue distribution experiments showed that L-SN38 made the drug more prone to distribute in liver, spleen, and lung, with their drug AUC0–6 h values being 3.95-, 6.7-, and 4.7-fold larger than those obtained by the drug solution, respectively. On the other hand, less drug accumulation in heart and kidney was also achieved by L-SN38. Namely, L-SN38 could not only improve the antitumor efficacy of SN-38 to liver, spleen, and lung cancers, but also reduce its toxicity to other key organs, resulting in a meaningful improvement in therapeutic index. Second, a highly lipophilic analog, 10-hydroxy-7-tert-butyldimethylsilyl camptothecin (DB-67), has been found to enhance the lactone stability and, thus, improve the antitumor activity. Zamboni et al. (2008) developed a DB-67-loaded liposome (L-DB67) and the in vivo assays indicated some similar results as L-SN38 in terms of the distribution into spleen and lung. In addition, Joguparthi et al. (2008) developed another liposome through shifting the lipid bilayer composition to distearoyl phosphatidylcholine (DSPC) and polyethylene glycol distearoyl phosphatidyl ethanolamine (m-PEG DSPE) (95:5) with the aim of encapsulating DB-67 within a rigid gel phase bilayer and thereby further keeping the active lactone structure from hydrolysis. As expected, the drug hydrolysis in aqueous buffer was significantly slowed down, and the drug apparent half-life was increased by approximately 6-fold compared to that of DB-67 added to blank liposomes. In addition, the hydrolysis assays in rat plasma also showed that the rigid gel phase bilayers of such liposomes effectively extended the half-life of DB-67. Namely, loading DB-67 in this type of liposome could significantly prolong its effect time in body. Finally, other analogs like 7-t-butyldimethylsilyl 10-hydroxycamptothecin (AR-67) might also be delivered via liposomes for the same purposes.
As for vincristine (VCR, a vinca alkaloid), it has been indicated to possess potent anticancer ability for a long time particularly in hematologic malignancies and solid tumors (Silverman & Deitcher, 2013). In addition, VCR has an inhibitory effect on angiogenic growth through hindering the secretion of angiogenic factors (Avramis et al., 2001). Moreover, VCR exerted its effect on acute lymphoblastic leukemia (ALL) by inhibiting cell division (Said & Tsimberidou, 2014). Unluckily, some factors have capped its application in clinic. Of particular importance is its neurotoxicity in a dose-dependent manner (Silverman et al., 2013). And, some PK behaviors (such as a rapid and extensive distribution phase followed by a long elimination phase) not only make the effective concentration of VCR be maintained in a relatively short period, but also reduce the amount of VCR distributed to tumor sites (Sethi et al., 1981).
VCR delivered by liposomes took the advantages of (i) significantly enhancing accumulation into tumor sites and, thus, obviously inhibiting tumor growth and (ii) retarding its release in plasma compared to the free drug (Mayer et al., 1995). Intriguingly, the ratio of the components consisting of lipid bilayers of liposomes had effect on the VCR release (Nakamura et al., 2012). The released percentage of VCR from liposomes made with hydrogenated soybean lecithin (HSPC):cholesterol (Chol) (65:35) was apparently smaller than from those consisting of HSPC:Chol (54:46) in the first 5 min (about 38 and 66%, respectively). Therefore, it seems possible to further prolong the in vivo release of VCR by virtue of shifting the lipid components of liposomes. Meanwhile, it is also worth mentioning that the single dosage of VCR entrapped in liposomes could be added up to 2.5 mg/kg for curing tumor, compared to only 1.5 mg/kg of free VCR. On the other hand, with an equivalent dose of VCR, VCR-loaded liposomes also exhibited larger efficacy (Shan et al., 2006).
Twenty clinical experiments and two charitable projects have been carried out to research VCR sulfate liposome injection (VSLI) used in the treatment of ALL, non-Hodgkin’s lymphoma (NHL), and so on, which included both of adults and children (Silverman & Deitcher, 2013). Initially, the VSLI was confirmed to effectively treat the relapsed ALL (Thomas et al., 2009). To verify this, VSLI was further tested in 65 adult patients suffering from ALL (Silverman et al., 2013). Fortunately, VSLI showed no new or unanticipated toxicities to them with the dose being almost 2-fold higher than that of the standard VCR sulfate. Namely, applying VSLI to treat ALL could boost the therapeutic index of VCR, which was consistent with the studies of O'Brien et al. (2013) and Deitcher et al. (2014). In addition, Kaplan et al. (2014) used VSLI to treat refractory diffuse large B-cell lymphoma (DBCL) as well as mantle cell lymphoma (MCL). The assays indicated that VSLI combined with rituximab could not only lead to a long-lasting effect on patients who suffered from advanced CD20+DBCL as well as MCL before, but also make the hematologic toxicity controllable. Finally, to assess the clinical PK profile of VCR-loaded liposomes, Yan et al. (2012) gave patients single and multiple dosage of VSLI, respectively. By virtue of measuring the VCR level via a LC-MS/MS method, the AUC value and the Cmax obtained from the patients who received VSLI were found to increase by 0.66- and 0.53-fold, respectively, compared to from the normal VCR group. On the other hand, an inverse trend (∼50% reduction) was achieved in terms of CL. Furthermore, the PK profiles of VSLI did not change after repetitively dosing, indicating that VSLI should not store up in body with the increasing of administration frequency. In a word, owing to not only boosted therapeutic effects, but also declined side effects, VSLI has been more and more popular with patients.
2.2. Long-circulating liposomes
Long-circulating liposomes, also called ‘stealth liposomes’, exert their long-lasting capabilities relying on importing a hydrophilic polymer, notably polyethylene glycol (PEG), onto lipid surfaces, thus, substantially helping them evade the uptake of mononuclear phagocyte system (MPS), enhancing their stability, and protecting the active substance loaded in them from being rapidly degraded (Gómez-Hens & Fernández-Romero, 2006; Chen et al., 2012). Recently, some problematic bioactive alkaloids from TCM, such as ligustrazine, berberine, and brucine (short half-life, low oral absorption, etc.) (Mei et al., 2008; Pirillo & Catapano, 2015), have already been delivered by long-circulating liposomes for the sake of strengthening their treatment efficiencies as well as reducing their undesirable side effects.
First, ligustrazine has the advantages of treating several vascular diseases and corresponding complications resulted from neurovascular and cardiovascular diseases (Chen & Chen, 1992; Li et al., 2012). However, the efficacy of ligustrazine is often restricted in clinic owing to its low oral bioavailability (10–30%) as well as a relatively short half-time (Mei et al., 2008), which results in the need of quite frequent administration and, thus, ascends the risk of its toxicity (Zhang et al., 2007). To surmount these challenges, Yang et al. (2016) designed a ligustrazine-loaded stealth liposome (LTSL). As a consequence, the drug AUC and half-life values were both enhanced in vivo, more specifically, being 15- and 7.5-fold larger than those achieved by the free drug, respectively. That was to say, LTSL had a much longer retention time in body, which was in accordance with the results of in vitro phagocytosis tests.
Second, berberine, an active ingredient isolated from Berberis spp., has been found to be anti-inflammatory, vasorelaxant, and so on (Pereira et al., 2007). Significantly, some investigations have proven that berberine could be used to treat the murine suffering from heart failure by virtue of its cardioprotective effects (Zhang et al., 2014a). To further enhance these effects, Allijn et al. (2017) developed a berberine-encapsulated stealth liposome (BBSL). Incubated with the RAW 246.7 macrophages, the BBSL did not fall and even ascend the interleukin-6 (IL-6) level compared to the free drug, which may indicate a boosted retention time of BBSL in vivo owing to dropped cell uptake. Furthermore, BBSL dramatically preserved the left ventricular ejection fraction of male C57BL/6J mice at day 28 following myocardial infarction (MI) by 64% in comparison to the free drug and control liposomes. To sum up, BBSL could markedly boost the treatment efficiency of berberine for adverse remodeling after MI. Furthermore, the similar effect of BBSL on liver cancer mainly HepG2 cells (human hepatoma cell line) was also achieved (Lin et al., 2013).
Third, another bioactive alkaloid, brucine, showed potent antitumor activities such as inhibiting Michigan Cancer Foundation-7 human breast cancer (MCF-7) cells growth (Philippe et al., 2004; Agrawal et al., 2011). On the other hand, its violent toxicity to central nervous system (CNS) has been a major obstacle to its clinical application (Chen et al., 2012). Moreover, high-dose brucine could lead to remarkable rise of blood pressure and even death (Malone et al., 1992). It is pretty advantageous for brucine to be delivered by long-circulating liposomes in terms of longer retention time in blood and thus, being less distributed into brain (Zhang et al., 2012). In addition, phosphatidylcholines (PCs) with high gel-liquid crystalline transition temperature (Tm) could enhance the stability of liposomes in the blood (Senior & Gregoriadis, 1982), due to the fact that at Tm the lipidic bilayer loses much of its ordered packing and, meanwhile, its fluidity and permeability increase. To prove these, Chen et al. (2012) developed a brucine-loaded stealth liposome (BSL) comprised of several types of membrane components. The in vitro assays showed that in rat plasma, HSPC-containing BSL showed the lowest drug release rate (15.56% ± 1.04%) at 10 h, merely accounting for about 1/5 of that from SPC-containing BSL. In principle, the in vivo result was in accordance with that found in vitro. Furthermore, the LD50 of HSPC-BSL intravenously administered to mice was about 1.37-fold larger than that obtained by the SPC-BSL group. In conclusion, HSPC-BSL with a higher Tm showed a more stable behavior compared to SPC-BSL. A similar trend was also found in the study of Li et al. (2013).
Finally, the effect of VCR on inhibiting tumor cell division was mainly based on the duration of the drug exposure (Camplejohn, 1980; Cui et al., 2011), and thus, being delivered by long-circulating liposomes might be a beneficial choice. To pick out the superior long-circulating material, Zhang et al. (2016) developed several types of VCR-entrapped long-circulating liposomes. The in vitro assays showed that the drug-loading amount of long-circulating liposomes made up of chitosan was only about 1/2 of that achieved by the PEG-1, 2-distearoyl sn-glycero-3-phosphatidylethanolamine (PEG-DSPE), or PEG-poly-lactide-co-glycolide (PEG-PLGA) formulation. What’s more, in terms of the in vivo PK behavior, the AUC0–∞ value of VCR from the PEG-DSPE formulation was substantially larger (approximately 2.3-fold) than the PEG-PLGA or chitosan formulation. Overall, PEG-DSPE appeared to be the best long-circulating material among the three studied. Meanwhile, to tackle the problem of drug leakage from liposomes composed of negatively charged PEG-DSPE (Zhu et al., 1996; Webb et al., 1998), Cui et al. (2011) introduced an intraliposomal trapping agent, negatively charged sulfobutyl ether cyclodextrin, which could attract alkaloids via both electrostatic and hydrophobic interactions. Accordingly, the stability and PK profile of VCR-loaded long-circulating liposomes were both further boosted. Furthermore, in terms of targeting tumor cells, drug combination may lead to a better treatment efficacy as it declines the resistance to drugs (Barenholz, 2007). However, it is quite difficult to fully exert their combined efficacy due to their independent PK profiles (Zucker et al., 2012). To solve the above dilemma, Zucker et al. (2012) developed VCR-and-topotecan-loaded long-circulating liposome (LViTo). As a consequence, both of the two drugs obtained a zero-order release kinetics with similar rates, and the efficacy of tumor treatment was apparently improved thanks to selective and simultaneous delivery of both the drugs to the tumor.
2.3. Targeted-release liposomes
As one of active liposomes, targeted-release liposomes exert their targeting capabilities by virtue of a series of affinity responses (such as that between antibodies and antigens) and release drugs specifically into the target location (Gómez-Hens & Fernández-Romero, 2006). Thus, it is undoubted that targeted-release liposomes are popular with the bioactive alkaloids such as oxymatrine, berberine, and VCR.
Arg-Gly-Asp (RGD)-modified liposomes have been used to deliver oxymatrine (OM) and matrine to enhance anti-tumor efficacy or further treat hepatic fibrosis (Gray & Lachance, 1956; Chai et al., 2012). OM has been found to inhibit hepatitis B and C viruses (Wu & Wang, 2004; Wang et al., 2011). What’s more, OM also played a vital role in hepatic fibrosis induced by CCl4 (Chai et al., 2012). To further enhance the efficacy of OM on anti-hepatic fibrosis, Chai et al. (2012) designed an OM-loaded RGD-labeled liposome (OM-RGD-L). The tests showed that the serum alkaline phosphatase (ALP) and collagen area values after intravenous administration of OM-RGD-L to SD rats dropped by 20.9 and 89.0%, respectively, compared to those achieved by the OM liposome. Moreover, the MTT assays showed that OM-RGD-L significantly inhibited the survival of hepatic stellate cells (HSCs). In contrast, the OM liposome had simply low cytotoxicity to HSCs. In other investigations, in order to enhance the anti-tumor activity of matrine (Liu et al., 2008), Liu et al. (2010) designed a matrine-loaded RGD-modified long-circulating liposomes (M-RGD-LCL). As a result, M-RGD-LCL showed markedly enhanced effects of anti-proliferation and pro-apoptosis on cancer cells (Bcap-37, HT-29, and A375) in comparison to the free matrine. In a word, RGD-modified liposomes have the advantage of accumulating more drug molecules within the targeting cells to induce a stronger therapeutic effect.
The anticancer and antibacterial activities of berberine were reported (Gray & Lachance, 1956; Liu et al., 2009). Furthermore, it’s thought that berberine might be a suitable modulating agent for use to circumvent drug efflux by the highly expressed membrane proteins and to initiate the apoptotic reactions of cancer stem cells (CSCs) that are responsible for breast cancer relapse (Ma et al., 2013). To enhance the efficacy to relapsed breast cancer, Ma et al. (2013) designed a berberine-encapsulated targeted-release liposome (BT-L) composed of dequlinium and carboxyl PEG2000-DSPE. As a result, BT-L was shown to enter the CSCs and accumulate in the mitochondria. Inhibition of the ATP-binding cassette (ABC) transporters overexpressed on the CSC membrane should be a contributor to the transportation. Furthermore, both the activation of the pro-apoptotic capability and the inhibition of the anti-apoptotic capacity of mitochondrial apoptosis-related proteins were observed. Significant efficacy was also observed after administration to mice. Therefore, BT-L showed obvious advantages in this treatment.
VCR is another important alkaloid for the treatment of cancers like brain glioma (Liang et al., 2008). However, the efficacy of VCR is always restricted by a series of obstacles in body such as blood brain barrier (BBB) and multidrug resistance (MDR) (Song et al., 2017). To handle these limitations, Song et al. (2017) designed a VCR-loaded transferrin (TF)-modified liposome (VT-L). As a consequence, VT-L had a stronger cytotoxic effect on C6 cells as well as C6/ADR cells compared to the conventional VCR liposome in vitro. Furthermore, the in vivo assays showed the AUC0–24 h of VT-L intravenously administrated to SD rats (2 mg/kg) was evidently larger (0.68-fold) than that of the conventional VCR liposome. Meanwhile, the CL of VT-L was substantially dropped by 93.1%. Overall, VT-L showed a much better efficacy to treat brain glioma. Moreover, the VCR-loaded targeted-release liposome was also used to treat human breast cancer (Zeng et al., 2015).
2.4. Triggered-release liposomes
So far, many researches have paid attention to developing triggered-release liposomes to obtain better drug efficacy (Andresen et al., 2005). By virtue of implanting a chemical component into the liposomes, the drug-loaded liposomes could be released in situ, being triggered by an external stimulus like light exposure and pH change (Gómez-Hens & Fernández-Romero, 2006). It is of apparent advantage for some bioactive alkaloids like berberine and VCR to be delivered by this type of liposomes.
To achieve the triggered-release capability of berberine, Zhou et al. (2012) designed a berberine-loaded pH- and thermo-triggered liposomes modified with poly (nisopropylacrylamide-co-methacrylic acid-co-octadecyl acrylate). As a consequence, a shell-core morphology made up of copolymer and liposome, respectively, was achieved. The loaded berberine could be released via either diffusion or phase transition. However, its maximum release rate was achieved on the duration of phase transition caused by phase transition pH and phase transition temperature. In a word, a thermo- and pH-triggered release behavior was obtained (Kono et al., 1999).
In addition, the triggered-release liposomes played a vital role in boosting the anti-tumor efficacy of VCR (Liu et al., 2015; Li et al., 2016). Liu et al. (2015) developed a liposome that encapsulated VCR-gold nanoparticle conjugates (VGC-L). The in vitro assays showed that VGC-L labeled with calcein indicated an increased fluorescence intensity in as well as higher cytotoxicity to HeLa cells illuminated with ultraviolet (UV) light compared to the VCR solution or the common VCR liposome. After intravenously administrated to BALB/c nude mice (0.6 mg/kg, each 2 days), VGC-L with UV exposure achieved an about 14.6% higher tumor inhibitory percentage than VCR or VGC-L without light exposure. Namely, the anti-tumor efficacy of VCR was improved by light-responsive controlled release. In another investigation, Li et al. (2016) designed a doxorubicin (DOX)- and VCR-loaded thermo-sensitive liposome (VD-TSL). The in vitro MTT assays showed that VD-TSL with hyperthermia (HT) had a strongest inhibitory proportion to PANG and sw-620 cell lines compared to the VCR and DOX solution and VD-TSL without HT. More importantly, the in vivo tests revealed that the tumor inhibition value of VD-TSL administrated intravenously to nude mice bearing tumor (VCR 0.2 mg/kg and DOX 0.8 mg/kg) under HT was 29.5% higher than that achieved by the VCR and DOX solution. In a word, the synergistic effects of VCR and DOX to tumor inhibition were exerted fully by virtue of this thermo-triggered drug delivery system.
2.5. Others
Liposomes modified with some specific moieties like N-trimethyl chitosan (TMC) have been used to deliver several bioactive alkaloids such as oxymatrine (OM) and harmine (HM) with the purpose of enhancing their oral bioavailability (Cao et al., 2009; Chen et al., 2016).
For example, Chen et al. (2016) designed a HM-loaded TMC-modified liposome (HM-TMCL). The oral bioavailability of HM-TMCL given intragastrically to SD rats was 2.51-fold larger than that obtained by the HM solution which was merely 29.2%. Similarly, the oral bioavailability of OM-encapsulated TMC-coated multivesicular liposome was 3.26-fold higher than that of the OM solution (Cao et al., 2009). In a word, these TMC-modified liposomes have the advantages of (i) being transported not only by the transcellular pathway, but also by the paracellular way by virtue of the opening of tight junctions between intestinal epithelial cells; and (ii) extending the retention time of bioactive alkaloids in gastrointestinal tract due to the bioadhesive property of TMC (Thanou et al., 2001; Van der Merwe et al., 2004; Huang et al., 2011).
In summary, specific delivery of TCM-derived bioactive alkaloids via various liposomes has exerted great advantages of (i) improving their PK profiles (e.g. higher bioavailability and longer half-life), (ii) strengthening their treatment efficacies (e.g. allowing larger single dosage of VCR to be used to cure tumors), and (iii) boosting their physicochemical properties (like declining the degradation of the active moiety of 10-hydroxycamptothecine in physiological pH). On the other hand, owing to deficient knowledge about the action mechanism, structure–efficacy relationship, and/or in vivo course of some TCM-derived alkaloids, it is relatively difficult to find out a tailor-made liposome to specifically deliver them.
3. Sustained delivery via nanoparticles, gels, emulsions, and others
To date, some novel carriers have been used as drug delivery systems to prolong the release of some bioactive alkaloids from TCM (Table 3). In addition, the nanocarriers modified with some specific moieties help such alkaloids achieve stronger capabilities of targeting to tumor cells. Moreover, some of the carriers could make these alkaloids more prone to enter lesion tissues or organs and, thus, enhance their efficiencies of curing the corresponding diseases and reduce their side effects.
Table 3.
Delivery of alkaloids derived from traditional Chinese medicine by sustained-release delivery systems.
| Carrier types | Alkaloids | Carrier composition | Preparation | Method of model | Method of dosing | Effects | References |
|---|---|---|---|---|---|---|---|
| Polymeric nanoparticles | Ligustrazine | PLGA | Hot-melting extrusion | NZW rabbits | Iv. | Relatively stable drug concentration for about 21 days, ideal zero-order in-vitro drug release; obvious inhibition to PVR for at least 5 weeks with only a 12.5% occurrence | (Xu et al., 2010) |
| Aconitine | PLGA | O/W single-emulsion/solvent-evaporation technique | – | – | Stability of aconitine ↑ ; slow-release behavior for 12 h | (Zhang et al., 2015b) | |
| Vincristine | PEG-PLGA or PLGA-PEG-folate | W/O/W emulsion solvent evaporation method | SD rats | Iv. | The NPs: AUC0–16 h ↑ 2.39-fold, MRT ↑ 4.32-fold, the CL↓69.7%; cytotoxicity to MCF-7 cells ↑ , IC50 ↑ 2.91-fold | (Chen et al., 2011a,b) | |
| Berberine | PCL | Nanoprecipitation method | SD rats | Ip. | T50% of the NPs ↑ 46-fold; stable at 25 °C storage | (Vuddanda et al., 2015) | |
| Chitosan | Ionic cross-linking method | The NPs: AUC0–96 h) ↑ 0.15-fold, MRT0–96 h ↑ 2.41-fold, Tmax ↑ 5- fold, Cmax↓70%, t1/2α ↑ 4.08-fold, t1/2β ↑ 3.42-fold; anti-apoptosis activity to chondrocyte ↑ | (Zhou et al., 2015) | ||||
| Tetrandrine | PVP-b-PCL | Nanoprecipitation method | – | – | Apoptosis to A549 cells ↑ , Bcl-2 protein↓, Bcl-xL protein↓, A549 cells migration and invasion↓, MMP-2 and MMP-9↓, MMP-3 ↑ | (Xu et al., 2014) | |
| Sustained-release gel system | Vincristine | Dextran, chitosan, β-glycerophosphate | Emulsion polymerization method and cold method | Swiss albino male mice | Sc. | The gels: AUC0–∞ ↑ 10.3-fold, MRT ↑ 9.9-fold , t1/2 ↑ 0.6-fold, CL↓91.1%, Vd↓85.7% , IC50↓ | (Thakur et al., 2016) |
| PLGA, PEG, PNIPAAm | W/O/W emulsion technique | SD rats | Medium survival period ↑ in brain tumor site | (Ozeki et al., 2012) | |||
| Sinomenine | Carbopol 940, HPMC (1:4) | – | White New Zealand rabbits | Eye drop | The gels: AUC0–8 h ↑ 1.7-fold, t1/2 ↑ 0.24-fold, MRT0–8 h ↑ 0.23-fold | (Song et al., 2013) | |
| Tetrandrine | Calcium alginate gel bead | – | Healthy dogs | Orally | The gels: Tmax ↑ 1.27-fold, t1/2 ↑ 0.58-fold, Cmax↓24.4% , 12 h of sustained release in vitro | (Ma et al., 2009) | |
| Emulsion | Ligustrazine | Soybean oil, oleic acid, lecithin, poloxamer 188, glycerol (240:12:20:12:45) | – | SD rats | Iv. | The emulsions: AUC0–10 h ↑ 0.61-fold, MRT ↑ 0.77-fold, t1/2 ↑ 0.76-fold, CL ↓ 40%; AUC0–3 h of the liver ↑ 10%, AUC0–3 h of the kidney ↑ 18%, AUC0–3 h of the brain ↑ 29% | (Wei et al., 2012) |
| Vincristine | Soybean lecithin, Solutol HS15, soybean oil (1:1:8) | Classical high-pressure homogenization | Wistar rats | Iv. | The emulsions: AUC0–∞ ↑ 0.46-fold, MRT0–∞ ↑ 0.22-fold, t1/2 ↑ 0.43-fold, Cmax ↑ 1.16-fold; cytotoxicity to MCF-7 cells ↑ | (Zhang et al., 2013) | |
| Others | Tetrandrine | Phospholipids, Solutol HS15, NaCl, and distilled water | Phase inversion method | SD rats | Oral gavage | The nanocapsules: AUC0–24 h ↑ 1.08-fold, MRT0–24 h ↑ 0.1-fold, Tmax ↑ 1.56-fold | (Zhao et al., 2013) |
| Sinomenine | Chitosan, gelatin, and alginate | Layer-by-layer technique | – | – | Light stability ↑ ; release rate↓ as the increase of chitosan/alginate bilayer number | (Shi et al., 2010a) | |
| Vincristine | Silk fibroin fibers | – | Female NCr nude mice | Iv. | Tumor growth↓ | (Harris et al., 2016) |
PLGA: dl-lactide-co-glycolide; Iv.: intravenously; NZW: New Zealand white; PVR: proliferative vitreoretinopathy; O/W: oil in water; PEG: poly (ethylene glycol); Folate: folic acid; W/O/W: water–oil–water; SD rats: male Sprague–Dawley rats; NPs: nanoparticles; AUC: area under the concentration-time curve; MRT: mean residence time; CL: clearance; MCF-7 cells: Michigan Cancer Foundation-7 human breast cancer cells; IC50: concentration of drug required to kill 50% of the cells; PCL: poly(ε-caprolactone); Ip.: intraperitoneally; T50%: the time required for 50% drug release; Cmax: maximum plasma concentration; Tmax: time to reach Cmax; t1/2α: distribution half-life; t1/2β: elimination half-life; PVP-b-PCL: poly (N-vinylpyrrolidone)-block-PCL; A549 cells: the non-small cell lung cancer cell; Bcl-2 and Bcl-xL: anti-apoptotic proteins; MMP-2 and MMP-9: matrix metalloproteinases; MMP-3: tissue inhibitor; Sc.: subcutaneous route of administration; Vd: apparent distribution volume; PNIPAAm: poly-N-isopropylacrylamide; HPMC: hydroxy propyl methyl cellulose; T1/2: elimination half-life; NaCl: sodium chloride.
3.1. Polymeric nanoparticles (NPs)
Polymeric NPs belong to solid NPs which could load the bioactive alkaloids into a core or onto the shell of the particles (Liu & Feng, 2015). Initially, the biodegradable poly (dl-lactide-co-glycolide) (PLGA) was found to effectively deliver some bioactive alkaloids, like ligustrazine, aconitine, and VCR for achieving a long-acting behavior (Xu et al., 2010; Chen et al., 2011a,b; Zhang et al., 2015b). For example, Xu et al. (2010) designed aconitine-loaded PLGA NPs. The in vitro assays showed that in acidic aqueous solution (pH =6.0), 46.7% of loaded aconitine was slowly released in next 50 h after 32.0% release occurred in the initial 6 h of incubation. In another investigation, Zhang et al. (2015b) designed ligustrazine-encapsulated PLGA NPs (the ligustrazine implants) with 30% drug loading, which were intravenously administrated to New Zealand white rabbits. The tests showed that the relatively stable drug concentration could be maintained for approximately 21 days after Cmax was reached. Moreover, the occurrence of proliferative vitreoretinopathy (PVR) in the ligustrazine implant-treated group was markedly lower than that in the untreated group (12.5% at day 35 versus 100% at day 14). To obtain the further long-acting capability, Chen et al. (2011a) also used PEG-modified PLGA NPs to load VCR, which were intravenously injected to SD rats. As a consequence, the AUC0–16 h and mean residence time (MRT) values of VCR were both evidently boosted (3.39- and 5.32-fold larger than those achieved by the VCR solution, respectively). Furthermore, Chen et al. (2011b) developed VCR-loaded folic acid-modified PEG-PLGA NPs in order to target VCR to tumor cells. Results showed that the uptake of such NPs by cancer cells overexpressing the folic acid receptor was higher than that of the NPs not containing folic acid. Correspondingly, they caused the highest cytotoxicity. Moreover, a longer retention time of alkaloids within tumor cells could also be achieved by such NPs via folic acid receptor-mediated endocytosis effect (Pinhassi et al., 2010; Yang et al., 2010). In general, the sustained release of active alkaloids from PLGA NPs mainly relies on the degradation of PLGA and its inherent viscosity rather than the drug itself (Zhang et al., 2015b).
Poly (ε-caprolactone) (PCL) was also used to deliver some bioactive alkaloids with the aim of achieving a sustained-release behavior (Xu et al., 2014; Vuddanda et al., 2015). For example, Vuddanda et al. (2015) designed PCL NPs to encapsulate berberine. As a result, the time for 50% release of berberine was roughly 46-fold longer than that of the free drug. In general, the nature of hydrophobicity of PCL could preserve the hydrophilic berberine chloride from fast releasing (Vuddanda et al., 2015). What’s more, Xu et al. (2014) developed poly (N-vinylpyrrolidone) (PVP)-block-PCL copolymer NPs to load tetrandrine (PP-NP-T). The assays showed that the PP-NP-T (with 20% drug loading) exerted the strongest sustained-release capability among the 10, 20, and 40% PP-NP-T. In addition, PP-NP-T showed stronger inhibition to lung cancer A549 cells’ migration and invasion, and also induced more apoptosis to A549 cells in a dose-dependent manner compared to the free drug. In conclusion, PCL with a hydrophilic moiety of PVP could more effectively deliver poorly soluble tetrandrine to exert its efficacy (Leiva et al., 2007).
Chitosan (CS) is another suited material to prepare NPs used for the loading of bioactive alkaloids due to its excellent biocompatibility and biodegradability (Shukla et al., 2013). For example, Zhou et al. (2015) designed berberine-loaded CS NPs, which were intravenously injected to SD rats (with 0.6 mg/ml). The tests indicated that the Tmax of berberine was 6-fold longer while the Cmax value was merely 0.3-fold lower than those of the berberine solution, respectively. Namely, a sustained-release behavior of berberine was achieved by employing CS NPs. Overall, polymeric NPs show great potential to significantly enhance the in vivo retention time and improve the efficacies of a variety of TCM alkaloids.
3.2. Gel systems
Several types of gel systems were used to deliver TCM alkaloids and helped them achieve long-acting capabilities (Ozeki et al., 2012; Sun et al., 2016; Thakur et al., 2016). Single gel system or gel system combined with microspheres has been developed to address some defects of VCR [e.g. short half-time (12 min) and frequent administration] (Silverman & Deitcher, 2013). For example, Thakur et al. (2016) designed a chitosan-β-glycerophosphate gel containing VCR-loaded dextran microspheres, which was subcutaneously administrated to Swiss albino male mice. As a consequence, loaded VCR exhibited a nearly 30-day slow release pattern in physiological medium (Nie et al., 2011). Correspondingly, the AUC0–∞, t1/2, and MRT values were all boosted (11.3-, 1.6-, and 9.9-fold larger than the free drug, respectively). On the other hand, the IC50 (concentration of drug required to kill 50% of the cells) of loaded VCR to cancer cells was significantly declined over time, which further verified the sustained-release effect of the gel system since no significant changes in IC50 were observed for the VCR solution during the same periods. Similarly, Ozeki et al. (2012) used a thermo-reversible gelation polymer hydrogel to carry VCR-encapsulated PLGA microspheres. After topically injected to the brain of SD rats bearing C6 glioma (30 μl and 10 μl/min), such gel system showed its distinctive advantages of treating brain tumor. As the results showed in vivo, the gel system significantly prolonged the survival time of the rats from 18 days (the untreated group) and 23.5 days (the microsphere-treated group) to 33 days. Namely, by virtue of such gel system composed of a unique thermo-reversible hydrogel, VCR-loaded PLGA microspheres could be localized in the site of brain tumor and then slowly release VCR there. In another research, a VCR-loaded β-hairpin peptide hydrogel was designed and one-month constant release was achieved (Sun et al., 2016).
Some other gel systems were also used to deliver TCM alkaloids (e.g. sinomenine hydrochloride and tetrandrine) (Ma et al., 2009; Song et al., 2013). Song et al. (2013) developed a sinomenine hydrochloride-loaded in situ gel system, which was dropped to White New Zealand rabbits’ eyes with 0.5% sinomenine to treat uveitis. As a consequence, the AUC0–8 h and MRT0–8 h values of sinomenine were both significantly improved (about 2.7- and 1.2-fold larger than those obtained by the sinomenine solution, respectively). In addition, Ma et al. (2009) designed calcium alginate gel beads to load tetrandrine with the aim of achieving an excellent sustained-release profile. The results showed that the Cmax value of tetrandrine after oral administration of the beads to healthy dogs only accounted for 3/4 of that achieved by the tetrandrine tablet with the same administration dosage (30 mg/kg). Moreover, the Tmax value was prolonged 2.27-fold compared to the tablet. As a whole, by virtue of various gel systems, the TCM alkaloids could better exert their efficacies in a prolonged-release manner.
3.3. Emulsions
Emulsions have two basic types (i.e. oil in water and water in oil). Among them, lipid emulsion and submicron emulsion are two important representatives to be used to deliver TCM alkaloids with the purpose of slowly releasing. For example, to solve the defects of ligustrazine (mainly low bioavailability and short half-life), Wei et al. (2012) used a lipid emulsion to deliver ligustrazine, which was intravenously administrated to SD rats (22 mg/kg). The results showed that the AUC0–10 h, MRT, and t1/2 values of ligustrazine were all boosted (1.61-, 1.77-, and 1.76-fold larger than those obtained by the ligustrazine solution, respectively). Namely, such an emulsion evidently enhanced the bioavailability and retention time of ligustrazine in vivo. Meanwhile, the bio-distribution tests indicated that this emulsion made the drug more prone to distribute into liver, kidney, and heart with the AUC0–3 h values being boosted by 10, 18, and 29% in comparison to the free ligustrazine, respectively. Thus, a better efficacy of ligustrazine on cardiovascular and hepatic illnesses has been achieved by this delivery technique (Liu et al., 2002; Li et al., 2012). In another research, Zhang et al. (2013) developed a VCR-oleic acid ion-pair complex-encapsulated submicron emulsion to enhance the anti-tumor efficacy of VCR. The results showed that the AUC0–∞, MRT, and t1/2 values after intravenous administration to Wistar rats (1.2 mg/kg) were enhanced by 46, 22, and 43% compared to the VCR solution, respectively. And, the uptake of this emulsion by MCF-7 cells was higher than that of the VCR solution. Therefore, due to (i) possessing higher bioavailability, (ii) being endocytosed into tumor cells, and (iii) inhibiting more mitotic activity of tumor cells, this emulsion induced more apoptosis to the tumor cells, showing a more potent efficacy on treating tumors. In a word, emulsions can not only improve the bioavailability of TCM alkaloids but also enhance their capabilities in terms of treating corresponding diseases.
3.4. Others
Other carriers used for sustained delivery of TCM alkaloids include micro/nanocapsules, silk film systems (SF), liquid crystals (LQ), and superparamagnetic anisotropic nano-assemblies (SANA). The oral bioavailability of tetrandrine-phospholipid complex loaded in lipid nanocapsules (mainly comprised of liquid core coated with a tensioactive membrane) was enhanced by 108% compared to the free drug (Zhao et al., 2013). And, sinomenine was loaded into microcapsules to enhance its efficacy of rheumatism and arthritis (Shi et al., 2010a). Moreover, SF, in situ LQ, and SANA were also fabricated to deliver some TCM alkaloids (e.g. sinomenine and VCR) with the aim of achieving sustained-release behaviors (Chen et al., 2015; Coburn et al., 2015; Harris et al., 2016; Xiong et al., 2016). At present, the researches about them are relatively limited, but it’s undoubted that they are worth being further studied due to their huge potentials in improving the delivery of alkaloids to achieve the effect of reducing toxicity and/or increasing curative efficacy (Liu & Feng, 2015).
4. Transdermal delivery via ethosomes, solid lipid nanoparticles, penetration enhancers, and others
Transdermal delivery has the advantages of (i) avoiding first-pass metabolism, (ii) reducing the fluctuation in plasma profile, and (iii) achieving better patient compliance owing to fewer dosing frequency (Brown et al., 2006) (Table 4). To improve percutaneous absorption of TCM alkaloids, many novel carriers, e.g. ethosomes and solid lipid nanoparticles, have been used alone or in combination with penetrating enhancers.
Table 4.
Delivery of alkaloids derived from traditional Chinese medicine by transdermal drug delivery systems.
| Carrier types | Alkaloids | Carrier composition | Preparation | Method of model | Method of dosing | Effects | References |
|---|---|---|---|---|---|---|---|
| Ethosomes | Ligustrazine | Phospholipid, cholesterol, ethanol (1:0.4:45) or egg phosphatidylcholine, 30% ethanol (2.5:30) | Ethanol injection-sonication | SD rats | Transdermally | The ethosome patches: AUC ↑ 1.09-fold, t1/2β ↑ 8.79-fold, Tmax ↑ 0.89-fold, Cmax ↓ 68.64%, CL ↓ 52.62%; whole blood viscosity ↓, plasma viscosity ↓, hematocrit ↓, RBC aggregation index ↓, RBC deformation index ↓, VF incidence ↓, times of ventricular premature beat ↓,SOD ↑, GSH-Px ↑, MDA ↓, the escape latency of amnesic rats ↓ | (Liu et al., 2011a,b; Shi et al., 2012b) |
| Tetrandrine | PC, ethanol, and propylene glycol | The pH gradient loading method | SD rats | Transdermally | The ethosomes: the drug flux of skin ↑ 1.1-fold, the drug deposition ↑ 0.7-fold; curing arthritis ↑ | (Fan et al., 2013) | |
| Penetration enhancers | Ligustrazine | Anethole or anisaldehyde or anisic acid or menthol and menthone | – | SD rats | Transdermally | The anisole compounds groups: percutaneous flux ↑, the apparent density ↑, Jss ↑, KP ↑ | (Zhang et al., 2015a; Wang et al., 2017) |
| Mesaconitine/Hypaconitine | M-OA | – | Male Wistar rats | Transdermally | The permeation ↑, desquamation of SC flake ↑, SC lipid fluidization ↑ | (Zhao et al., 2011) | |
| Solid lipid nanoparticles | Aconitine | Compritol® 888 ATO, Cremophor® EL), TranscotolP) | Microemulsion precursor method | SD rats | Transdermally | The SLNs: Cmax ↑ 2.56-fold, AUC0–10 h ↑ 1.26-fold, Tmax ↑ 3-fold than the ethanol tinctures; the AUC0–10 h and Cmax of the SLNs in scapular regin > abdomen > chest; the toxicity ↓, transdermal permeability ↑ | (Zhang et al., 2014b, 2015c) |
| Others | Ligustrazine | Oleic acid, Cremophor RH40, ethanol,1,8-cineole | The lamination technique | SD rats | Transdermally | The penetration ↑ | (Shi et al., 2012a) |
| Evodiamine/Rutaecarpine | Cremophor® EL, PEG400,Ethyl oleate,Water | – | SD rats | Transdermally | Evodiamine- and rutaecarpine-loaded microemulsions: the fluxes ↑ ; AUC0–10 h ↑ 2.06 and 3.23-fold | (Zhang et al., 2011) |
SD rats: male Sprague–Dawley rats; AUC: area under the concentration–time curve; t1/2β: elimination half-life; Cmax: maximum plasma concentration; Tmax: time to reach Cmax; CL: clearance; RBC: red blood cells; VF: ventricular fibrillation; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; MDA: Malondialdehyde; PC: Phosphatidylcholine; Jss: percutaneous flux; KP: permeability coefficients; M-OA: (E)-2-isopropyl-5-methylcyclohexyl octadec-9-enoate; SC: stratum corneum; SLNs: Solid lipid nanoparticles.
4.1. Ethosomes
Ethosomes, consisting of soft phospholipid vesicles with high concentrations of ethanol, could facilitate the delivery of TCM alkaloids into deep skin layers (Fang et al., 2008; Mishra et al., 2010). They were found to deliver ligustrazine to achieve a long-acting behavior and improve its efficacy in terms of curing Alzheimer’s disease and myocardial ischemia (Liu et al., 2011a,b; Shi et al., 2012b). For instance, Liu et al. (2011a) loaded ligustrazine into an ethosome patch using an ethanol injection-sonication method. When the patch was transdermally given to SD rats (100 mg/kg), the AUC value of ligustrazine was increased by 109% compared to the intragastric group. Meanwhile, the same patch was also used to evaluate other properties (Liu et al., 2011b). The assays indicated that the Cmax value of ligustrazine accounted for merely 31.4% of that of the intragastric group; however, the Tmax was prolonged by 1.89 times. In general, such a patch substantially boosted the bioavailability of ligustrazine probably due to helping the drug avoid the first-pass metabolism induced by oral delivery. What’s more, the ventricular fibrillation of the SD rats receiving this patch did not occur for 3 days and the times of ventricular premature beat significantly declined compared to the single ischemia-reperfusion control group (0% versus 20% and 4.05 ± 4.92 times versus 8.09 ± 6.82 times, respectively). Furthermore, in terms of curing acute ischemic myocardium, the plasma viscosity and aggregation of red blood cells of the rats that received the patch were both essentially lower than those of the ischemic control group.
In another study, another kind of ligustrazine ethosomal system was fabricated to treat Alzheimer’s disease. And, a good ability of penetration and drug deposition to skin was achieved owing to the interaction between the ethanol of such ethosomes and the lipid of the stratum corneum (Liu et al., 2011a; Shi et al., 2012b). Moreover, other researchers also used ethosomes to encapsulate tetrandrine, using a pH gradient loading method (Fan et al., 2013). The flux of drug across skin and the amount of drug deposition were enhanced by 110 and 70%, respectively, in comparison to those obtained by the liposome group. In addition, such ethosomes also showed more potent efficacy of treating arthritis.
4.2. Solid lipid nanoparticles (SLNs)
After topical application, SLNs could effectively form a lipid film, which boosts the adhesion on the surface of the skin and enhances the permeability of TCM alkaloids (Wissing & Müller, 2002; Zhang et al., 2015c).
To date, SLNs are used to deliver aconitine with the purpose of boosting its transdermal permeability, prolonging its release and, thus, reducing its toxicity (Zhang et al., 2014b, 2015c). For instance, Zhang et al. (2015c) designed a kind of aconitine-encapsulated SLNs. After topical administration to SD rats, the SLNs resulted in larger percutaneous fluxes and deposition of aconitine compared to an ethanol tincture. The drug deposition’s amount of such SLNs (with three different ratios of total surfactant and co-surfactant to oil matrix) was 1.5- to 5.6-fold higher than that obtained by the tincture. Moreover, the Cmax, Tmax, and AUC0–10 h values of aconitine loaded in such SLNs were enhanced by 256, 400, and 126% in comparison to those achieved by the tincture, respectively. Correspondingly, the anti-inflammatory and analgesic efficacies of such SLNs on the mice with induced inflammation were much stronger than those of the tincture. Meanwhile, the capability of transdermal delivery of aconitine-loaded microemulsion was also compared with that of the SLNs using the same formulation except the type of oil matrix (Zhang et al., 2014b). The studies showed that the drug deposition’s amount of the SLNs to the skin was larger than that of the microemulsion. And, the uptake efficiency of the SLNs by human immortalized keratinocyte cells was also higher than that of the microemulsion. Moreover, aconitine encapsulated in SLNs achieved a more long-acting release behavior and, thus, remarkably decreased the toxicity of the drug. In a word, SLNs showed their particular benefits of delivering aconitine, not only improving its bioavailability, but also reducing its toxicity.
4.3. Penetration enhancers
Penetration enhancers could facilitate the delivery of TCM alkaloids into deep skins by virtue of shifting the organization of intercellular lipid domain of stratum corneum (SC) (e.g. extracting lipids, changing lipids fluidity, and forming hydrogen bonds) (Moghimi et al., 1996; Zhang et al., 2015a).
Some penetration enhancers (e.g. anisole compounds and monocyclic monoterpenes) were used as promoters to improve the transdermal absorption of ligustrazine (Zhang et al., 2015a; Wang et al., 2017). For example, Zhang et al. (2015a) employed anisole compounds to enhance the transdermal delivery of ligustrazine. The penetration flux of porcine skin’s SC dealt with ligustrazine plus anisic acid was the highest among the anisic acid, anisaldehyde, anethole, and free ligustrazine groups (11.9 versus 9.9 versus 8.0 versus 0.75 μg/cm2/h, respectively). Moreover, the apparent density of the SC flake treated with anisole compounds was significantly larger than that with the free drug, indicating that the higher quantities of desquamated flakes were achieved in the penetration enhancer groups. In another investigation, Wang et al. (2017) applied another type of enhancer, i.e. ‘monocyclic monoterpenes’ (menthol and menthone) to helping ligustrazine deliver into deeper skins. As a consequence, the percutaneous flux and permeability coefficient values of SC of porcine skin treated with such enhancers were both larger than those of the control group. In addition, the desquamation extent of the SC flake treated with menthone was the most significant among the menthol, menthone, and control groups. In general, the improvements achieved by anisole compounds and monocyclic monoterpenes were both related to removing the intercellular lipids of SC. And, the further mechanism might be that the hydrogen-bonding action between the penetration enhancers and amides of SC was stronger than that between SC amides themselves and, thus, disrupted the originally existing hydrogen bonding that was responsible for the bilayer structure and integrity of SC lipids (Narishetty & Panchagnula, 2004).
Moreover, (E)-2-isopropyl-5-methylcyclohexyl octadec-9-enoate (M-OA), an another enhancer, was also used to improve the transdermal capability of Aconitum alkaloids (Zhao et al., 2011). As a result, the permeation abilities of mesaconitine and hypaconitine were both markedly improved after the skin was treated with M-OA.
4.4. Others
Others, including microemulsions (MS), nanostructured lipid carriers (NLCs), and liposomes, were also used to facilitate the transdermal delivery of TCM alkaloids. MS is a homogeneous system dispersed with nano-scaled liquid droplets (Tenjarla, 1999; Liu & Feng, 2015). The permeation rates of MS-loading huperzine A and ligustrazine phosphate were both markedly enhanced in comparison to the free drugs in treatment of amnesia (Shi et al., 2012a). Moreover, both of evodiamine and rutaecarpine filled into MS showed larger bioavailabilities than the tinctures (Zhang et al., 2011). NLCs, consisting of solid and liquid lipids, are derived from SLNs (Tiwari & Pathak, 2011; Liu & Feng, 2015). It was found that the transdermal cumulative amounts of NLC-loading lappacontine and ranaconitine were both larger than the SLN group (Guo et al., 2015).
5. Conclusions
A variety of carriers have shown their immense advantages in the delivery of the bioactive alkaloids derived from TCM, resulting in not only the improved PK behaviors and physicochemical characters of such alkaloids, but also the reduced adverse effects and enhanced curative efficacies. In more detail, by virtue of a host of novel nano-scaled carriers, the undesirable PK profiles of TCM alkaloids have been significantly improved. For example, the in vivo AUC value of such alkaloids loaded in such carriers was remarkably boosted (more specifically, was about 1.1- to 15-fold as large as the free drug). Namely, the bioavailability of such alkaloids was evidently enhanced by improved absorption and/or reduced in vivo elimination. More specifically, (i) specific moiety-modified carriers could facilitate the delivery of the alkaloids by opening the tight junctions between intestinal epithelial cells and/or employing the bioadhesive features of the moieties, (ii) some specific carriers probably help such alkaloids avoid the first-pass metabolism, and (iii) the half-life value of such alkaloids could increase by approximately 40–650% by virtue of more or less declined CL.
Moreover, the Tmax and MRT values of such alkaloids were boosted by about 0.2- to 9-fold, and 0.9- to 5-fold, respectively, compared to the drug solution with the equal administration dosage, while the Cmax value was decreased by approximately 25–70%. It means such alkaloids achieve the capability of long-lasting release, which plays a vital role in decreasing the dosing frequency, reducing the occurrence of cumulative toxicity, and thus, improving their therapeutic index. In general, the sustained-release behavior of TCM alkaloids depends on the properties of the carriers (like solubility, viscosity, polarity, and degradation rate) more than the drugs’. Meanwhile, by virtue of some targeted-release carriers, some TCM alkaloids (e.g. hydroxycamptothecin and brucine) could not only be more prone to distribute into lesion locations, but also less accumulate into the normal tissues and, thus, reduce their potential toxicities and improve their treatment efficiencies as well as their undesirable stabilities.
So far, many other types of bioactive components from TCM (e.g. flavonoids, glycosides, polysaccharides, terpenes, etc.) also exhibit great potentials in treating some diseases. However, most of them face some similar limitations that trouble TCM alkaloids. Thus, this review could also be regarded as a useful and informative reference for these TCM components. With a reasonable choice of delivery systems, their drug-like properties will be definitely improved, resulting in ultimately improved patient benefits.
Funding Statement
This work was supported by Program for New Century Excellent Talents in University (NCET-13-0906); the funds from Shanghai Municipal Commission of Health and Family Planning (ZY3-CCCX-3-5001) and Science and Technology Commission of Shanghai Municipality (15DZ2292000); and the Xinglin Scholar Program of Shanghai University of Traditional Chinese Medicine.
Disclosure statement
The authors report no conflicts of interest in this work.
References
- Agrawal SS, Saraswati S, Mathur R, Pandey M. (2011). Cytotoxic and antitumor effects of brucine on Ehrlich ascites tumor and human cancer cell line. Life Sci 89:147–58. [DOI] [PubMed] [Google Scholar]
- Allijn IE, Czarny BM, Wang X, et al. (2017). Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J Control Release 247:127–33. [DOI] [PubMed] [Google Scholar]
- Andresen TL, Jensen SS, Jørgensen K. (2005). Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res 44:68–97. [DOI] [PubMed] [Google Scholar]
- Avramis IA, Kwock R, Avramis VI. (2001). Taxotere and vincristine inhibit the secretion of the angiogenesis inducing vascular endothelial growth factor (VEGF) by wild-type and drug-resistant human leukemia T-cell lines. Anticancer Res 21:2281–6. [PubMed] [Google Scholar]
- Barenholz Y.2007. Amphipathic weak base loading into preformed liposomes having a transmembrane ammonium ion gradient: From the bench to approved DOXIL In: Gregoriadis G, ed. Liposome technology. 3rd ed., Vol. 2 New York (NY): Informa Healthcare, 1–25. [Google Scholar]
- Brown MB, Martin GP, Jones SA, Akomeah FK. (2006). Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–87. [DOI] [PubMed] [Google Scholar]
- Cai XH, Wang S, Chen BA. (2011). Research advances on the pharmacological effects of tetrandrine. Chin J Nat Med 9:473–80. [Google Scholar]
- Camplejohn RS. (1980). A critical review of the use of vincristine (VCR) as a tumour cell synchronizing agent in cancer therapy. Cell Tissue Kinet 13:327–35. [DOI] [PubMed] [Google Scholar]
- Cao J, Sun J, Wang X, et al. (2009). N-trimethyl chitosan-coated multivesicular liposomes for oxymatrine oral delivery. Drug Dev Ind Pharm 35:1339–47. [DOI] [PubMed] [Google Scholar]
- Chai NL, Fu Q, Shi H, et al. (2012). Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells. World J Gastroenterol 18:4199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, He H, Li S, Shen Q. (2011a). An HPLC method for the pharmacokinetic study of vincristine sulfate-loaded PLGA-PEG nanoparticle formulations after injection to rats. J Chromatogr B Analyt Technol Biomed Life Sci 879:1967–72. [DOI] [PubMed] [Google Scholar]
- Chen J, Li S, Shen Q, et al. (2011b). Enhanced cellular uptake of folic acid-conjugated PLGA-PEG nanoparticles loaded with vincristine sulfate in human breast cancer. Drug Dev Ind Pharm 37:1339–46. [DOI] [PubMed] [Google Scholar]
- Chen J, Yan GJ, Hu RR, et al. (2012). Improved pharmacokinetics and reduced toxicity of brucine after encapsulation into stealth liposomes: role of phosphatidylcholine. Int J Nanomedicine 7:3567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KJ, Chen K. (1992). Ischemic stroke treated with Ligusticum chuanxiong. Chin Med J 105:870–3. [PubMed] [Google Scholar]
- Chen WL, Yuan ZQ, Liu Y, et al. (2016). Liposomes coated with N-trimethyl chitosan to improve the absorption of harmine in vivo and in vitro. Int J Nanomedicine 11:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liang X, Ma P, et al. (2015). Phytantriol-based in situ liquid crystals with long-term release for intra-articular administration. AAPS PharmSciTech 16:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourpa I, Millot JM, Sockalingum GD, et al. (1998). Kinetics of lactone hydrolysis in antitumor drugs of camptothecin series as studied by fluorescence spectroscopy. Biochim Biophys Acta 1379:353–66. [DOI] [PubMed] [Google Scholar]
- Coburn JM, Na E, Kaplan DL. (2015). Modulation of vincristine and doxorubicin binding and release from silk films. J Control Release 220:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Li C, Wang C, et al. (2011). Development of pegylated liposomal vincristine using novel sulfobutyl ether cyclodextrin gradient: is improved drug retention sufficient to surpass DSPE-PEG-induced drug leakage?. J Pharm Sci 100:2835–48. [DOI] [PubMed] [Google Scholar]
- Deitcher OR, Glaspy J, Gonzalez R, et al. (2014). High-dose vincristine sulfate liposome injection (Marqibo) is not associated with clinically meaningful hematologic toxicity. Clin Lymphoma Myeloma Leuk 14:197–202. [DOI] [PubMed] [Google Scholar]
- Douer D. (2016). Efficacy and safety of vincristine sulfate liposome injection in the treatment of adult acute lymphocytic leukemia. Oncologist 21:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Li X, Zhou Y, et al. (2013). Enhanced topical delivery of tetrandrine by ethosomes for treatment of arthritis. Biomed Res Int 2013:161943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YP, Tsai YH, Wu PC, Huang YB. (2008). Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int J Pharm 356:144–52. [DOI] [PubMed] [Google Scholar]
- Gómez-Hens A, Fernández-Romero JM. (2006). Analytical methods for the control of liposomal delivery systems. Trac-Trend Anal Chem 25:167–78. [Google Scholar]
- Gray PH, Lachance RA. (1956). Assimilation of berberine by bacteria. Nature 177:1182–3. [DOI] [PubMed] [Google Scholar]
- Guo T, Zhang Y, Zhao J, et al. (2015). Nanostructured lipid carriers for percutaneous administration of alkaloids isolated from Aconitum sinomontanum. J Nanobiotechnol 13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JC, Coburn JM, Kajdacsy-Balla A, et al. (2016). Sustained delivery of vincristine inside an orthotopic mouse sarcoma model decreases tumor growth. J Pediatr Surg 51:2058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Makhlof A, Ping Q, et al. (2011). N-trimethyl chitosan-modified liposomes as carriers for oral delivery of salmon calcitonin. Drug Deliv 18:562–9. [DOI] [PubMed] [Google Scholar]
- Joguparthi V, Xiang TX, Anderson BD. (2008). Liposome transport of hydrophobic drugs: gel phase lipid bilayer permeability and partitioning of the lactone form of a hydrophobic camptothecin, DB-67. J Pharm Sci 97:400–20. [DOI] [PubMed] [Google Scholar]
- Kaplan LD, Deitcher SR, Silverman JA, Morgan G. (2014). Phase II study of vincristine sulfate liposome injection (Marqibo) and rituximab for patients with relapsed and refractory diffuse large B-Cell lymphoma or mantle cell lymphoma in need of palliative therapy. Clin Lymphoma Myeloma Leuk 14:37–42. [DOI] [PubMed] [Google Scholar]
- Kono K, Henmi A, Yamashita H, et al. (1999). Improvement of temperature-sensitivity of poly(N-isopropylacrylamide)-modified liposomes. J Control Release 59:63–75. [DOI] [PubMed] [Google Scholar]
- Leiva A, Quina FH, Araneda E, et al. (2007). New three-arm amphiphilic and biodegradable block copolymers composed of poly(epsilon-caprolactone) and poly(N-vinyl-2-pyrrolidone). Synthesis, characterization and self-assembly in aqueous solution. J Colloid Interface Sci 310:136–43. [DOI] [PubMed] [Google Scholar]
- Li J, Chen J, Cai BC, Yang T. (2013). Preparation, characterization and tissue distribution of brucine stealth liposomes with different lipid composition. Pharm Dev Technol 18:772–8. [DOI] [PubMed] [Google Scholar]
- Li JS, Wang HF, Bai YP, et al. (2012). Ligustrazine injection for chronic pulmonary heart disease: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2012:792726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Wang S. (2016). Preparation, pharmacokinetic profile, and tissue distribution studies of a liposome-based formulation of SN-38 using an UPLC-MS/MS method. AAPS PharmSciTech 17:1450–6. [DOI] [PubMed] [Google Scholar]
- Li M, Li Z, Yang Y, et al. (2016). Erratum to: thermo-sensitive liposome co-loaded of vincristine and doxorubicin based on their similar physicochemical properties had synergism on tumor treatment. Pharm Res 33:2307. [DOI] [PubMed] [Google Scholar]
- Li ZW, Ma XY, Wang MN, et al. (2004). Investigation of pharmacokinetics of oxymatrine soft capsule in healthy volunteers. Northwest Pharm J 19:149–151. [Google Scholar]
- Liang GW, Lu WL, Wu JW, et al. (2008). Enhanced therapeutic effects on the multi-drug resistant human leukemia cells in vitro and xenograft in mice using the stealthy liposomal vincristine plus quinacrine. Fundam Clin Pharmacol 22:429–437. [DOI] [PubMed] [Google Scholar]
- Lin YC, Kuo JY, Hsu CC, et al. (2013). Optimizing manufacture of liposomal berberine with evaluation of its antihepatoma effects in a murine xenograft model. Int J Pharm 441:381–388. [DOI] [PubMed] [Google Scholar]
- Liu CF, Lin CC, Ng LT, Lin SC. (2002). Hepatoprotective and therapeutic effects of tetramethylpyrazine on acute econazole-induced liver injury. Planta Med 68:510–514. [DOI] [PubMed] [Google Scholar]
- Liu JH, Li WD, Teng HL, Lin ZB. (2005). Immunopharmacological action of sinomenine, an alkaloid isolated from Sinomenium acutum, and its mechanism of action in treating rheumatoid arthritis. Acta Pharm Sin 40:127–131. [PubMed] [Google Scholar]
- Liu X, Liu H, Liu J, et al. (2011a). Preparation of a ligustrazine ethosome patch and its evaluation in vitro and in vivo. Int J Nanomedicine 6:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu H, Zeng Z, et al. (2011b). Pharmacokinetics of ligustrazine ethosome patch in rats and anti-myocardial ischemia and anti-ischemic reperfusion injury effect. Int J Nanomedicine 6:1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Fang H, Yang ZG, et al. (2008). Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int J Dermatol 47:448–456. [DOI] [PubMed] [Google Scholar]
- Liu XY, Ruan LM, Mao WW, et al. (2010). Preparation of RGD-modified long circulating liposome loading matrine, and its in vitro anti-cancer effects. Int J Med Sci 7:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Feng N. (2015). Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv Colloid Interface Sci 221:60–76. [DOI] [PubMed] [Google Scholar]
- Liu Y, He M, Niu M, et al. (2015). Delivery of vincristine sulfate-conjugated gold nanoparticles using liposomes: a light-responsive nanocarrier with enhanced antitumor efficiency. Int J Nanomedicine 10:3081–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu Q, Xu B, et al. (2009). Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res 662:75–83. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhou J, Zhang CX, et al. (2013). Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials 34:4452–4465. [DOI] [PubMed] [Google Scholar]
- Ma Y, Li WZ, Guan SX, et al. (2009). Evaluation of tetrandrine sustained release calcium alginate gel beads in vitro and in vivo. Yakugaku Zasshi 129:851–854. [DOI] [PubMed] [Google Scholar]
- Malone MH, St John-Allan KM, Bejar E. (1992). Brucine lethality in mice. J Ethnopharmacol 35:295–297. [DOI] [PubMed] [Google Scholar]
- Mayer LD, Masin D, Nayar R, et al. (1995). Pharmacology of liposomal vincristine in mice bearing L1210 ascitic and B16/BL6 solid tumours. Br J Cancer 71:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei D, Mao S, Sun W, et al. (2008). Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur J Pharm Biopharm 70:874–881. [DOI] [PubMed] [Google Scholar]
- Mishra D, Mishra PK, Dabadghao S, et al. (2010). Comparative evaluation of hepatitis B surface antigen-loaded elastic liposomes and ethosomes for human dendritic cell uptake and immune response. Nanomedicine 6:110–118. [DOI] [PubMed] [Google Scholar]
- Moghimi HR, Williams AC, Barry BW. (1996). A lamellar matrix model for stratum corneum intercellular lipids. II. Effect of geometry of the stratum corneum on permeation of model drugs 5-fluorouracil and oestradiol. Int J Pharm 131:117–129. [Google Scholar]
- Nakamura K, Yoshino K, Yamashita K, Kasukawa H. (2012). Designing a novel in vitro drug-release-testing method for liposomes prepared by pH-gradient method. Int J Pharm 430:381–387. [DOI] [PubMed] [Google Scholar]
- Narishetty ST, Panchagnula R. (2004). Transdermal delivery of zidovudine: effect of terpenes and their mechanism of action. J Control Release 95:367–379. [DOI] [PubMed] [Google Scholar]
- Nie S, Hsiao WL, Pan W, Yang Z. (2011). Thermoreversible pluronic F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studies. Int J Nanomedicine 6:151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S, Schiller G, Lister J, et al. (2013). High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol 31:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki T, Kaneko D, Hashizawa K, et al. (2012). Improvement of survival in C6 rat glioma model by a sustained drug release from localized PLGA microspheres in a thermoreversible hydrogel. Int J Pharm 427: 299–304. [DOI] [PubMed] [Google Scholar]
- Pal A, Khan S, Wang YF, et al. (2005). Preclinical safety, pharmacokinetics and antitumor efficacy profile of liposome-entrapped SN-38 formulation. Anticancer Res 25:331–341. [PubMed] [Google Scholar]
- Pereira GC, Branco AF, Matos JAC, et al. (2007). Mitochondrially targeted effects of berberine [Natural Yellow 18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a) quinolizinium] on K1735-M2 mouse melanoma cells: comparison with direct effects on isolated mitochondrial fractions. J Pharmacol Exp Ther 323:636–649. [DOI] [PubMed] [Google Scholar]
- Philippe G, Angenot L, Tits M, Frédérich M. (2004). About the toxicity of some Strychnos species and their alkaloids. Toxicon 44:405–416. [DOI] [PubMed] [Google Scholar]
- Ping YH, Lee HC, Lee JY, et al. (2006). Anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. Oncol Rep 15: 1273–1279. [PubMed] [Google Scholar]
- Pinhassi RI, Assaraf YG, Farber S, et al. (2010). Arabinogalactan − folic acid − drug conjugate for targeted delivery and target-activated release of anticancer drugs to folate receptor-overexpressing cells. Biomacromolecules 11:294–303. [DOI] [PubMed] [Google Scholar]
- Pirillo A, Catapano AL. (2015). Berberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studies. Atherosclerosis 243:449–461. [DOI] [PubMed] [Google Scholar]
- Rao S, Sikdar SK. (2000). Modification of alpha subunit of RIIA sodium channels by aconitine. Pflugers Arch 439:349–355. [DOI] [PubMed] [Google Scholar]
- Said R, Tsimberidou AM. (2014). Pharmacokinetic evaluation of vincristine for the treatment of lymphoid malignancies. Expert Opin Drug Metab Toxicol 10:483–494. [DOI] [PubMed] [Google Scholar]
- Senior J, Gregoriadis G. (1982). Is half-life of circulating liposomes determined by changes in their permeability? FEBS Lett 145:109–114. [DOI] [PubMed] [Google Scholar]
- Sethi VS, Jackson DV Jr., White DR, et al. (1981). Pharmacokinetics of vincristine sulfate in adult cancer patients. Cancer Res 41:3551–3555. [PubMed] [Google Scholar]
- Shan S, Flowers C, Peltz CD, et al. (2006). Preferential extravasation and accumulation of liposomal vincristine in tumor comparing to normal tissue enhances antitumor activity. Cancer Chemother Pharmacol 58:245–255. [DOI] [PubMed] [Google Scholar]
- Shi D, Greever R, Chen Y. (2010a). Preparation and characterization of novel sinomenine microcapsules for oral controlled drug delivery. Drug Dev Ind Pharm 36:482–489. [DOI] [PubMed] [Google Scholar]
- Shi J, Cong WJ, Wang YM, et al. (2012a). Microemulsion-based patch for transdermal delivery of huperzine A and ligustrazine phosphate in treatment of Alzheimer's disease. Drug Dev Ind Pharm 38:752–761. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang YM, Luo GA. (2012b). Ligustrazine phosphate ethosomes for treatment of Alzheimer's disease, in vitro and in animal model studies. AAPS PharmSciTech 13:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K, Tian Y, Jiang Y, et al. (2010b). Modified hydrolysis kinetics of the active lactone moiety of 10-hydroxycamptothecin by liposomal encapsulation. Pharm Dev Technol 15:644–652. [DOI] [PubMed] [Google Scholar]
- Shukla SK, Mishra AK, Arotiba OA, Mamba BB. (2013). Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol 59:46–58. [DOI] [PubMed] [Google Scholar]
- Silverman JA, Deitcher SR. (2013). Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol 71:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JA, Reynolds L, Deitcher SR. (2013). Pharmacokinetics and pharmacodynamics of vincristine sulfate liposome injection (VSLI) in adults with acute lymphoblastic leukemia. J Clin Pharmacol 53:1139–1145. [DOI] [PubMed] [Google Scholar]
- Song J, Bi H, Xie X, et al. (2013). Preparation and evaluation of sinomenine hydrochloride in situ gel for uveitis treatment. Int Immunopharmacol 17:99–107. [DOI] [PubMed] [Google Scholar]
- Song XL, Liu S, Jiang Y, et al. (2017). Targeting vincristine plus tetrandrine liposomes modified with DSPE-PEG2000-transferrin in treatment of brain glioma. Eur J Pharm Sci 96:129–140. [DOI] [PubMed] [Google Scholar]
- Sun JE, Stewart B, Litan A, et al. (2016). Sustained release of active chemotherapeutics from injectable-solid β-hairpin peptide hydrogel. Biomater Sci 4:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang L, Sun S, et al. (2008). The effect of 10-hydroxycamptothecine in preventing fibroblast proliferation and epidural scar adhesion after laminectomy in rats. Eur J Pharmacol 593:44–48. [DOI] [PubMed] [Google Scholar]
- Tenjarla S. (1999). Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst 16:461–521. [PubMed] [Google Scholar]
- Thakur V, Kush P, Pandey RS, et al. (2016). Vincristine sulfate loaded dextran microspheres amalgamated with thermosensitive gel offered sustained release and enhanced cytotoxicity in THP-1, human leukemia cells: in vitro and in vivo study. Mater Sci Eng C Mater Biol Appl 61:113–122. [DOI] [PubMed] [Google Scholar]
- Thanou M, Verhoef JC, Junginger HE. (2001). Chitosan and its derivatives as intestinal absorption enhancers. Adv Drug Deliv Rev 50:S91–S101. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Kantarjian HM, Stock W, et al. (2009). Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia. Cancer 115:5490–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R, Pathak K. (2011). Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm 415:232–243. [DOI] [PubMed] [Google Scholar]
- Van der Merwe SM, Verhoef JC, Verheijden JH, et al. (2004). Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur J Pharm Biopharm 58:225–235. [DOI] [PubMed] [Google Scholar]
- Vuddanda PR, Mishra A, Singh SK, Singh S. (2015). Development of polymeric nanoparticles with highly entrapped herbal hydrophilic drug using nanoprecipitation technique: an approach of quality by design. Pharm Dev Technol 20:579–587. [DOI] [PubMed] [Google Scholar]
- Wang J, Dong C, Song Z, et al. (2017). Monocyclic monoterpenes as penetration enhancers of ligustrazine hydrochloride for dermal delivery. Pharm Dev Technol 22:571–577. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li XK. (2011). Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol 11:373–376. [DOI] [PubMed] [Google Scholar]
- Wang XW, Xie H. (1999). Alkaloids of Chinese aconitum plants. Drugs Fut 24:877–882. [Google Scholar]
- Wang YP, Zhao W, Xue R, et al. (2011). Oxymatrine inhibits hepatitis B infection with an advantage of overcoming drug-resistance. Antiviral Res 89:227–231. [DOI] [PubMed] [Google Scholar]
- Webb MS, Saxon D, Wong FM, et al. (1998). Comparison of different hydrophobic anchors conjugated to poly(ethylene glycol): effects on the pharmacokinetics of liposomal vincristine. Biochim Biophys Acta 1372:272–282. [DOI] [PubMed] [Google Scholar]
- Wei L, Marasini N, Li G, et al. (2012). Development of ligustrazine-loaded lipid emulsion: formulation optimization, characterization and biodistribution. Int J Pharm 437:203–212. [DOI] [PubMed] [Google Scholar]
- Wissing S, Müller R. (2002). The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int J Pharm 242:377–379. [DOI] [PubMed] [Google Scholar]
- Wu XN, Wang GJ. (2004). Experimental studies of oxymatrine and its mechanisms of action in hepatitis B and C viral infections. Chin J Dig Dis 5:12–16. [DOI] [PubMed] [Google Scholar]
- Xiong F, Tian J, Hu K, et al. (2016). Superparamagnetic anisotropic nano-assemblies with longer blood circulation in vivo: a highly efficient drug delivery carrier for leukemia therapy. Nanoscale 8:17085–17089. [DOI] [PubMed] [Google Scholar]
- Xu H, Hou Z, Zhang H, et al. (2014). An efficient Trojan delivery of tetrandrine by poly(N-vinylpyrrolidone)-block-poly(ε-caprolactone) (PVP-b-PCL) nanoparticles shows enhanced apoptotic induction of lung cancer cells and inhibition of its migration and invasion. Int J Nanomedicine 9:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Shi DZ. (2003). The clinical application and pharmacological effect of Ligustrazine. Chin J Integr Tradit West Med 23:376–377. [PubMed] [Google Scholar]
- Xu M, Liu L, Qi C, et al. (2008). Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med 74:1423–1429. [DOI] [PubMed] [Google Scholar]
- Xu X, Xiang Q, He Z, et al. (2010). Crystalline drug aconitine-loaded poly(d,l-lactide-coglycolide) nanoparticles: preparation and in vitro release. Yakugaku Zasshi 130:409–418. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhu ZL, Qian ZZ, et al. (2012). Pharmacokinetic characteristics of vincristine sulfate liposomes in patients with advanced solid tumors. Acta Pharmacol Sin 33:852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ni B, Liu J, et al. (2011). Application of liposome-encapsulated hydroxycamptothecin in the prevention of epidural scar formation in New Zealand white rabbits. Spine J 11:218–223. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Lin FH, Tsai KC, et al. (2010). Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjug Chem 21:679–689. [DOI] [PubMed] [Google Scholar]
- Yang YH, Wang QQ, Li J, et al. (2016). Ligustrazine-loaded stealth liposomes: cellular uptake in murine phagocyte cell model and pharmacokinetics in rats. Lat Am J Pharm 35:32–37. [Google Scholar]
- Zamboni WC, Jung LL, Strychor S, et al. (2008). Plasma and tissue disposition of non-liposomal DB-67 and liposomal DB-67 in C.B-17 SCID mice. Invest New Drugs 26:399–406. [DOI] [PubMed] [Google Scholar]
- Zeng F, Ju RJ, Liu L, et al. (2015). Application of functional vincristine plus dasatinib liposomes to deletion of vasculogenic mimicry channels in triple-negative breast cancer. Oncotarget 6: 36625–36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CF, Yang ZL, Luo JB, et al. (2007). Effects of cinnamene enhancers on transdermal delivery of ligustrazine hydrochloride. Eur J Pharm Biopharm 67:413–419. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Y, Li X, et al. (2016). The influence of different long-circulating materials on the pharmacokinetics of liposomal vincristine sulfate. Int J Nanomedicine 11:4187–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Han L, Sun X, et al. (2012). The use of PEGylated liposomes to prolong the circulation lifetime of salvianolic acid B. Fitoterapia 83:678–689. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zheng Y, Peng Q, et al. (2013). A novel submicron emulsion system loaded with vincristine-oleic acid ion-pair complex with improved anticancer effect: in vitro and in vivo studies. Int J Nanomedicine 8:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Wang JY, Li H, et al. (2015a). Novel application of natural anisole compounds as enhancers for transdermal delivery of ligustrazine. Am J Chin Med 43:1231–1246. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wei J, Ma P, et al. (2015b). Preparation and evaluation of a novel biodegradable long‐acting intravitreal implant containing ligustrazine for the treatment of proliferative vitreoretinopathy. J Pharm Pharmacol 67:160–169. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Yang SH, Li MH, et al. (2014a). Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: role of autophagy. Clin Exp Pharmacol Physiol 41:995–1002. [DOI] [PubMed] [Google Scholar]
- Zhang YT, Han MQ, Shen LN, et al. (2015c). Solid lipid nanoparticles formulated for transdermal aconitine administration and evaluated in vitro and in vivo. J Biomed Nanotechnol 11:351–361. [DOI] [PubMed] [Google Scholar]
- Zhang YT, Wu ZH, Zhang K, et al. (2014b). An in vitro and in vivo comparison of solid and liquid-oil cores in transdermal aconitine nanocarriers . J Pharm Sci 103:3602–3610. [DOI] [PubMed] [Google Scholar]
- Zhang YT, Zhao JH, Zhang SJ, et al. (2011). Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion. Int J Nanomedicine 6:2469–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Fang L, Li Y, et al. (2011). Effect of (E)-2-isopropyl-5-methylcyclohexyl octadec-9-enoate on transdermal delivery of aconitum alkaloids. Drug Dev Ind Pharm 37:290–299. [DOI] [PubMed] [Google Scholar]
- Zhao YQ, Wang LP, Ma C, et al. (2013). Preparation and characterization of tetrandrine-phospholipid complex loaded lipid nanocapsules as potential oral carriers. Int J Nanomedicine 8:4169–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WT, An XQ, Wang JZ, et al. (2012). Characteristics, phase behavior and control release for copolymer–liposome with both pH and temperature sensitivities. Colloids Surf A 395: 225–232. [Google Scholar]
- Zhou Y, Liu SQ, Peng H, et al. (2015). In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int Immunopharmacol 28:34–43. [DOI] [PubMed] [Google Scholar]
- Zhu G, Oto E, Vaage J, et al. (1996). The effect of vincristine-polyanion complexes in STEALTH liposomes on pharmacokinetics, toxicity and anti tumor activity. Cancer Chemother Pharmacol 39:138–142. [DOI] [PubMed] [Google Scholar]
- Zucker D, Andriyanov AV, Steiner A, et al. (2012). Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: relating structure and pharmacokinetics to therapeutic efficacy. J Control Release 160:281–289. [DOI] [PubMed] [Google Scholar]