Abstract
Purpose of review
For over 60 years, chelation therapy with disodium ethylene diamine tetraacetic acid (EDTA, edetate) had been used for the treatment of cardiovascular disease (CVD) despite lack of scientific evidence for efficacy and safety. The Trial to Assess Chelation Therapy (TACT) was developed and received funding from the National Institutes of Health (NIH) to ascertain the safety and efficacy of chelation therapy in patients with CVD.
Recent findings
This pivotal trial demonstrated an improvement in outcomes in postmyocardial infarction (MI) patients. Interestingly, it also showed a particularly large reduction in CVD events and all-cause mortality in the prespecified subgroup of patients with diabetes. The TACT results may support the concept of metal chelation to reduce metal-catalyzed oxidation reactions that promote the formation of advanced glycation end products, a precursor of diabetic atherosclerosis.
Summary
In this review, we summarize the epidemiological and basic evidence linking toxic metal accumulation and diabetes-related CVD, supported by the salutary effects of chelation in TACT. If the ongoing NIH-funded TACT2, in diabetic post-MI patients, proves positive, this unique therapy will enter the armamentarium of endocrinologists and cardiologists seeking to reduce the atherosclerotic risk of their diabetic patients.
Keywords: cardiovascular disease, chelation, diabetes, metals, Trial to Assess Chelation Therapy
INTRODUCTION
In November 2012, we presented the results of the NIH-funded Trial to Assess Chelation Therapy (TACT) at the American Heart Association Scientific Sessions in Anaheim, California. Metal chelation for atherosclerosis had long been thought to be quackery by traditional cardiologists, yet persisted in clinical use. In spite of the absence of actionable data, a safety and efficacy trial of edetate disodium (disodium ethylene diamine tetra acetic acid, Na2EDTA) chelation in patients following myocardial infarction was funded in 2002 by the NIH, and completed over a decade [1,2].
The results of the primary analysis of TACT were surprising. Instead of debunking a quack therapy, the results of this double-blind, placebo-controlled clinical trial demonstrated benefit of 40 intravenous edetate disodium-based chelation infusions in reducing cardiovascular disease events in a secondary prevention population [1,2] (Fig. 1a). The effect of metal chelation on diabetic patients with cardiovascular disease (CVD, defined as prior myocardial infarction) is the focus of this review.
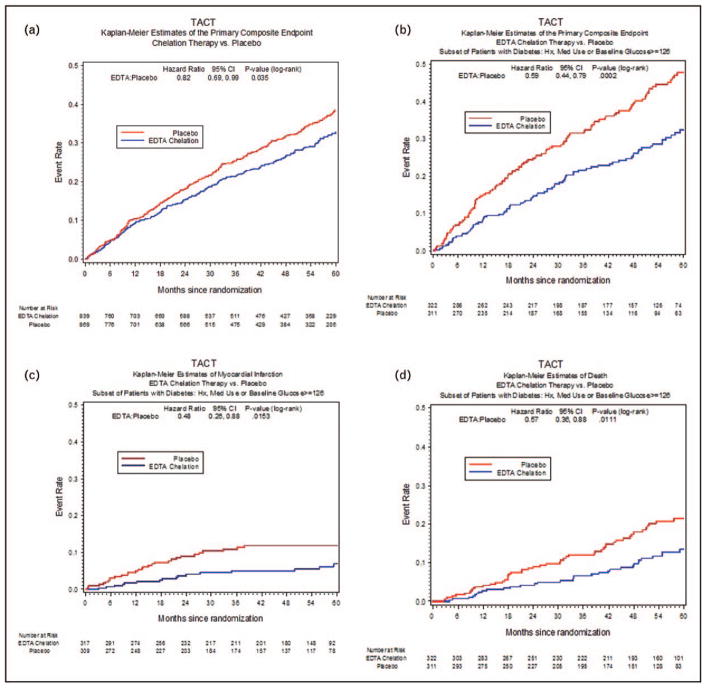
FIGURE 1.
The Trial to Assess Chelation Therapy Kaplan–Meier estimates of the primary composite endpoint: edetate disodium (EDTA) chelation therapy versus placebo (a), primary composite end point: edetate disodium chelation therapy versus placebo, subset of patients with diabetes mellitus (b), myocardial infarction in patients with diabetes mellitus by infusion group (c), mortality in patient with diabetes mellitus by infusion group (d). CI, confidence interval; TACT, Trial to Assess Chelation Therapy.
Of the 1708 post-MI patients studied in TACT, 633 had diabetes [3▪▪]. Diabetic patients assigned to chelation therapy demonstrated a 41% relative risk reduction in combined cardiovascular events compared with placebo infusions (P = 0.0002, 5-year number needed to treat to prevent one event = 6.5) 6.5) (Fig. 1b). There was a 52% relative reduction in the risk of recurrent myocardial infarction (P = 0.015; Fig. 1c); and a 43% relative risk reduction in mortality (P = 0.011, 5-year number needed to treat to prevent one death = 12; Fig. 1d) [3▪▪]. This review will touch upon the key role that essential and toxic metals, may play upon the development of vascular complications, particularly in patients with diabetes, the results of a clinical trial of metal chelation, particularly in diabetic patients, and ongoing research.
BRIEF HISTORY OF CHELATION BEFORE THE TRIAL TO ASSESS CHELATION THERAPY
Chelation, etymologically from chelos, the Greek word meaning claw, refers to the pincer like manner in which cations are incorporated into an organic molecule referred to as a chelating agent, forming a complex ring structure. The chelate–chelator complex is usually more stable, soluble and resistant to dissociation, allowing for effective removal from tissue and excretion in the urine or in bile [4].
A nearly ideal chelator, edetate is a synthetic amino acid with high affinity for metallic and non-metallic cations. EDTA is water soluble and excreted in the urine, forming strong coordinate bonds with certain ions such as calcium, zinc, cadmium and lead [5]. It was first developed in Germany in the late 1930s for chelation of calcium stains from textiles, and later applied to the treatment of heavy metal poisoning and hypercalcemia. The strong bond of EDTA with calcium led early proponents to hypothesize that it might reduce the calcium burden of atheromatous plaque [6,7].
In 1956, Clarke et al. [8] described the first use of chelation therapy and showed that 19 out of 20 patients with severe angina had improvement of symptoms and/or electrocardiographic findings after edetate disodium chelation infusions. The beneficial effects reported in this small study were followed by other small case reports and case series that failed to corroborate the efficacy of chelation in treating atherosclerosis. By the 1970s, chelation therapy had firmly moved into the realm of complementary and alternative medicine (CAM). Clinical trials were eventually performed, but were too small individually or in aggregate to exclude a small-to-moderate beneficial effect [9–13]. Yet over the decades, patients continued to seek and receive chelation from practitioners. Owing to the unsupported use of chelation, the NIH released a Request for Applications (RFAs) in 2001 for a safety and efficacy clinical trial of edetate disodium chelation in cardiovascular disease. The Trial to Assess Chelation Therapy (TACT) was the result. The positive results in patients with diabetes suggest that toxic metals may have a role in the excess atherosclerotic risk of such patients. In order to better understand the TACT results, it is worthwhile; therefore, to consider the epidemiological and mechanistic links between toxic metals and atherosclerosis.
TOXIC METALS AND VASCULAR DISEASE MECHANISMS AND EVIDENCE
Essential metals are crucial in maintaining cellular homeostasis, but excessive exposure may lead to metal-induced toxicity by increasing production of reactive oxygen species (ROS). ROS can overwhelm and deplete the cell’s intrinsic antioxidant defenses leading to oxidative stress. Iron and copper are the two best-known metals that have the potential of generating ROS. They are both effectively chelated by the edetates. Other metals, referred to as xenobiotic or toxic metals, have no role in human physiology and, like lead, may be toxic even in dilute concentrations. Some of the most commonly found toxic metals in humans include lead, cadmium, arsenic, and mercury, all of which cause – or at the very least are associated with – CVD.
Diabetes-related macrovascular complications, particularly CVD, have been found to be mediated by advanced glycation end products (AGEs), advanced lipoxygenation end products (ALEs), and protein oxidation products (PrOPs) [14]. AGEs are heterogeneous cross-linked complexes formed by the nonenzymatic glycation and oxidation of aldose sugars with proteins, lipids and nucleic acids [15]. There is also convincing evidence that formation of chemically active, cross-linked aldose sugars requires metal catalyzed oxygen chemistry to produce oxygen and hydroxyl radicals. AGEs have a deleterious effect on the integrity of the vessel walls through several mechanisms, including activation of the receptor for AGE (RAGE) [16]. RAGE activation triggers rapid generation of ROS and a pro-inflammatory signaling cascade with expression of pro-atherogenic adhesion molecules [17]. This interaction between AGE and its receptor perpetuates chronic vascular injury, which may play a role in the progression of diabetic atherosclerosis. These reactions may require metals that donate or accept electrons, in order to create oxygen and hydroxyl-free radicals. There is abundant evidence linking toxic metal accumulation and CVD [18–24]. We restrict our discussion below to lead and cadmium because of their effective chelation by the edetates.
Lead is a toxic metal found in contaminated water, air, food and soil because of its nonbiodegradable nature and continuous use. Human exposure to lead occurs mainly through leaded gasoline still used in piston engine aircraft, lead-containing pipes, smelting of lead and its combustion, lead-based paints and battery recycling (Fig. 2) [25]. Over the past three decades, there has been a dramatic drop in mean lead blood levels in the United States because of banning lead-based paints and leaded gasoline used by ground vehicles. However, environmental exposure continues, as evident in the recent public health crisis in Flint, Michigan because of lead seepage into drinking water. Lead contamination of drinking water is typically caused by metal corrosion of water pipes, particularly lead service lines. The incidence of elevated blood lead levels after the city of Flint changed its water source to the Flint River increased from 2.4 to 4.9% (P < 0.05) [26]. Lead can be inhaled or ingested, and then absorbed into the bloodstream [27]. Once absorbed, lead moves into the red cell compartment, where it is bound by metallothionein-like protein, a thiol-containing protein. Lead has a half-life of about 28–36 days in blood, 1–1.5 months in soft tissue, and about 25–30 years in bone [28–29]. When lead completes its lifespan in blood, it is released and displaces calcium in bone. Bone then acts as an endogenous source of lead, releasing lead back into the bloodstream years after exposure [29]. A single disodium edetate infusion of 3 g will increase lead excretion by nearly 4000% in a few hours [30,31▪].
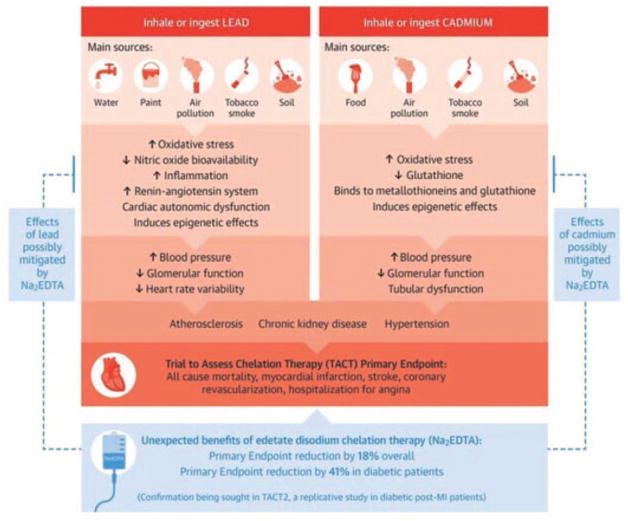
FIGURE 2.
Central illustration. Heavy metals as a risk factor for atherosclerotic cardiovascular disease and the benefits of chelation therapy. Major sources and routes of exposure, mechanisms at the molecular and cellular level, responses at the tissue and organ level, and subclinical and clinical cardiovascular effects on the basis of experimental and epidemiological evidence are shown for lead and cadmium. Arrows denote direction of flow. Na2EDTA, disodium ethylene diamine tetra acid; NO, nitric oxide; TACT, Trial to Assess Chelation Therapy.
Epidemiological data from various National Health and Nutrition Examination Surveys (NHANES) support an association between toxic metal exposure and CVD events. The prospective analysis of NHANES III participants showed an increase in all-cause and cardiovascular mortality, with cause specific deaths highest for myocardial infarction and stroke at lead blood levels greater than 0.10 μmol/l [32,33]. Several possible mechanisms have been postulated for the association of lead accumulation and its direct and indirect effect on CVD. In-vivo and in-vitro studies have shown that one mechanism by which chronic lead exposure causes CVD is by increasing production of ROS leading to oxidative stress, limiting nitric oxideavailability and disrupting the nitric oxide signaling cascade [34–37]. Nitric oxide has important cardioprotective roles including regulation of blood pressure and vascular homeostasis such as regulation of vascular dilation, local cell growth and protection of the vessel from injury because of platelet aggregation. Therefore, the impaired synthesis or excessive oxidative degradation of nitric oxide leads to endothelial dysfunction, a starting point for atherosclerosis.
Lead increases protein kinase C (PKC) activity and promotes atherogenicity [38,39]. PKC regulates many functions including cell growth, vascular smooth muscle contraction, blood flow and permeability [39]. Lead additionally promotes inflammation, fibrosis and apoptosis via activation of nuclear factor-κB. Lead also increases the production of endothelin, vasoconstrictor peptides primarily synthesized and secreted by endothelial cells that can raise arterial pressure. Khalil-Manesh et al. [40] found that rats exposed to low levels of lead for 1–12 months had a significant rise in arterial pressure and marked increase in plasma endothelin 3 concentrations. These and other proposed mechanisms may explain how lead causes vascular injury in humans. If more than one of these mechanisms is relevant to human diabetes, then lead may act as a risk factor multiplier, perhaps at least partially explaining the effect of lead chelation.
Cadmium is another toxic metal that accumulates in the environment and is associated with atherosclerotic disease. Environmental sources of cadmium include rechargeable batteries, electronics, building construction, jewelry, toys, plastic production, paint pigments and metal coating [41–43]. Tobacco leaves, spinach and root vegetables naturally accumulate and concentrate high levels of cadmium from the soil, dependent on the cadmium content of fertilizers. Tobacco use, therefore, is one of the single most important sources of cadmium exposure [44]. Cadmium accumulates in both the liver and kidneys, protein bound to metallothionein [45,46]. Because of its slow urinary excretion, cadmium has a half-life up to 38 years [41]. A single disodium edetate infusion of 3 g will increase cadmium excretion by about 700% in a few hours [30,31▪].
Similar to lead, there are several mechanisms that have been suggested to explain the role of cadmium in atherosclerosis. In a systematic review, Tellez-Plaza et al. [47] found evidence supporting cadmium as a cardiovascular risk factor. Cadmium can indirectly interfere with antioxidant response by binding to metalloproteins, thereby increasing ROS causing lipid peroxidation and cellular injury and DNA damage [48]. The Atherosclerosis Risk Factors in Female Youngsters study examined carotid intima–media thickness in 195 young healthy women and measured serum metal concentrations, including cadmium. They reported an independent association between high cadmium levels and intima–media thickness, exceeding the 90th percentile in distribution, representing early atherosclerotic vessel wall thickening [49]. Cadmium has also been found to raise blood pressure, an established risk factor for cardiovascular disease [50,51]. In a large population study of NHANES III participants between 1988 and 1994, creatinine-corrected urinary cadmium levels in men were associated with an increase in risk of all-cause and cardiovascular mortality, including coronary artery disease-associated mortality [52]. Finally, Tellez-Plaza et al. [53▪] examined 3348 American Indian adults ages 45–74 years who participated in the Strong Heart Study from 1989 to 1991 and measured urine cadmium levels. Urine cadmium levels were associated with an increased incidence of cardiovascular disease and mortality. The diabetic subgroup in this study showed a stronger, statistically significant association between cadmium and cardiovascular disease endpoints when compared with those without diabetes.
THE TRIAL TO ASSESS CHELATION THERAPY
Coincident with the interest of the diabetes scientific community in metal-catalyzed oxygen chemistry as an important part of the formation of AGEs and complications of diabetes, the cardiology community was in the final phases of planning a metal chelation trial. As noted above, edetate disodium chelation had been used to treat atherosclerotic complications for many years. A literature review in 2000 concluded that there was insufficient evidence for or against the practice [12]. This was followed by a more formal 2002 Cochrane Review again concluding that there was insufficient evidence to decide on the effectiveness of edetate sodium to treat atherosclerotic disease [13]. This conclusion sparked the interest of the NIH to release a RAF for a randomized double-blind, placebo-controlled trial to test the efficacy and safety of chelation therapy in patients with coronary artery disease. The study that resulted, TACT, was widely expected to be negative. During the planning phase, high-dose oral multivitamins and minerals (OMVM) were added in a factorial design, as most chelation practitioners also recommended that patients take large doses of OMVM during intravenous chelation [1]. The four randomized groups in the 2 × 2 factorial trial included:
active chelation and active OMVM
active chelation and placebo OMVM
placebo chelation and active OMVM
placebo chelation and placebo OMVM
Eligible patients had to be at least 50 years of age, have had prior myocardial infarction at least 6 weeks prior to enrollment, and have a serum creatinine level 2.0 mg/dl or less. Enrollment took place between 2003 and 2010. There were 1708 patients enrolled in the clinical trial across 134 sites in the United States and Canada. The blind was broken and analyses were completed, in 2012.
STUDY TREATMENTS
Intravenous chelation regimens developed organically through the years, and although the consistent chelating drug was edetate disodium, other components were often added (Table 1). The TACT infusion regimen included 30 weekly infusions followed by 10 maintenance infusions, the latter 2 weeks to 2 months apart. By 18 months after randomization, over 90% of the infusions had been administered. The randomized OMVM or oral placebo was taken throughout the duration of the trial and consisted of three caplets twice daily, containing 28 individual components (Table 2).
Table 1.
The Trial to Assess Chelation Therapy chelation components
| Up to 3 g of disodium EDTAa |
| 2 g of magnesium chloride |
| 100 mg of procaine HCl |
| 2500 U of heparin |
| 7 g of ascorbate |
| 2 mEq KCl |
| 840 mg sodium bicarbonate |
| 250 mg pantothenic acid |
| 100 mg of thiamine |
| 100 mg of pyridoxine |
| Add with sterile water to 500 ml |
The maximum dose of EDTA was 3 g for patients who have at least 60 kg of lean body weight and normal kidney function. Reduction in kidney function and/or lower lean body weight led to a reduction in the total EDTA dose infused.
Table 2.
High-dose oral contents used in TACT
| High-dose regimen (taken twice daily) | Total amount for 6 pills | % RDA |
|---|---|---|
| Vitamin A | 25 000 IU | 500% |
| Vitamin C | 1200 mg | 2000% |
| Vitamin D3 | 100 IU | 25% |
| Vitamin E | 400 IU | 1333% |
| Vitamin K1 | 60 μg | 75% |
| Thiamin | 100 mg | 6667% |
| Niacin | 200 mg | 1000% |
| Vitamin B6 | 50 mg | 2500% |
| Folate | 800 μg | 200% |
| Vitamin B12 | 100 μg | 1667% |
| Biotin | 300 μg | 100% |
| Pantothenic acid | 400 mg | 4000% |
| Calcium | 500 mg | 50% |
| Iodine | 150 μg | 100% |
| Magnesium | 500 mg | 125% |
| Zinc | 20 mg | 133% |
| Selenium | 200 μg | 286% |
| Copper | 2 mg | 100% |
| Manganese | 20 mg | 400% |
| Chromium | 200 μg | 167% |
| Molybdenum | 150 μg | 200% |
| Potassium | 99 mg | 3% |
| Choline | 150 mg | a |
| Inositol | 50 mg | a |
| PABA (para-aminobenzoic acid) | 50 mg | a |
| Boron | 2 mg | a |
| Vanadium | 39 μg | a |
| Citrus bioflavanoids | 100 mg | a |
RDA, recommended daily allowance.
Recommended daily allowance not established. Other ingredients: croscarmellose sodium, microcrystalline cellulose, magnesium stearate, hydroxypropyl cellulose, and silicon dioxide.
ENDPOINTS AND STATISTICAL POWER
The primary end point was a composite of all-cause mortality, myocardial infarction, stroke, coronary revascularization and hospitalization for angina. The principal secondary end point was cardiovascular mortality, recurrent myocardial infarction and stroke.
OVERALL RESULTS
The intention-to-treat analyses revealed a statistically significant reduction in the primary endpoint (hazard ratio 0.82; 95% CI 0.69–0.99; P = 0.035). Although the individual components of the primary endpoint did not reach statistical significance, all but total mortality had point estimates favoring active chelation treatments.
TRIAL TO ASSESS CHELATION THERAPY DIABETES SUBGROUP
Patients with diabetes were defined as those who self-reported diabetes, were receiving pharmacotherapy for diabetes, or had a fasting glucose of at least 126 mg/dl at baseline. Of the 1708 participants enrolled in the study, 633 (37.1%) patients had diabetes mellitus.
BASELINE CHARACTERISTICS
Among the patients with diabetes, 322 were randomized to receive edetate disodium-based infusions and 311 received placebo infusions. There were no significant between-group differences in important baseline characteristics. The median age was 65 years, with 19% women and 11% minorities. The trial encouraged patients to maintain standard, evidence-based treatments for patients with prior MI and diabetes.
Mean fasting blood sugar was 132 mg/dl, and low-density lipoprotein (LDL) cholesterol was 81 mg/dl. Within the diabetic population, 81% had undergone prior coronary revascularization (either percutaneous or surgical). There were 92% of patients on aspirin, clopidogrel or warfarin, and 76% on statins. Treatment of diabetes required insulin in 26%, and oral hypoglycemics in 61%.
RESULTS OF TRIAL TO ASSESS CHELATION THERAPY IN PATIENTS WITH DIABETES
In spite of the arduous nature of the study therapy, 73% of patients completed 30 infusions, and 61% completed all 40. There was a statistically significant interaction (P of interaction of the primary endpoint = 0.004) between edetate disodium therapy effect and the presence of diabetes mellitus at baseline. There was a striking benefit of the edetate disodium infusions in the diabetic patients randomly assigned to receive active infusions compared with placebo with 25% vs. 38% incidence of the primary outcome (hazard ratio 0.59; 95% CI 0.44–0.79; P = 0.0002). There was a 15% absolute decrease in the 5-year Kaplan–Meier primary event rate. The number of patients that needed to be treated to prevent a single event over 5 years was 6.5 (Fig. 1b). The prespecified major secondary end point, cardiovascular death, recurrent myocardial infarction or stroke, was also reduced in those randomized to the active chelation treatment, with a 40% relative risk reduction (hazard ratio 0.60; 95% CI 0.39–0.91; P = 0.017). Patients with diabetes randomized to EDTA chelation treatment had a reduction in recurrent MI (hazard ratio 0.60; 0.48; 95% CI 0.26–0.88; P = 0.015) (Fig. 1c), all-cause mortality (hazard ratio 0.57; 95% CI 0.36–0.88; P = 0.011; Fig. 1d), and coronary revascularization (hazard ratio 0.68; 95% CI 0.47–0.99; P = 0.042; Table 3). When compared with diabetic patients, the patients without diabetes did not have this treatment effect in primary or secondary event outcomes. In patients with diabetes, the Kaplan–Meier curves continued to separate after infusions were completed, a legacy effect unlike most in cardiology, and perhaps supporting the beneficial effect of toxic metal removal by EDTA.
Table 3.
Clinical end points by infusion arms for patients with diabetes mellitus in TACT
| End point | EDTA chelation (n = 322) | Placebo (n = 311) | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Primary end point | 80 (25%) | 117 (38%) | 0.59 (0.44–0.79) | < 0.001 |
| Death | 32 (10%) | 50 (16%) | 0.57 (0.36–0.88) | 0.011 |
| Ml | 16 (5o/o) | 30 (10%) | 0.48 (0.26–0.88) | 0.015 |
| Stroke | 4 (1o/o) | 3 (1%) | 1.19 (0.27–5.30) | 0.829 |
| Coronary | 48 (15%) | 62 (20%) | 0.68 (0.48–0.99) | 0.042 |
| Revascularization | ||||
| Hospitalization for angina | 5 (2%) | 6 (2%) | 0.72 (0.22–2.36) | 0.588 |
| Secondary end point | 35 (11%) | 52 (17%) | 0.60 (0.39–0.91) | 0.017 |
| Cardiovascular death | 19 (6%) | 27 (9%) | 0.63 (0.35–1.13) | 0.118 |
CI, confidence interval; MI, myocardial infarction.
DISCUSSION
In the United States, 23.3 million people have diabetes mellitus [54]. Another 7.2 million are estimated to have undiagnosed diabetes. Diabetes is a key risk factor for cardiovascular disease with a two-fold to three-fold increased likelihood of vascular death. The cause of this excess cardiovascular morbidity and mortality is not completely clear, but the results of TACT inevitably point to mechanistic speculation. The chelation infusion did not treat hyperglycemia. In TACT, there were no differences in mean fasting blood glucose levels from baseline to the 30th infusion in neither the placebo nor the active chelation groups. There were no differences in pharmacotherapy for diabetes between the treatment groups throughout the study. This evidence suggests that although hyperglycemia, the hallmark of diabetes, is a risk factor for cardiovascular disease, it is clearly not the only modifiable risk factor in diabetic patients.
CONCLUSION
Edetate disodium chelation, far from standard therapy, is currently a Class 2B indication in the Stable Ischemic Heart Disease Guidelines [55]. As such, the results of TACT require replication and mechanistic investigation. With this in mind, NIH (NCCIH, NHLBI, NIDDK, NIEHS) has funded the second Trial to Assess Chelation Therapy, TACT2. TACT2 will have identical inclusion criteria as TACT, with the exception of including only diabetic patients post-MI.
TACT2 is currently enrolling with a target sample size of 1200 post-MI diabetic patients. We welcome potentially interested sites, investigators and patients to contact us for more details, or log on to www.tact2.org.
KEY POINTS.
TACT showed a marked reduction in CVD events and all-cause mortality in the subgroup of patients with diabetes.
The results may support the concept that metal chelation reduces metal-catalyzed oxidation reactions that promote the formation of advanced glycation end products.
TACT2, in diabetic post-MI patients, is currently enrolling patients in an effort to replicate these findings, and define a mechanism of benefit.
Acknowledgments
We gratefully acknowledge the advice and critical review of our manuscript by Dr Ana Navas-Acien, MD, PhD and Dr David M. Nathan, MD.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
None.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Lamas GA, Goertz C, Boineau R, et al. Design of the trial to assess chelation therapy (TACT) Am Heart J. 2012;163:7–12. doi: 10.1016/j.ahj.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT Randomized Trial. JAMA. 2013;309:1241–1250. doi: 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪▪.Escolar E, Lamas GA, Mark DB, et al. The effect of an EDTA-based chelation regimen on patients with diabetes and prior myocardial infarction in TACT. Circ Cardiovasc Qual Outcomes. 2014;7:15–24. doi: 10.1161/CIRCOUTCOMES.113.000663. Results of this subgroup analysis of the diabetic population in the TACT trial suggest a novel mechanism and potential new target to treat atherosclerosis in diabetic patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen O. Principles and recent developments in chelation treatment of metal intoxication. Chem Rev. 1999;99:2683–2710. doi: 10.1021/cr980453a. [DOI] [PubMed] [Google Scholar]
- 5.Cranton EM, editor. A textbook on EDTA chelation therapy. 2. Hampton Roads Publishing; 2001. pp. 503–539. [Google Scholar]
- 6.Bellin J, Laszlo D. Metabolism and removal of Ca45 in man. Science. 1953;117:331–334. doi: 10.1126/science.117.3039.331. [DOI] [PubMed] [Google Scholar]
- 7.Clarke NE, Clarke CN, Mosher RE. The in vivo dissolution of metastatic calcium. An approach to atherosclerosis. Am J Med Sci. 1955;229:142–149. doi: 10.1097/00000441-195502000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetra acetic acid. Am J Med Sci. 1956;232:654–666. doi: 10.1097/00000441-195612000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Guldager B, Jelnes R, Jorgensen SJ, et al. EDTA treatment of intermittent claudication - a double-blind, placebo-controlled study. J Intern Med. 1992;231:261–267. doi: 10.1111/j.1365-2796.1992.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Rij AM, Solomon C, Packer SGK, Hopkins WG. Chelation Therapy for intermittent claudication: a double-blind, randomized, controlled trial. Circulation. 1994;90:1194–1199. doi: 10.1161/01.cir.90.3.1194. [DOI] [PubMed] [Google Scholar]
- 11.Lamas GA, Ackermann A. Clinical evaluation of chelation therapy: is there any wheat amidst the chaff? Am Heart J. 2000;140:4–5. doi: 10.1067/mhj.2000.107549. [DOI] [PubMed] [Google Scholar]
- 12.Knudtson ML, Wyse DG, Galbraith PD, et al. Program to Assess Alternative Treatment Strategies to Achieve Cardiac Health (PATCH) Investigators. Chelation therapy for ischemic heart disease. A randomized controlled trial. JAMA. 2002;287:481–486. doi: 10.1001/jama.287.4.481. [DOI] [PubMed] [Google Scholar]
- 13.Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev. 2002;(4):CD002785. doi: 10.1002/14651858.CD002785. [DOI] [PubMed] [Google Scholar]
- 14.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt AM, Hori O, Brett J, et al. Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- 16.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt AM, Hori O, Cao R, et al. RAGE: a novel cellular receptor for advanced glycation end products. Diabetes. 1996;45(Suppl 3):S77–S80. doi: 10.2337/diab.45.3.s77. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder HA, Vinton WH., Jr Hypertension induced in rats by small doses of cadmium. Am J Physiol. 1962;202:515–518. doi: 10.1152/ajplegacy.1962.202.3.515. [DOI] [PubMed] [Google Scholar]
- 19.Carroll RE. The relationship of cadmium in the air to cardiovascular disease death rates. JAMA. 1966;198:267–269. [PubMed] [Google Scholar]
- 20.Hu H, Aro A, Payton M, et al. The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA. 1996;275:1171–1176. [PubMed] [Google Scholar]
- 21.Navas-Acien A, Selvin E, Sharrett AR, et al. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 22.Schober SE, Mirel LB, Graubard BI, et al. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III Mortality Study. Environ Health Perspect. 2006;114:1538–1541. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solenkova NV, Newman JD, Berger JS, et al. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014;168:812–822. doi: 10.1016/j.ahj.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamas GA, Navas-Acien A, Mark DB, Lee KL. Heavy metals, cardiovascular disease, and the unexpected benefits of edetate disodium chelation therapy. J Am Coll Cardiol. 2016;67:2411–2418. doi: 10.1016/j.jacc.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease control (CDC) Preventing Lead Poisoning in Young children: a statement by the Centers for Disease Control. Atlanta, GA: 1991. [Google Scholar]
- 26.Attisha MH, Lachnace J, Sadler RC, Schnepp AC. Elevated blood lead levels in children associated with the flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106:283–290. doi: 10.2105/AJPH.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minot AS. The physiological effects of small amounts of lead: an evaluation of the lead hazard of the average individual. Physiol Rev. 1938;18:554–577. [Google Scholar]
- 28.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Lead. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2007. [Google Scholar]
- 29.Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976;58:260–270. doi: 10.1172/JCI108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters RS, Bryden NA, Patterson KY, et al. EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc. Biol Trace Elem Res. 2001;83:207–221. doi: 10.1385/BTER:83:3:207. [DOI] [PubMed] [Google Scholar]
- 31▪.Arenas IA, Navas-Acien A, Erqui I, Lamas GA. Enhanced vasculotoxic metal excretion in post-MI patients after edetate disodium therapy. J Am Coll Cardiol. 2016;67(13 Suppl):A2125. This study showed that edetate disodium-based infusions markedly enhanced the urinary excretion of lead and cadmium in post-MI patients. [Google Scholar]
- 32.Menke A, Muntner P, Batuman V, et al. Blood lead below 0. 48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 33.Nawrot TS, Staessen JA. Low-level environmental exposure to lead unmasked as silent killer. Circulation. 2006;114:1347–1349. doi: 10.1161/CIRCULATIONAHA.106.650440. [DOI] [PubMed] [Google Scholar]
- 34.Gonick HC, Ding Y, Bondy SC, et al. Lead-induced hypertension: interplay of nitric oxide and reactive oxygen species. Hypertension. 1997;30:1487–1492. doi: 10.1161/01.hyp.30.6.1487. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Vaziri ND, Gonick HC. Lead-induced HTN. II, Response to sequential infusions of l-arginine, superoxide dismutase, and nitroprusside. Environ Res. 1998;76:107–113. doi: 10.1006/enrs.1997.3796. [DOI] [PubMed] [Google Scholar]
- 36.Ding Y, Gonick HC, Vaziri ND. Lead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothelial cells. Am J Hypertens. 2000;13(5 Pt 1):552–555. doi: 10.1016/s0895-7061(99)00226-5. [DOI] [PubMed] [Google Scholar]
- 37.Vaziri N, Ding Y. Effect of lead on nitric oxide synthase expression in coronary endothelial cells: role of superoxide. Hypertension. 2001;37:223–226. doi: 10.1161/01.hyp.37.2.223. [DOI] [PubMed] [Google Scholar]
- 38.Watts SW, Chai S, Webb RC. Lead acetate-induced contraction in rabbit mesenteric artery: interaction with calcium and protein kinase C. Toxicology. 1995;99:55–65. doi: 10.1016/0300-483x(94)03003-k. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Q, Slavkovich V, Zheng W. Lead exposure promotes translocation of protein kinase C activities in rat choroid plexus in vitro, but not in vivo. Toxicol Appl Pharmacol. 1998;149:99–106. doi: 10.1006/taap.1997.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalil-Manesh F, Gonick HC, Weiler EWJ, et al. Lead induced HTN: possible role of endothelial factors. Am J Hypertens. 1993;6:723–729. doi: 10.1093/ajh/6.9.723. [DOI] [PubMed] [Google Scholar]
- 41.Agency for Toxic Substances & Disease Registry (ATSDR) [Accessed 20 November 2017];Toxicological Profile for Cadmium. 2012 Available at: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=48&tid=15.
- 42.Bernhoft RA. Scientific World Journal. 2013. Cadmium toxicity and treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordberg GF, Nogawa K, Nordberg M, Friberg LT. Cadmium. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the Toxicology of Metals. 3. Boston: Elsevier; 2007. pp. 445–486. [Google Scholar]
- 44.Wagner GJ, Yeargan R. Variation in cadmium accumulation potential and tissue distribution of cadmium in tobacco. Plant Physiol. 1986;82:274–279. doi: 10.1104/pp.82.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kagi JH, Himmelhoch SR, Whanger PD, et al. Equine hepatic and renal metallothioneins purification. molecular weight, amino acid composition, and metal content. J Biol Chem. 1974;249:3537–3541. [PubMed] [Google Scholar]
- 46.Nordberg M. General aspects of cadmium: transport, uptake and metabolism by the kidney. Environ Health Perspect. 1984;54:13–20. doi: 10.1289/ehp.845413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, et al. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15:356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messner B, Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals. 2010;23:811–822. doi: 10.1007/s10534-010-9314-4. [DOI] [PubMed] [Google Scholar]
- 49.Messner B, Knoflach M, Seubert A, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanism and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;9:1392–1398. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- 50.Franceschini N, Fry R, Balakrishnan P, et al. Cadmium Body Burden and Increased Blood Pressure in Middle-Aged American Indians: The Strong Heart Study. J Hum Hypertens. 2017;31:225–230. doi: 10.1038/jhh.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:1676–1684. doi: 10.1289/ehp.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menke A, Muntner P, Silbergeld EK, et al. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117:190–196. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Tellez-Plaza M, Guallar E, Howard BV, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–429. doi: 10.1097/EDE.0b013e31828b0631. A large population cohort study analyzing the association of urine cadmium concentration with cardiovascular disease incidence and mortality. In this study, diabetic participants showed stronger associations with most cardiovascular endpoints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 55.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749–1767. doi: 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]