Abstract
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease. Long-term, high-dose glucocorticoid therapy can be used to treat the disease, but the fact that the drug distributes systemically can give rise to severe adverse effects. Here we develop a targeted system for treating RA in which the glucocorticoid prednisolone (PD) is encapsulated within solid lipid nanoparticles (SLNs) coated with hyaluronic acid (HA), giving rise to HA-SLNs/PD. HA binds to hyaluronic receptor CD44, which is over-expressed on the surface of synovial lymphocytes, macrophages and fibroblasts in inflamed joints in RA. As predicted, HA-SLNs/PD particles accumulated in affected joint tissue after intravenous injection into mice with collagen-induced arthritis (CIA), and HA-SLNs/PD persisted longer in circulation and preserved bone and cartilage better than free drug or drug encapsulated in SLNs without HA. HA-SLNs/PD reduced joint swelling, bone erosion and levels of inflammatory cytokines in serum. These results suggest that encapsulating glucocorticoids such as PD in HA-coated SLNs may render them safe and effective for treating inflammatory disorders.
Keywords: Rheumatoid arthritis, hyaluronic acid, solid lipid nanoparticles, drug delivery, prednisolone
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder that results in systemic autoimmune destruction of bone and cartilage. Glucocorticoids are widely used to manage this disease as well as other chronic inflammatory diseases (Smolen & Steiner, 2003). However, these drugs distribute systemically in the body, necessitating the use of sustained high-dose therapy, which often leads to severe side effects, such as muscle weakness, increased risk of infection, growth retardation, and peptic ulcer disease (Yuan et al., 2012).
The intra-articular injection could achieve the most directly targeted therapy, but this administration often needs repeated joint needling that might increase the infection risk and result in the reduction of patient compliance. Moreover, as a polyarthritic and systemic disease, RA often involving all joints of the whole body, and local injection to the inflamed joints is not an optimum selection (Ulmansky et al., 2012). By packaging glucocorticoids into liposomes, nanoparticles, micelles, nanoemulsions, or SLNs could enhance drug accumulation preferentially at target sites (Crielaard et al., 2012). Among these packaging systems, SLNs are particularly attractive because of their good biocompatibility, high drug loading capacity, excellent physical stability, and ability to protect labile drugs from degradation (Wissing et al., 2004). Moreover, the increasing joints-targeting of glucocorticoids-loaded SLNs by systemic administration could improve the blood circulation time, enhance therapeutic effect, and reduce severe systemic toxicity. So far, enveloping glucocorticoids into SLNs has not been extensively investigated in RA therapy. In this paper, we designed to examine the possibility of encapsulating the widely used glucocorticoid PD into SLNs that would target RA-affected tissues.
Activated macrophages are the main effectors of inflammation in RA: they continuously release several pro-inflammatory cytokines, which aggravated the progress of RA (Lee et al., 2007; Yang et al., 2017). CD44 is over-expressed on the surface of synovial lymphocytes, macrophages, and fibroblasts in inflamed joints in RA (Nedvetzki et al., 1999), and this protein promotes RA progression by facilitating inflammatory cell migration and activation of various effector cells (Wang & Sun, 2017). HA, a naturally charged linear polysaccharide, binds to CD44 and has shown excellent biocompatibility, biodegradability and selectivity for targeting drugs to inflammatory cells (Naor & Nedvetzki, 2003; Shen et al., 2015). Therefore, we wanted to design SLNs coated with HA to ensure that the glucocorticoid cargo would be released selectively at inflamed tissues.
In order for these coated SLNs to reach inflammation sites, we relied on the demonstrated ability of SLNs to undergo ‘extravasation via leaky vasculature followed by inflammatory cell sequestration’ (ELVIS) (Wang et al., 2016; Li et al., 2017; Wang & Sun, 2017), a process analogous to the classical enhanced permeability and retention (EPR) effect that allows encapsulated anticancer drugs to accumulate in solid tumors (Sun et al., 2011; Li et al., 2015; Si et al., 2016). Our hypothesis was that HA-SLNs/PD reached to inflamed tissue through the natural ELVIS effect, then HA could combine with CD44 on the surface of macrophage, releasing glucocorticoid to affected tissues, thereby reducing systemic drug toxicity and improving therapeutic efficacy.
In this paper, we studied the drug release and stability of HA-SLNs/PD particles in vitro. Then, the biodistribution of HA-SLNs/PD in vivo was observed. We also investigated the therapeutic effect of HA-SLNs/PD particles after intravenous injection into mice with CIA.
2. Materials and methods
2.1. Materials
PD was obtained from Henan Lihua Pharmaceutical Co. Ltd. (Henan, China) with purity of 99.0%. Hyaluronic acid (molecular weight, 300 kDa) was provided by Shandong Freda Biochem Co. (Shandong, China). Bovine Type II collagen and complete or incomplete Freund’s adjuvant were obtained from Chondrex (Washington, DC); Cholesterol (Chol), soy phosphatidylcholine (SPC), and glyceryl monostearate (GMS), from Taiwei Pharmaceutical Co., Ltd (Shanghai, China); Dimethyldioctadecylammonium bromide (DDAB), from Sigma-Aldrich (St. Louis, MO). All other chemicals and reagents were obtained commercially and were of analytical grade.
2.2. Cell lines
RAW264.7 cells were obtained from the American Type Culture Collection (Rockville, MD) and cultured in Dulbecco's Modified Eagle Medium (DMEM; Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (Hyclone) and 100 IU/mL penicillin and 100 μg/mL streptomycin. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2, and the medium was changed every other day.
2.3. Animals
Male DBA/1 mice (6 weeks old, 20 ± 2 g) were obtained from Beijing Huafukang Co. Ltd (Beijing, China). All animal experiments were approved by the Animal Ethics Committee of Southwest Medical University, and all procedures with animals were conducted according to the guidelines of the Local Animal Use and Care Committee of Luzhou and the National Animal Welfare Law of China.
2.4. Preparation of PD-loaded solid lipid nanoparticles (SLNs/PD)
SLNs/PD was prepared by film ultrasonication. Briefly, GMS (7.5 mg), SPC (7.5 mg), Chol (5 mg), DDAB (5.5 mg), and PD (1 mg) were dissolved in chloroform, and the solution was added to a round-bottom flask. Solvent was removed by distillation under low pressure, and the resulting film was sonicated (80 W, 60 s) in the presence of 5 mL of ultrapure water. The resulting SLNs/PD were stored at 4 °C.
2.5. Determination of entrapment efficiency and drug loading
Encapsulation efficiency (EE) and drug loading (DL) were measured using the sodium citrate ultracentrifugation method. The desired amount of SLNs/PD was dispersed in 10% (w/v) sodium citrate solution and centrifuged for 40 min at 40,000 rpm (Beckman-Coulter, Brea, CA) to separate free PD from SLNs/PD. The same amount of SLNs/PD was dissolved in methanol, and the amount of PD was determined by high-performance liquid chromatography (HPLC; Agilent, Santa Clara, CA) using a Kromasil ODS-1 C18 column (150 × 4.6 mm, 5 µm) and a mobile phase of acetonitrile–water.
2.6. Coating SLNs/PD with hyaluronic acid (HA-SLNs/PD)
HA-SLNs/PD was prepared based on electrostatic attraction. Briefly, 1 mL of a 0.02% (w/v) solution of hyaluronic acid (300 kDa) was slowly added under vigorous stirring to a dispersion of SLNs/PD in a 12:1 (w/w) ratio. The resulting preparation was stored at 4 °C.
2.7. Characterization of SLNs/PD and HA-SLNs/PD
Size of SLNs/PD and HA-SLNs/PD (n = 3) in ddH2O (pH 7.4) was measured using a Zetasizer Nano ZS90 (Malvern Instruments, UK). The morphology of HA-SLNs/PD was also analyzed using transmission electron (TEM). Stability of HA-SLNs/PD in vitro was examined by storing them at 4 or 37 °C for 8 Days and periodically measuring size. Release of drug from HA-SLNs/PD was investigated in vitro using dialysis. Initially, 1 mL of free PD in solution (0.2 mg/mL) and the same volume of SLNs/PD or HA-SLNs/PD were loaded into three separate dialysis bags. The bags were incubated at 37 °C with constant shaking at 100 rpm, and at selected times, 300 μL of outer dialysis solution was withdrawn and analyzed by HPLC as described above, while the same amount of fresh buffer was immediately added to the outer dialysis solution. Drug release was determined for all samples within 100 h (n = 3).
2.8. Uptake of SLNs/PD and HA-SLNs/PD in vitro
We investigated uptake of SLNs/PD or HA-SLNs/PD by RAW264.7 cells. Cells were cultured overnight in 12-well plates (5 × 105 cells per well) in the absence or presence of LPS (100 ng/mL), then two preparations were added into the cultures, which were incubated another 1 h. Cells were washed with phosphate-buffered saline (PBS) three times and analyzed by HPLC.
2.9. Tissue distribution and bioavailability of free drug, SLNs/PD and HA-SLNs/PD in a mouse model ofcollagen-induced arthritis
Male DBA/1 mice were acclimated to their housing for at least 1 week, and then subcutaneously injected at the base of the tail with 100 μL of an emulsion of 2 mL bovine type II collagen (1 mg/mL) in 2 mL of complete Freund’s adjuvant (1 mg/mL). On day 21 after the first injection, animals received a booster injection in the tail, followed by subcutaneous injection at the base of the tail with 50 μL of an emulsion of 1 mL of bovine type II collagen in 1 mL of incomplete Freund’s adjuvant. On day 50 after the first set of injections, an articular index (AI) score was calculated for the hind paws of each mouse. Scores were calculated as follows: 0, no signs of swelling or erythema; 1, minor erythema and/or swelling; 2, moderate edema and signs of tarsal involvement; 3, remarkable edema, limited use of the joint and signs extending to the metatarsals; and 4, excessive edema with joint rigidity and serious signs involving the entire hind paw.
Mice with an AI score >4 points were used in subsequent experiments to analyze the in vivo distribution of free drug, SLNs/PD and HA-SLNs/PD. In these experiments, mice received a single intravenous injection of free drug, SLNs/PD or HA-SLNs/PD at a dose of 15 mg PD/kg body weight. At 0.25, 0.5, 1, 2, and 4 h after injection, mice were sacrificed, blood was sampled via the eye sockets, and plasma, various organs (heart, liver, spleen, lung, and kidney) as well as entire hind limbs were immediately harvested. Six animals per treatment condition were sacrificed at each time point. Samples were analyzed on a Shimadzu analyzer (Chiyoda-Ku, Kyoto, Japan) equipped with a 50-μL injector loop, CTO-10 A column thermostat, two LC-10AT pumps and an SPD-10 A UV detector. Separations were carried out on a Diamonsil C18 reverse-phase column (150 × 4.6 mm, 5 μm) protected by a Shimadzu Shim-Pack guard column. Elution was performed using a 70-to-30% gradient of 50 mM trisodium citrate adjusted to pH 4.10 using phosphoric acid. The detector monitored the signal at 254 nm. To further evaluate the joint targeting property of SLNs/PD and HA-SLNs/PD, the parameters including relative uptake efficiency (Rejoint), concentration efficiency (Cejoint) were defined as follows:
2.10. Therapeutic efficacy of SLNs/PD and HA-SLNs/PD in a mouse model of collagen-induced arthritis
Arthritic mice were injected in the tail vein with saline, free drug, SLNs/PD, or HA-SLNs/PD at a dose of 15 mg PD/kg body weight (n = 5 for each condition). The first injection was delivered on the same day as the booster injection of collagen emulsion, and thereafter once every three days for a total of four injections. Healthy DBA/1 mice were maintained in parallel as a normal control group. Once every three days, the mice were weighed and inflammation in their joints was assessed as described. AI scores for the different extremities were calculated as described (Wang & Goldring, 2011), and the individual scores were summed to obtain an overall score for each animal (total possible score =16 points).
At the end of this observation period, three mice were randomly selected from each of the four treatment groups and from the normal control group. These mice were sacrificed, blood was sampled via the eye socket, and serum was quickly harvested and stored at −40 °C until use. Hind limbs were fixed in 4% paraformaldehyde for 3 days, decalcified with 10% EDTA decalcifying fluid for 15 days, embedded in paraffin, cut into sections 5 μm thick, and stained with hematoxylin and eosin for histological evaluation. The following signs of joint inflammation were scored using the scales indicated in parentheses (Liu et al., 2010; Shin et al., 2014): synovial cell lining hyperplasia (0–2), villous hyperplasia (0–2), polymorphonuclear leukocyte infiltration in periarticular soft tissue (0–3), cellular infiltration and bone erosion at astragalus and distal tibia (0–3), and cartilage cell infiltration (0–1). The researcher scoring the sections (JT) was blind to treatment group.
2.11. Statistical analysis
All quantitative data were expressed as mean ± SD from triplicate measurements, unless otherwise noted. Differences between treatment groups were assessed for significance using single-factor analysis of variance (ANOVA), followed by Student’s t-test. Significance was determined at the following thresholds: *p < .05, **p < .01, ***p < .001.
3. Results and discussion
3.1. Characteristics of SLNs/PD and HA-SLNs/PD
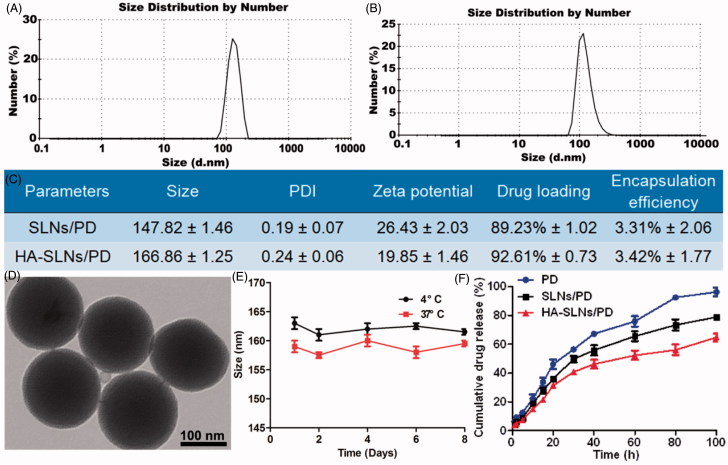
We coated SLNs with hyaluronic acid through electrostatic attraction, a simple yet controllable method that avoids the chemical reactions and organic solvents needed for covalently attaching hyaluronic acid to the nanoparticle surface. Cationic SLNs/PD was prepared using film ultrasonication. Average size was 147.8 ± 1.46 nm; zeta potential, 26.4 ± 2.03 mV; and PDI, 0.19 ± 0.07 (Figure 1(A,B)). The DL and EE of SLNs/PD were, respectively, 89.23 ± 1.02% and 3.31 ± 2.06%. Simply mixing hyaluronic acid and SLNs/PD (1:10, w/w) generated HA-SLNs/PD through electrostatic interactions. These complete particles had an average size of 166.86 ± 1.25 nm, zeta potential of 19.8 ± 1.46 mV, and PDI of 0.24 ± 0.06. They showed high DL (92.61% ± 0.73) and EE (3.42% ± 1.77) (Figure 1(C)). Transmission electron microscopy showed that the average size of HA-SLNs/PD was approximately 150 nm (Figure 1(D)).
Figure 1.
Size distribution of SLNs/PD (A) and HA-SLNs/PD (B) based on dynamic light scattering. (C) Encapsulation efficiency and drug loading yield of SLNs/PD and HA-SLNs/PD (n = 3). (D) Transmission electron micrographs of HA-SLNs/PD. Bar, 100 nm. (E) In vitro stability of HA-SLNs/PD at 4 °C or 37 °C (n = 3). (F) In vitro cumulative PD release profiles of free PD, SLNs/PD and HA-SLNs/PD at 37 °C in PBS (pH 7.4) (n = 5).
Average size of HA-SLNs/PD remained nearly unchanged over 6 Days at 4 or 37 °C (Figure 1(E)). These results suggest good colloidal stability. Drug release from SLNs/PD and HA-SLNs/PD was analyzed using dynamic dialysis. PD was released much more slowly from HA-SLNs/PD than from SLNs/PD or a solution of free drug. During the first 30 h, 56.22% of drug was released from the free drug solution, compared to only 37.6% from HA-SLNs/PD. These results indicate that encapsulating PD in HA-SLNs can slow drug release at 37 °C and pH 7.4 (Figure 1(F)).
3.2. Uptake of SLNs/PD and HA-SLNs/PD in vitro
Uptake of the particles by non-activated or LPS-activated Raw264.7 macrophages was analyzed by HPLC (Figure 2). The results showed that the uptake of HA-SLNs/PD was much higher than SLNs/PD when the RAW264.7 cells were activated with LPS. The reason for this phenomenon is that hyaluronic acid coating could help facilitate CD44 receptor-mediated endocytosis of the SLNs into target cells.
Figure 2.
HPLC analysis showing uptake of SLNs/PD or HA-SLNs/PD in Raw264.7 cells with or without LPS (100 ng/mL) activation (mean ± SD, n = 3). *p < .05.
3.3. Biodistribution of HA-SLNs/PD in arthritic mice
In order to reduce the need for long-term, high-dose systemic glucocorticoid therapy in RA (Baschant et al., 2012; Krasselt & Baerwald, 2014), we developed SLNs coated with hyaluronic acid that can persist in the circulation and accumulate at sites of inflammation, where they release their drug cargo.
The concentration of PD in circulation decreased with time in arthritic mice injected with either free drug or HA-SLNs/PD. At 4 h after injection, animals injected with free drug showed significantly lower plasma drug concentrations than animals injected with HA-SLNs/PD (Figure 3). These results suggest that HA-SLNs/PD persist for long periods in circulation.
Figure 3.
In vivo biodistribution of free PD, SLNs/PD and HA-SLNs/PD in mice with collagen-induced arthritis. Mice were injected with either preparation via the tail vein and sacrificed at 0.5, 1, 2 and 4 h afterwards. Samples of plasma, heart, liver, spleen, lung, kidney, and joints were immediately collected and analyzed by HPLC. Data shown are mean ± SD (n = 5). *p < .05 vs mice treated with free PD or SLNs/PD.
As shown as Figure 3, the PD concentration in joints of SLNs/PD group was higher than that of free PD group after intravenous injection, revealing that packaging PD into SLNs could enhance the drug accumulation into arthritic joints. Furthermore, the PD level in joints of HA-SLNs/PD group was significantly higher than both free PD and SLNs/PD group at all times points under tested. These results suggested that coating SLNs with HA could help facilitate the SLNs/PD selectively accumulation into arthritic joints.
Coating our SLNs with HA led to much greater accumulation in inflamed joint tissue than the uncoated SLNs, this largely due to the ‘extravasation via leaky vasculature followed by inflammatory cell sequestration’ (ELVIS) effect and the ability of HA to bind CD44 specifically in inflamed joints.
3.4. Bioavailability of HA-SLNs/PD in mouse plasma and joints
As shown as Figure 4, compared to PD group, the area under the curve (AUC0–t) and the maximal concentration (Cmax) of SLNs/PD group were enhanced in joints and in plasma. Moreover, the results showed that the bioavailability of HA-SLNs/PD in joints was higher than that of PD. The AUC0–t and Cmax of HA-SLNs/PD were 2.62- and 1.93-fold higher than those of PD, respectively (Figure 4). In addition, AUC0–t, plasma was 2.35-fold higher for HA-SLNs/PD than for PD, Cmax, plasma was 1.90-fold higher, and T1/2 in plasma was 1.49-fold longer (Figure 5). Furthermore, significantly higher AUC0–t and Cmax values of HA-SLNs/PD than SLNs/PD in joints and in plasma was observed. All these results indicated that capsuled PD with SLNs could improve the bioavailability. However, coating SLNs with HA help further enhanced the bioavailability both in joints and in plasma.
Figure 4.
Concentration-vs-time curves (A) and pharmacokinetic parameters (B) of PD, SLNs/PD and HA-SLNs/PD in joints after intravenous injection into arthritis mice (n = 5).
Figure 5.
Concentration-vs-time curves (A) and pharmacokinetic parameters (B) of PD, SLNs/PD and HA-SLNs/PD in plasma after intravenous injection into arthritis mice (n = 5).
3.5. Therapeutic efficacy of SLNs/PD and HA-SLNs/PD in vivo
Arthritic mice were treated by tail vein injection with saline, PD, SLNs/PD, or HA-SLNs/PD, and then evaluated for changes in body weight, mean arthritis scores, and histology scores. Histological sections of joints in the right hind limb were analyzed under the microscope. Healthy, untreated animals were also analyzed as a control.
Body weight in mice injected with HA-SLNs/PD increased continuously from day 21 (booster injection) to day 30, similar to body weight in healthy, untreated mice (Figure 6(A)). Mean AI scores were lowest in animals treated with HA-SLNs/PD (Figure 6(B)). In the joints of mice treated with saline, histological examination of right hind limbs revealed massive synovial cell lining, extensive pannus formation, and severe bone destruction (Figure 6(C)); the histopathology score was highest (> 7.0) in this group. Animals treated with free drug showed abundant synovial cell lining and significant bone destruction; the histopathology score for this group was similar to that of the saline-treated group. Animals treated with SLNs/PD showed less synovial cell lining and less severe bone destruction, with a histopathology score of 4.0. Mice treated with HA-SLNs/PD showed minor pannus formation and obvious bone protection, with a histopathology score of 2.0.
Figure 6.
Therapeutic efficacy of HA-SLNs/PD in vivo. (A) Body weight in different treatment groups. Data are mean ± SD (n = 5). (B) Mean arthritis scores were calculated for the joints in the right hind limb following different treatments. Data shown are mean ± SD (n = 5). (C) Photomicrographs of histological sections of ankle joints from animals after different treatments. Arrows indicate finger-like pannus formation; asterisks, bone destruction. Bar, 100 nm.
3.6. Effects of HA-SLNs/PD on pro-inflammatory cytokine production in vivo
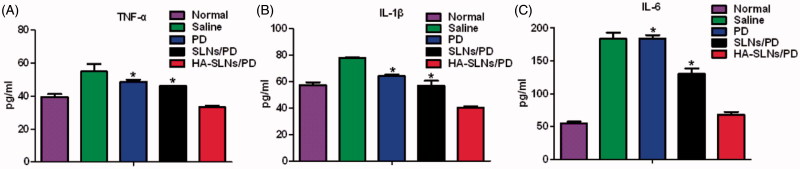
Several pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 contribute to RA pathogenesis and drive RA progression (Feldmann et al., 1996; Sattar et al., 2003; McInnes & Schett, 2007). In fact, their levels in serum can be used as surrogate markers of therapeutic efficacy for anti-arthritis drugs. We measured their levels in arthritic mice treated with HA-SLNs/PD as another index of therapeutic efficacy. Levels of all three cytokines in serum were significantly lower in animals treated with HA-SLNs/PD than in animals treated with free drug or SLNs/PD (*p < .05, Figure 7(A–C)). These results indicate greater therapeutic efficacy with HA-SLNs/PD than with free drug or SLNs/PD. This effect depended on the hyaluronic acid coating, since SLNs/PD reduced cytokine levels to a much smaller extent.
Figure 7.
Production of pro-inflammatory cytokines in arthritic mice treated with saline, free PD, SLNs/PD, or HA-SLNs/PD. Healthy, untreated animals served as a control (Normal). Serum levels of (A) TNF- α, (B) IL-1β, and (C) IL-6 were assayed. Data shown are mean ± SD (n = 5). *p < .05 vs animals treated with free PD or SLNs/PD. CIA, mice with collagen-induced arthritis.
4. Conclusion
Here we describe a novel delivery system of HA-SLNs/PD that remains stable in circulation for long periods and that selectively accumulates in inflamed tissue, where the drug is selectively released. This targeted drug delivery system shows good therapeutic efficacy in a mouse model of collagen-induced arthritis, based on analysis of joints, pannus formation, bone preservation, and measurements of pro-inflammatory cytokines. In fact, the therapeutic effects of HA-SLNs/PD are superior to those observed with SLNs/PD and PD, consistent with the ability of HA-SLNs/PD to target arthritic joints and release their drug cargo selectively at those locations. This drug delivery system shows potential for targeted, glucocorticoid-based treatment of RA, and it may be adaptable to treating other inflammatory diseases.
Funding Statement
This work was supported by the Fund of Sichuan Province Science and Technology Agency Innovation Talent Project [2017RZ0048, 2017].
Acknowledgements
The authors are thankful for the financial support of the Fund of Sichuan Province Science and Technology Agency Innovation Talent Project (2017RZ0048, 2017).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Baschant U, Lane NE, Tuckermann J. (2012). The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol 8:645–55. [DOI] [PubMed] [Google Scholar]
- Crielaard BJ, Rijcken CJ, Quan L, et al. (2012). Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis. Angew Chem 124:7366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. (1996). Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14:397–440. [DOI] [PubMed] [Google Scholar]
- Krasselt M, Baerwald C. (2014). The current relevance and use of prednisone in rheumatoid arthritis. Expert Rev Clin Immunol 10:557–71. [DOI] [PubMed] [Google Scholar]
- Lee MK, Lim SJ, Kim CK. (2007). Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials 28:2137–42. [DOI] [PubMed] [Google Scholar]
- Li HM, Fu Y, Zhang T, et al. (2015). Rational design of polymeric hybrid micelles with highly tunable properties to co-deliver MicroRNA-34a and vismodegib for melanoma therapy. Adv Funct Mater 25:7457–69. [Google Scholar]
- Li CH, Li HM, Wang Q, et al. (2017). pH-sensitive polymeric micelles for targeted delivery to inflamed joints. J Control Release 246:133–41. [DOI] [PubMed] [Google Scholar]
- Liu XM, Quan L, Tian J, et al. (2010). Syntheses of click PEG-Dexamethasone conjugates for the treatment of rheumatoid arthritis. Biomacromolecules 11:2621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Schett G. (2007). Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7:429–42. [DOI] [PubMed] [Google Scholar]
- Nedvetzki S, Walmsley M, Alpert E, et al. (1999). CD44 involvement in experimental collagen-induced arthritis (CIA). J Autoimmun 13:39–47. [DOI] [PubMed] [Google Scholar]
- Naor D, Nedvetzki S. (2003). CD44 in rheumatoid arthritis. Arthritis Res Ther 5:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, McCarey DW, Capell H, McInnes IB. (2003). Explaining how “High-Grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 108:2957–63. [DOI] [PubMed] [Google Scholar]
- Shen HX, Shi SJ, Zhang ZR, et al. (2015). Coating solid lipid nanoparticles with hyaluronic acid enhances antitumor activity against melanoma stem-like cells. Theranostics 5:755–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JM, Kim SH, Thambi T, et al. (2014). A hyaluronic acid-methotrexate conjugate for targeted therapy of rheumatoid arthritis. Chem Commun (Camb) 50:7632–5. [DOI] [PubMed] [Google Scholar]
- Si J, Shao S, Shen Y, Wang K. (2016). Macrophages as active nanocarriers for targeted early and adjuvant cancer chemotherapy. Small 12:5108–17. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Steiner G. (2003). Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov 2:473–88. [DOI] [PubMed] [Google Scholar]
- Sun TM, Du JZ, Yao YD, et al. (2011). Simultaneous delivery of siRNA and paclitaxel via a “Two-in-One” micelleplex promotes synergistic tumor suppression. Acs Nano 5:1483–94. [DOI] [PubMed] [Google Scholar]
- Ulmansky R, Turjeman K, Baru M, et al. (2012). Glucocorticoids in nano-liposomes administered intravenously and subcutaneously to adjuvant arthritis rats are superior to the free drugs in suppressing arthritis and inflammatory cytokines. J Control Release 160:299–305. [DOI] [PubMed] [Google Scholar]
- Wang D, Goldring SR. (2011). The bone, the joints and the Balm of Gilead. Mol Pharm 8:991–3. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun X. (2017). Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater Sci 5:1407–20. [DOI] [PubMed] [Google Scholar]
- Wang Q, Jiang JY, Chen WF, et al. (2016). Targeted delivery of low-dose dexamethasone using PCL-PEG micelles for effective treatment of rheumatoid arthritis. J Control Release 230:64–72. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Kayser O, Muller RH. (2004). Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev 56:1257–72. [DOI] [PubMed] [Google Scholar]
- Yuan F, Quan LD, Cui L, et al. (2012). Development of macromolecular prodrug for rheumatoid arthritis. Adv Drug Deliv Rev 64:1205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Feng X, Ding J, et al. (2017). Nanotherapeutics relieve rheumatoid arthritis. J Control Release 252:108–24. [DOI] [PubMed] [Google Scholar]