Abstract
Purpose
The RPE cells have a major role in the development of dry age-related macular degeneration (AMD). We present novel evidence that βA3/A1-crystallin, encoded by the Cryba1 gene, a protein known to be important for lysosomal clearance in the RPE, also has a role in epithelial-to-mesenchymal transition (EMT) of RPE cells.
Methods
RPE from dry AMD globes, genetically engineered mice lacking Cryba1 globally or specifically in the RPE, spontaneous mutant rats (Nuc1) with a loss-of-function mutation in Cryba1, and the melanoma OCM3 cell line were used. Spatial localization of proteins was demonstrated with immunofluorescence, gene expression levels were determined by quantitative PCR (qPCR), and protein levels by Western blotting. Cell movement was evaluated using wound healing and cell migration assays. Co-immunoprecipitation was used to identify binding partners of βA3/A1-crystallin.
Results
βA3/A1-crystallin is upregulated in polarized RPE cells compared to undifferentiated cells. Loss of βA3/A1-crystallin in murine and human RPE cells resulted in upregulation of Snail and vimentin, downregulation of E-cadherin, and increased cell migration. βA3/A1-crystallin binds to cortactin, and loss of βA3/A1-crystallin resulted in increased P-cortactinY421. The RPE from AMD samples had increased Snail and vimentin, and decreased E-cadherin, compared to age-matched controls.
Conclusions
We introduced a novel concept of dry AMD initiation induced by lysosomal clearance defects in the RPE and subsequent attempts by RPE cells to avoid the resulting stress by undergoing EMT. We demonstrate that βA3/A1-crystallin is a potential therapeutic target for AMD through rejuvenation of lysosomal dysfunction and potentially, reversal of EMT.
Keywords: crystallin, lysosomes, EMT, dry AMD, autophagy
Crystallins, the highly abundant proteins of the lens, are essential determinants of the transparency and refractivity required for lens function.1–3 Initially thought to be lens-specific and to have evolved as lens proteins, crystallins are now known to be recruited to the lens from proteins that existed before lenses evolved.3 Crystallins are expressed outside of the lens and most have been shown to have cellular functions distinct from their roles as structural elements in the lens. For, βA3/A1-crystallin, one member of the β/γ-crystallin family, it appears that its “nonlens” function is mediated via signaling pathways.4,5
In the past several years, we explored possible functions for the polypeptides (βA3- and βA1-crystallins) encoded by Cryba1, one of the six β-crystallin genes. In a spontaneous rat mutant (Nuc1)6–10 and genetically engineered mouse models (Cryba1 knockout [KO]11–13 and Cryba1 conditional [RPE-specific] knockout [cKO]),14,15 a striking age-related macular degeneration (AMD)-like phenotype with compromised lysosome-mediated clearance12,13,15 was observed. Our findings suggested that βA3/A1-crystallin localizes to the lysosomal lumen of RPE cells and binds with the V0 subunit of V-ATPase, the proton pump responsible for endo-lysosomal acidification.8,14 βA3/A1-crystallin also regulates amino acid transport via the proton-assisted amino acid transporter 4 (PAT4)/solute-linked carrier 36A4 (SLC36A4).13 Therefore, it has an important role in the complex process of recruiting and assembling the mechanistic target of rapamycin, complex 1 (mTORC1) signaling platform to the lysosomal surface.13,14 As a result, βA3/A1-crystallin influences the clearance functions of lysosomes, both phagocytosis and autophagy.
Lysosomal efficiency declines with age, and this decline has been implicated in age-related diseases, such as Parkinson's and Huntington's diseases, and recently, AMD.5,16–18 In dry AMD, lysosomal dysfunction may drive RPE cells into epithelial-mesenchymal transition (EMT) to survive a stressful microenvironment. Different forms of EMT are associated with three distinct biological settings, with varying functional consequences. While, type 1 EMT has a role during development and type 3 EMT occurs in most cancers, type 2 EMT is associated with wound healing and tissue regeneration.19 It now is well documented that in AMD, some RPE cells appear to degenerate, losing normal cell shape, exhibiting migratory behavior, and losing their epithelial function.20,21 This degeneration is especially evident in the transition zone of geographic atrophy (GA), the advanced dry form of AMD.22 Previous studies have described these RPE cells as severely dysmorphic, often multilayered, with migration into the retina and sub-RPE space.23 While described classically as “degeneration,” a closer examination of these “degenerating” cells suggests that some are not dying, but instead may have transformed into mesenchymal cells to survive the harsh microenvironment during disease progression.23–25 While cells undergoing Type 2 EMT would lose critical epithelial function, they also become resistant to cell death.26 Since EMT is reversible, these cells are logical targets for novel therapies aimed at reversing dry AMD. Such treatments would greatly benefit patients who currently have very limited prevention or treatment options.
We report that βA3/A1-crystallin is highly expressed in polarized, differentiated (RPE) cells, but is not detected in undifferentiated cells, and further, that the absence of βA3/A1-crystallin causes RPE cells to display molecular and functional features of type 2 EMT. Therefore, βA3/A1-crystallin, through its regulatory role on lysosomes, may influence EMT in the RPE, and may offer a novel approach to therapy for AMD.
Materials and Methods
Human Samples
Fresh postmortem eyes obtained from the Portland, Oregon Eye Bank or the National Disease Research Interchange (Philadelphia, PA, USA) were processed within 14 hours after death. Donor information has been summarized previously.27 The disease conditions were determined by medical record, and the globes were examined further by an experienced retinal physician with expertise in AMD (JTH). The retinas were defined as normal when there were no abnormalities observed using a dissecting microscope. Early-stage AMD was defined by the presence of any RPE pigmentary changes and/or large-size drusen (>125 μm diameter). Late-stage AMD was defined by areas of geographic atrophy due to loss of the RPE. We only included dry AMD and excluded wet AMD. Under direct visualization with a dissecting microscope, the RPE was mechanically separated from the choroid and used for Western analysis.
All research including human samples followed the tenets of the Declaration of Helsinki, informed consent was obtained from the study subjects and the research was conducted under protocols approved by the respective institutional review boards.
Generation of Cryba1 cKO and Cryba1 KO Animals
βA3/A1-crystallin cKO (Cryba1 cKO) and corresponding Cryba1fl/fl control mice were generated as described previously.14 Cryba1 complete KO mice were generated as explained previously and wild type (WT) mice were used as controls.11 All studies including animals were performed in adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and under approved Institutional Animal Care and Use Committee (IACUC) protocols.
Human RPE Cell Culture
Human RPE cells were isolated from postmortem fetal eyes (gestational age 16–18 weeks) obtained from Advanced Bioscience Resources, Inc. (Alameda, CA, USA) and cultured according to published protocols.28,29 Primary cultures of nonpolarized RPE cells were established as described previously.30,31 Isolation of nonpolarized and polarized cells were performed according to the previously described protocol.28
Culture of OCM3 Cell Line
OCM3 is a human primary uveal melanoma cell line that is fibroblastic in nature with adherent properties.32 These cells were cultured following a previously reported method.33 The cells were grown in RPMI medium with 10% fetal bovine serum (FBS), HEPES buffer, sodium pyruvate, penicillin/streptomycin, and L-glutamine to 90% confluency.
RNA Isolation and Real-Time RT-PCR
Total RNA was isolated and cDNA was generated as described previously.13 Gene expression levels were normalized relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and reported as fold change over controls. The primer sequences used were as follows: βA3/A1-crystallin, 5′- CCCTGAAGGTGGAAAGTGG-3′ and 5′-CATCCCAGCGAGGGTATTC-3′; and GAPDH, 5′-CCACATCGCTCAGAACACC-3′ and 5′-GCCCAATACGACCAAATCC-3′. The mRNA expression of mouse Snai1 and Snai2 genes was evaluated by using Taqman probes, for which the Hprt gene was used as an internal control.13
Microarray Analysis
Microarray was performed on cultured nonpolarized and polarized RPE cells with a mean TER of 385 ± 13.1 Ω·cm2 grown to confluency in RtEGM (Retinal Pigment Epithelial Cell Growth Medium; Lonza, Walkersville, MD, USA) with 1% FBS. Total RNA was isolated using RNeasy kit (Qiagen, Germantown, MD, USA). Analysis was performed using a commercial array service (Agilent Expression Array; Agilent, Tokyo, Japan).
Immunofluorescence Staining and Immunohistochemistry
Human RPE cells were cultured in 4-well multichamber slides. Cells were subjected to immunofluorescence staining using primary βA3/A1-crystallin goat polyclonal antibody (Santa Cruz Biotechnology, Dallas, TX, USA). Mouse RPE flat mounts (Cryba1fl/fl, Cryba1 cKO, Cryba1 KO, and WT) from freshly enucleated eyes were immunostained as described previously.13,14 Images were acquired by a Zeiss LSM 710 (Carl Zeiss Meditec, Jena, Germany) confocal workstation.
Transfection Studies
Cryba1 siRNA or Cryba1 pcDNA was transfected using a previously reported protocol.14
Co-Immunoprecipitation
Co-immunoprecipitation was performed using the Pierce Co-Immunoprecipitation Kit (Thermo Fisher Scientific, Waltham, MA, USA).12,13 RPE-choroid preparations from seven rats of each genotype (WT and Nuc1) were sonicated in IP Lysis/Wash Buffer (provided in the kit) with 1% protease inhibitors (Millipore Sigma, St. Louis, MO, USA) and the procedure was performed according to manufacturer's instructions.
Western Blot Analysis
Lysates were prepared from RPE-choroid preparations from freshly dissected mice (Cryba1fl/fl, Cryba1 cKO), rat (WT and Nuc1), normal, and AMD RPE cells from human tissues, fetal human RPE cell cultures, and OCM3 cell line. Total protein (50 μg) was loaded for fetal human RPE cell culture and 10 μg protein was loaded for the other samples. Western blots were performed as described previously.12,13 The primary antibodies used in this study were βA3/A1-crystallin (Cat# sc-22398; Santa Cruz Biotechnology and Cat# ab151722; Abcam, Cambridge, MA, USA) as well as a polyclonal antibody developed in our laboratory and previously characterized,7 E-cadherin (Cat# 610181, BD Biosciences, Franklin Lakes, NJ, USA), Vimentin (Cat# 550513; BD Biosciences and Cat# MA5-11883; Thermo Fisher Scientific, Waltham, MA, USA), β-Catenin (Cat# ab16051; Abcam), Cortactin (Cat# ab33333; Abcam), phospho(Y421)-cortactin (Cat# 4569S; Cell Signaling Technology, Danvers, MA, USA), Snail1 (Cat# 14-9859-82; Invitrogen, Carlsbad, CA, USA), and Actin (Cat# A2066; Sigma Aldrich Corp., St. Louis, MO, USA). Secondary antibodies were peroxidase labeled goat anti-rabbit (Cat# 074-1506; KPL, Gaithersburg, MD, USA) and peroxidase labeled goat anti-mouse (Cat# 074-1806; KPL).
Wound Healing Assay
Wound healing assay to assess the migration of OCM3 cells was performed following previously reported methods.34
Cell Migration Assay
Cell migration assay was performed by following the manufacturer's instructions for the Cell Migration Assay Kit (M1000; Biocolor Ltd, Carrickfergus, United Kingdom).
Statistics
Statistical analysis was performed using Microsoft Excel and GraphPad 5.0 software. The P values were determined using the Mann-Whitney U test in at least four biological replicates representative of three independent experiments. Multiple comparisons were made using the Kruskal-Wallis test. Significance was defined as P ≤ 0.05. Results are presented as mean ± SD. Each biological replicate has at least three technical replicates.
Results
βA3/A1-Crystallin Is Expressed in Polarized RPE Cells
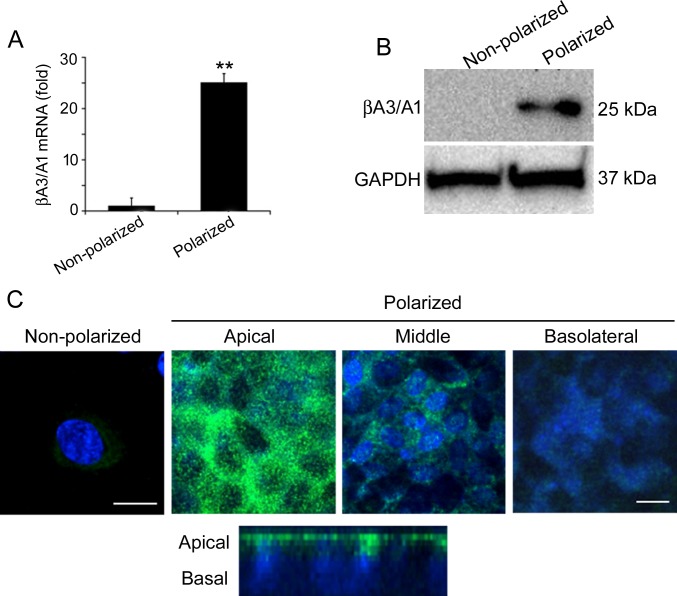
A variety of RPE cell functions are dependent on its polarized state, including trafficking of ions, fluid, and metabolites across the RPE monolayer.35 Recently it has been shown that polarized RPE cells exhibit distinct proteomes on the apical and basal plasma membranes.35 We report the expression profile of βA3/A1-crystallin in polarized and nonpolarized human fetal RPE cells.36 The differentiation of cultured fetal human RPE cells was assessed by the detection of RPE-65, a marker of terminal differentiation in the RPE, which was 6.8 times higher in polarized than nonpolarized fetal human RPE cells (data not shown). Using microarray analysis, the expression of 12 crystallin genes, from the α- and β/γ-crystallin families was compared in polarized and nonpolarized fetal human RPE cells (Table). While most crystallins showed little, if any, increased expression in polarized cells, βA3/A1-crystallin expression was increased 23.75-fold compared to nonpolarized cells (Table). RNA (Fig. 1A) and protein levels (Fig. 1B) were significantly increased in polarized fetal human RPE cells as validated by quantitative RT-PCR (qRT-PCR) and Western analysis, respectively. Our data also showed that αB-crystallin, a small heat shock protein with known regulatory function in the RPE,37 did not show significant expression change with polarization.
Table.
Gene Expression of Crystallins in Polarized RPE Cells Normalized to Nonpolarized RPE Cells Determined by Microarray Analysis
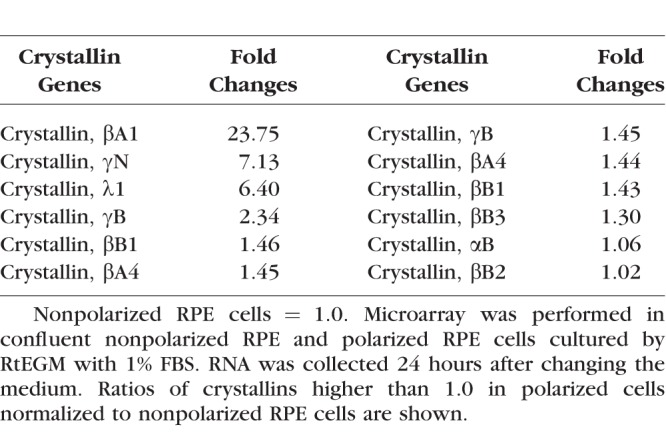
Figure 1.
βA3/A1-crystallin is differentially expressed in nonpolarized and polarized human RPE cells. (A, B) Polarization of RPE cells results in a highly significant increase in the mRNA and protein expression of βA3/A1-crystallin, compared to nonpolarized RPE cells. All values are represented as mean ± S.D. **P < 0.01 (Mann-Whitney U test), n = 4. (C) The distribution of βA3/A1-crystallin in polarized cultured human RPE cells as seen by confocal microscopy (upper) indicates that βA3/A1-crystallin is predominantly localized to the apical region of RPE cells. This was confirmed by a confocal cross-section image (lower). Scale bar: 20 μm for nonpolarized cells, 60 μm for polarized cells.
Immunofluorescence studies showed that βA3/A1-crystallin was localized predominantly to the apical side of polarized fetal human RPE cells in contrast with nonpolarized RPE cells, which have very weak expression of βA3/A1-crystallin that was distributed throughout the cytosol (Fig. 1C). Cultured RPE cells from WT mouse or rats only express detectable βA3/A1-crystallin when the culture is confluent and the cells are polarized (data not shown).
Loss of Cryba1 Leads to EMT in RPE Cells
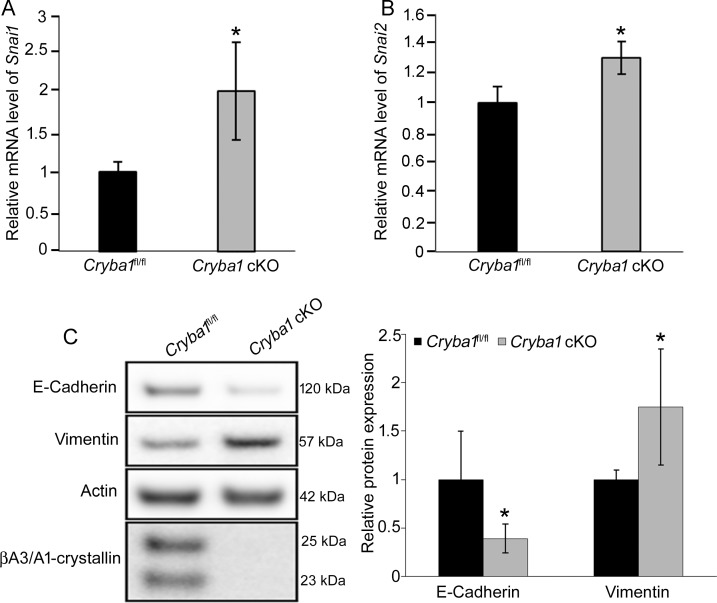
EMT is a process that allows a polarized epithelial cell, which normally interacts with its basement membrane via its basal surface, to assume a mesenchymal cell phenotype, conferring increased migratory capacity, invasiveness, resistance to apoptosis, and production of ECM (extracellular matrix) components.38,39 It is known that RPE cells undergoing EMT assume a mesenchymal morphology, lose cell-cell adhesion, deposit ECM proteins, and migrate to the retina or subduct into Bruch's membrane.39,40 In EMT, the transcription factor Snail1 is associated with upregulation of vimentin, an intermediate filament protein that is expressed in mesenchymal cells, and downregulation of E-cadherin within tight junctions despite its potential for mosaic expression in certain epithelial cells, such as the RPE.41–45 Therefore, we investigated whether βA3/A1-crystallin might have a role in the onset of EMT in RPE cells. RPE cells from Cryba1 cKO mice have elevated mRNA expression of the EMT transcription factors Snai1 and Snai2 (Figs. 2A, 2B), elevated Vimentin protein expression, and loss of E-cadherin (Fig. 2C).
Figure 2.
Cryba1 is involved in the onset of EMT in the mouse RPE cells. (A, B) The mRNA levels of Snai1 and Snai2 were increased in the RPE from 5-month-old Cryba1 cKO mice compared to age-matched Cryba1fl/fl controls, as assessed by qRT-PCR. Hprt was used as internal reference gene. (C) Western blotting revealed reduced E-cadherin and increased vimentin with no noticeable expression of βA3/A1-crystallin in 5-month-old Cryba1 cKO RPE cells compared to age-matched Cryba1fl/fl RPE cells. Actin was used as internal control. The graphs show mean ± SD and *P < 0.05 (Mann-Whitney U test), n = 4.
Dynamic reorganization of the actin cytoskeleton is a prerequisite for the migration and invasion of cells undergoing EMT.46 This led us to evaluate whether the actin cytoskeleton in the RPE of Cryba1 KO mice was reorganized compared to that in WT controls. We found that staining for the high-affinity filamentous actin probe Phalloidin13 indicated significant change in the size and shape of RPE cells from Cryba1 KO compared to WT mice (Fig. 3A).
Figure 3.
Cryba1 regulates cytoskeletal organization during EMT. (A) RPE flat mounts prepared from the eyes of 20-month-old Cryba1 KO and WT mice were stained with phalloidin (green) and DAPI (blue). Phalloidin staining revealed changes in the shape of Cryba1 KO RPE cells compared to WT control mice (white arrows). Scale bar: 20 μm. (B) RPE-choroid-sclera flat mounts prepared from Cryba1 cKO and Cryba1fl/fl control mice were stained with anti-β-catenin antibody (red) and 4′,6-diamidino-2-phenylendole (DAPI; blue). At 13 months of age, Cryba1 cKO RPE cellular architecture shows highly variable geometry, where some cells are enlarged while, others show significantly smaller size (asterisk in c and d) at central and peripheral regions of the RPE layer compared to Cryba1fl/fl control mice (a, b). In control RPE cells, β-catenin is located mainly at the cell membrane, especially at the central region of the RPE layer; while in cKO cells, β-catenin is not restricted to the plasma membrane, but also is located in the cytoplasm (arrowheads). Double-layer staining also is seen in some cKO cells (arrows in c). At 23 months of age, there is noticeable enlargement of cells especially at the peripheral region of RPE layer (arrows in g and h). Scale bar: 20 μm.
In normal epithelial tissues with high E-cadherin expression, β-catenin is sequestered at the membrane, preventing it from being released into the cytoplasm and entering the nucleus to activate genes required for cell proliferation and survival.43 It also is known that complexes of cadherin and catenins connect adjacent epithelial cells through adherens junctions, which then link to the actin cytoskeleton via α-catenin and its interacting protein, α-actinin.43 However, in proliferating or migrating cells, due to decreased expression of cadherins (E-cadherin), the sequestration of β-catenin to the membrane is lost, enabling its translocation to the nucleus.43 We observed that in floxed controls, β-catenin was predominantly localized to the cell membrane, especially in the central region of the RPE cell, while in the Cryba1 cKO mice, it was distributed at the plasma membrane and in the cytoplasm (Fig. 3B).
Loss of βA3/A1-Crystallin Induces EMT by Acquisition of Vimentin and Migratory Properties
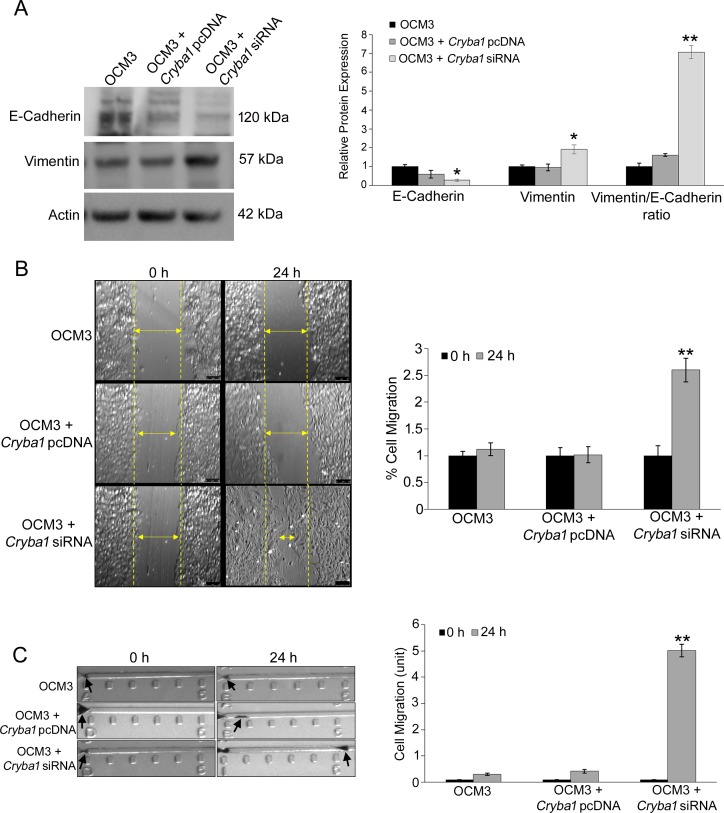
It is well documented that cancer cells have intrinsic migratory properties and are susceptible to EMT.19 Thus, as a model system for evaluating how βA3/A1-crystallin might modulate EMT in cells, we used uveal melanoma cells (OCM3),33 which are susceptible to EMT and express βA3/A1-crystallin (Supplementary Fig. S1). When OCM3 cells were transfected with Cryba1 short interfering RNA (siRNA) to knockdown the expression of βA3/A1-crystallin8 (Supplementary Fig. S2), we observed loss of E-cadherin and acquisition of vimentin (Fig. 4A), two critical steps in EMT. EMT is a cellular differentiation regime and is associated with cellular migration.47 We sought to evaluate whether loss of βA3/A1-crystallin could potentiate migration in the OCM3 cells undergoing EMT. We performed Cryba1 siRNA silencing studies to downregulate βA3/A1-crystallin and found that there was increased migration of the cells as evident from wound healing (Fig. 4B) and horizontal cellular migration experiments (Fig. 4C), further emphasizing the fact that loss of Cryba1 is responsible for EMT-induced cell migration. As a control, we attempted the converse experiment to upregulate expression of βA3/A1-crystallin by transfecting with the expression vector Cryba1 pcDNA. Since this did not generate any increase in βA3/A1-crystallin (not shown), probably because OCM3 cells already are producing it, this had no effect on EMT-related parameters (Figs. 4A, 4C).
Figure 4.
Knockdown of Cryba1 triggers EMT-like phenotype in ocular melanoma cells. (A) Knockdown of Cryba1 (OCM3 cells transfected with Cryba1 siRNA) resulted in decrease in E-cadherin expression with an increase in vimentin expression, while overexpression of βA3/A1-crystallin (OCM3 cells transfected with Cryba1 pcDNA) did not cause any changes in the expression of these proteins in OCM3 cells. Representative Western blot images are shown. The graphs show mean ± S.D; *P < 0.05, **P < 0.001 (Mann-Whitney U test), n = 4. (B) Wound healing assay revealed that OCM3 cells transfected with Cryba1 siRNA showed increased wound healing after 24 hours of exposure compared to OCM3 cells transfected with Cryba1 pcDNA. The migration rate of control cells was taken as 1% and healing rate of other plates were compared with respect to control cells. (C) Increased cell migration is evident after 24 hours in Cryba1 siRNA transfected cells compared to cells transfected with Cryba1 pcDNA, suggesting the role of βA3/A1-crystallin in inhibiting ocular melanoma cell migration. The migration strips are divided into 5 units (each unit = 100 μm), black arrows indicate the cluster of cells. The migration was quantified under microscope and replicated thrice for each group. The data are represented as ‘units' migrated. All values are mean + SD and **P < 0.01 indicates significant change compared to Cryba1 siRNA transfected OCM3 cells at 0 hours.
Association of Cortactin and βA3/A1-Crystallin Is Necessary for EMT-Induced Migration
To further delineate how βA3/A1-crystallin controls EMT-mediated migration, we re-evaluated our previous proteome high-throughput microarray data to identify proteins associated with βA3/A1-crystallin.13 We identified cortactin, a protein known to have a major role in cell migration.48 Co-immunoprecipitation assay using OCM3 cells supported the microarray data and demonstrated that βA3/A1-crystallin was associated with cortactin (Fig. 5A). Further, we observed significant increase in p-CortactinY421 expression in OCM3 cells transfected with Cryba1 siRNA (Fig. 5B), with no change in the total cortactin level (Fig. 5B). Cortactin, a substrate of sarcoma (Src) kinases, is an actin-binding protein that is involved in cytoskeletal regulation.48 The tyrosine phosphorylation (Y421) of cortactin increases its turnover at focal adhesions to promote cell motility.49 Binding to the actin-related protein 2/3 (Arp2/3) complex stimulates cortactin activity, which promotes F-actin nucleation and assembly.48 Cortactin promotes cancer cell migration and invasion, and has a pivotal role in invadopodia formation and ECM degradation.48 The cortactin N-terminus associates with F-actin, while its C-terminus interacts with focal adhesions, dynamic protein complexes through which the cytoskeleton of a cell connects to the ECM.49
Figure 5.
Association between βA3/A1-crystallin and cortactin regulates cell migration. (A) Pull-down assay using antibody to βA3/A1-crystallin demonstrates that cortactin and βA3/A1-crystallin interact in OCM3 lysates. (B) Western blot analysis revealed that phosphorylated (Y421) cortactin is present at a higher level in cultured OCM3 cells transfected with Cryba1 siRNA compared to untreated control. (C) Pull down assay followed by Western blot showed increased cortactin and βA3/A1-crystallin association in the RPE+Choroid from WT (control) compared to Nuc1 (mutation in Cryba1 gene, forming nonfunctional βA3/A1-crystallin protein) rats. (D) Western blotting demonstrated increased expression of p-cortactinY421 in RPE+Choroid from Nuc1 rat compared to WT, with no change in the total cortactin level. These results suggested that mutated βA3/A1-crystallin loses its ability to bind cortactin and the loss of this association triggers phosphorylation of cortactin at Y421 resulting in actin disassembly and cell migration. All values are mean ± S.D and are evaluated from three independent experiments. *Denotes significant change (P < 0.05) with respect to control OCM3 cells or RPE cell lysates from WT rats (Mann-Whitney U test), n = 4.
To demonstrate the involvement of βA3/A1-crystallin and the cortactin complex in RPE cell migration, we performed a co-immunoprecipitation assay and evaluated the expression of p-CortactinY421 in RPE+Choroid lysates harvested from spontaneous mutant Nuc1 rats, which lack functional βA3/A1-crystallin. We found that there was decreased association of cortactin and βA3/A1-crystallin (Fig. 5C), along with increased phosphorylation of cortactin (Fig. 5D) in the RPE+Choroid of Nuc1 compared to WT rats. Taken together, our data suggested that functional βA3/A1-crystallin is required for regulating cortactin phosphorylation, which may be a critical regulatory mechanism in migration of RPE cells.
The RPE of Nonneovascular AMD Expresses EMT Markers
Finally, to provide molecular evidence of EMT in human dry AMD, we found that the RPE from nonneovascular AMD eyes had increased Snail1 and vimentin and decreased E-cadherin, as detected by Western analysis compared to age-matched controls (Fig. 6), which is consistent with the onset of EMT.
Figure 6.
Prevalence of EMT-like phenotype in the RPE cells of human AMD tissues. RPE cell lysates from human AMD samples showed increased Snail1 and vimentin expression concomitant with decreased expression of E-cadherin compared to age-matched controls. Representative Western blot images are shown. The graphs show mean ± SD and *P < 0.05 (Mann-Whitney U test), n = 4.
Discussion
AMD is a major cause of vision loss in the elderly.50 With the increasing human life span, the prevalence of aging-related diseases, including AMD, is increasing.51 Despite this growing population of afflicted individuals, no definitive treatment other than the Age-Related Eye Disease Study (AREDS) II formulation for intermediate AMD, or prevention for dry AMD currently is available. Therefore, a new treatment that targets dry AMD before significant loss of vision occurs would have great benefit for patients.
The RPE, which is critically important to retinal homeostasis, is a central cell type involved in dry AMD pathobiology.27 As AMD progresses, some RPE cells appear to degenerate while others appear to be normal. The morphologic changes observed are characteristic of type 2 EMT, a process that can be activated by oxidative stress-induced impairment of autophagy and lysosomal function.52–54 EMT is a transcriptional program allowing cells to survive stresses, such as oxidative stress, but at the expense of losing their specialized function. We showed that the RPE obtained from AMD patients and genetically engineered mouse models with an AMD-like phenotype express key molecular markers of EMT. RPE dysfunction undeniably drives AMD pathophysiology.27,50 Since EMT is reversible and cells in EMT are resistant to death, targeting RPE cells in EMT is a promising novel strategy to reverse AMD pathobiology and ultimately stabilize vision.
EMT in the RPE of Cryba1 deficient mice probably is due to impaired autophagy, as we have shown previously that autophagy flux and autophagy-mediated clearance is compromised in these mice.14 In RPE cells, βA3/A1-crystallin is absolutely essential for normal lysosomal-mediated clearance by the processes of autophagy and phagocytosis.13,14 The literature indicates that lysosomal dysfunction is involved in AMD since autophagosome clearance and autophagy flux are significantly impaired in tissue sections from human donor AMD eyes,55 and circulating autoantibodies from AMD patients point to compromised autophagy.56 In fact, autophagy can prevent EMT by clearing Snail and Twist.57 In differentiated hepatocytes, autophagy degrades Snail while lysosomal inhibition impairs the autophagic clearance of Snail and Twist to cause EMT.57,58 It is highly likely that in our genetically engineered mice, impaired autophagic clearance also leads to upregulation of Snail, as shown in this study. Moreover, our results suggest that βA3/A1-crystallin is required for regulating cortactin phosphorylation, which is critical for quality control (QC) autophagy.59 QC autophagy has been shown to selectively remove toxic protein aggregates and damaged organelles, the accumulation of which may lead to various forms of neurodegenerative disease.60 Since our previous and recent data suggested that βA3/A1-crystallin is a key component of starvation-induced and QC autophagy, it is highly likely that loss of this protein in RPE cells is linked to EMT.
In summary, we introduced a new concept regarding one aspect of dry AMD pathology. We believe that RPE cells take refuge from the stressful microenvironment produced by oxidative insult via initiation of Type 2 EMT, a mechanism that the cells adopt to avoid cell death. However, in AMD patients,61,62 as well as in our genetically engineered animal models,15 the cells ultimately die due to the chronic nature of the insult as the disease progresses. The RPE may switch on EMT due to malfunctioning of various pathways/processes that are required to maintain homeostasis of the postmitotic RPE cells. One such process that is immensely important for RPE cell survival is lysosomal-mediated clearance by phagocytosis and autophagy. It now is becoming increasingly clear that defects in these clearance processes can contribute to AMD. We suggest that there is a defined window of time during which patients suffering from dry AMD may be amenable to treatment aimed at reversing the subpopulation of RPE that have entered EMT. While malfunction of a myriad of proteins involved in the cellular processes that maintain healthy RPE can lead to such dysfunction, our data indicated that βA3/A1-crystallin is an attractive target for rejuvenation of lysosomal function, and potentially, for reversal of EMT. Thus, it may provide a novel and effective means of preventing or delaying the progression of the disease.
Supplementary Material
Acknowledgments
The authors thank Arkasubhra Ghosh, MSc, PhD, Head of GROW Research Laboratory, Narayana Nethralaya Foundation, India for critical reading and discussions regarding this manuscript, David R. Hinton, MD, Keck School of Medicine of University of Southern California for his expert advice, and Laura Asnaghi, PhD, Wilmer Eye Institute, The Johns Hopkins University School of Medicine for the OCM3 cell line.
Supported by University of Pittsburgh start-up funds (DS), Jennifer Salvitti Davis, M.D. Chair in Ophthalmology (DS), BrightFocus, Inc. Foundation (DS), RPB/IRRF Catalyst Award for Innovative Research Approaches for AMD (DS), National Institutes of Health (NIH; Bethesda, MD, USA) Grants EY019037-S (DS) and NIH R01EY027691 (JTH), Macular Degeneration Foundation (JTH), Research to Prevent Blindness (Wilmer Eye Institute) and Wilmer Core Grant EY001765. JTH is the Robert Bond Welch Professor.
Disclosure: S. Ghosh, None; P. Shang, None; H. Terasaki, None; N. Stepicheva, None; S. Hose, None; M. Yazdankhah, None; J. Weiss, None; T. Sakamoto, None; I.A. Bhutto, None; S. Xia, None; J.S. Zigler Jr, None; R. Kannan, None; J. Qian, None; J.T. Handa, None; D. Sinha, None
References
- 1.Slingsby C., Wistow GJ. Functions of crystallins in and out of lens: roles in elongated and post-mitotic cells. Prog Biophys Mol Biol. 2014;115:52–67. doi: 10.1016/j.pbiomolbio.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piatigorsky J. Gene Sharing and Evolution: The Diversity of Protein Functions. Cambridge, MA: Harvard University Press;; 2007. [Google Scholar]
- 4.Zigler JS, Jr,, Sinha D. βA3/A1-crystallin: more than a lens protein. Prog Retin Eye Res. 2015;44:62–85. doi: 10.1016/j.preteyeres.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha D., Valapala M., Shang P., et al. Lysosomes: regulators of autophagy in the retinal pigmented epithelium. Exp Eye Res. 2016;144:46–53. doi: 10.1016/j.exer.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha D., Klise A., Sergeev Y., et al. βA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol Cell Neurosci. 2008;37:85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zigler JS, Jr,, Zhang C., Grebe R., et al. Mutation in the βA3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J Cell Sci. 2011;124:523–531. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valapala M., Hose S., Gongora C., et al. Impaired endolysosomal function disrupts Notch signalling in optic nerve astrocytes. Nat Commun. 2013;4:1629. doi: 10.1038/ncomms2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma B., Sen T., Asnaghi L., et al. βA3/A1-Crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways through the PKD/Bit1-signaling axis. Cell Death Dis. 2011;2:e217. doi: 10.1038/cddis.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C., Asnaghi L., Gongora C., et al. A developmental defect in astrocytes inhibits programmed regression of the hyaloid vasculature in the mammalian eye. Eur J Cell Biol. 2011;90:440–448. doi: 10.1016/j.ejcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valapala M., Edwards M., Hose S., et al. βA3/A1-crystallin is a critical mediator of STAT3 signaling in optic nerve astrocytes. Sci Rep. 2015;5:8755. doi: 10.1038/srep08755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh S., Shang P., Yazdankhah M., et al. Activating the AKT2-nuclear factor-κB-lipocalin-2 axis elicits an inflammatory response in age-related macular degeneration. J Pathol. 2017;241:583–588. doi: 10.1002/path.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang P., Valapala M., Grebe R., et al. The amino acid transporter SLC36A4 regulates the amino acid pool in retinal pigmented epithelial cells and mediates the mechanistic target of rapamycin, complex 1 signaling. Aging Cell. 2017;16:349–359. doi: 10.1111/acel.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valapala M., Wilson C., Hose S., et al. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valapala M., Edwards M., Hose S., et al. Increased lipocalin-2 in the retinal pigment epithelium of Cryba1 cKO mice is associated with a chronic inflammatory response. Aging Cell. 2014;13:1091–1094. doi: 10.1111/acel.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vilchez D., Saez I., Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun. 2014;5:5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- 17.Guha S., Coffey EE, Lu W., et al. Approaches for detecting lysosomal alkalinization and impaired degradation in fresh and cultured RPE cells: evidence for a role in retinal degenerations. Exp Eye Res. 2014;126:68–76. doi: 10.1016/j.exer.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golestaneh N., Chu Y., Xiao YY, et al. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8:e2537. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R., Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radeke MJ, Radeke CM, Shih YH, et al. Restoration of mesenchymal retinal pigmented epithelial cells by TGFβ pathway inhibitors: implications for age-related macular degeneration. Genome Med. 2015;7:58. doi: 10.1186/s13073-015-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura K., Orita T., Liu Y. Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-γ agonist. J Mol Med (Berl) 2015;93:749–758. doi: 10.1007/s00109-015-1289-8. [DOI] [PubMed] [Google Scholar]
- 22.Zanzottera EC, Ach T., Huisingh C., Messinger JD, Freund KB, Curcio CA. Visualizing retinal pigment epithelium phenotypes in the transition to geographic atrophy in age-related macular degeneration. Retina. 2016;36(suppl 1):S12–S25. doi: 10.1097/IAE.0000000000001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 24.Guidry C., Medeiros NE, Curcio CA. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:267–273. [PubMed] [Google Scholar]
- 25.Zanzottera EC, Messinger JD, Ach T., Smith RT, Curcio CA. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:3269–3278. doi: 10.1167/iovs.15-16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tennakoon AH, Izawa T., Kuwamura M., Yamate J. Pathogenesis of type 2 epithelial to mesenchymal transition (EMT) in renal and hepatic fibrosis. J Clin Med. 2015;5:E4. doi: 10.3390/jcm5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Zibetti C., Shang P., et al. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat Comm. 2018;9:1364. doi: 10.1038/s41467-018-03856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonoda S., Spee C., Barron E., Ryan SJ, Kannan R., Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonoda S., Sreekumar PG, Kase S., et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY) 2009;2:28–42. doi: 10.18632/aging.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa K., Sreekumar PG, Spee C., et al. αB-Crystallin regulates subretinal fibrosis by modulation of epithelial-mesenchymal transition. Am J Pathol. 2016;186:859–873. doi: 10.1016/j.ajpath.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sreekumar PG, Zhou J., Sohn J., et al. N-(4-hydroxyphenyl) retinamide augments laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2008;49:1210–1220. doi: 10.1167/iovs.07-0667. [DOI] [PubMed] [Google Scholar]
- 32.Rajaii F., Asnaghi L., Enke R. The demethylating agent 5-Aza reduces the growth, invasiveness, and clonogenicity of uveal and cutaneous melanoma. Invest Ophthalmol Vis Sci. 2014;55:6178–6186. doi: 10.1167/iovs.14-13933. [DOI] [PubMed] [Google Scholar]
- 33.Asnaghi L., Gezgin G., Tripathy A., et al. EMT-associated factors promote invasive properties of uveal melanoma cells. Mol Vis. 2015;21:919–929. [PMC free article] [PubMed] [Google Scholar]
- 34.Chang CW, Hsieh YH, Yang WE, Yang SF, Chen Y., Hu DN. Epigallocatechingallate inhibits migration of human uveal melanoma cells via downregulation of matrix metalloproteinase-2 activity and ERK1/2 pathway. Biomed Res Int. 2014;2014:141582. doi: 10.1155/2014/141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khristov V., Wan Q., Sharma R., Lotfi M., Maminishkis A., Bharti K. Polarized human retinal pigment epithelium exhibits distinct surface proteome on apical and basal plasma membranes. Methods Mol Biol. 2018;1722:223–247. doi: 10.1007/978-1-4939-7553-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshihara N., Terasaki H., Shirasawa M., et al. Permeability and anti-vascular endothelial growth factor effects of bevacizumab, ranibizumab, and afliercept in polarized retinal pigment epithelial layer in vitro. Retina. 2017;37:179–190. doi: 10.1097/IAE.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 37.Fort PE, Lampi KJ. New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp Eye Res. 2011;92:98–103. doi: 10.1016/j.exer.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S., Zhan M., Wang J. Epithelial-to-mesenchymal transition in gallbladder cancer: from clinical evidence to cellular regulatory networks. Cell Death Discov. 2017;3:17069. doi: 10.1038/cddiscovery.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mony S., Lee SJ, Harper JF, Barwe SP, Langhans SA. Regulation of Na,K-ATPase β1-subunit in TGF-β2-mediated epithelial-to-mesenchymal transition in human retinal pigmented epithelial cells. Exp Eye Res. 2013;115:113–122. doi: 10.1016/j.exer.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JW, Kang KH, Burrola P., Mak TW, Lemke G. Retinal degeneration triggered by inactivation of PTEN in the retinal pigment epithelium. Genes Dev. 2008;22:3147–3157. doi: 10.1101/gad.1700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z., Rayala S., Nguyen D., Vadlamudi RK, Chen S., Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 42.Myong NH. Loss of E-cadherin and acquisition of vimentin in epithelial-mesenchymal transition are noble indicators of uterine cervix cancer progression. Korean J Pathol. 2012;46:341–348. doi: 10.4132/KoreanJPathol.2012.46.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Katsunuma S., Honda H., Shinoda T., et al. Synergistic action of nectins and cadherins generates the mosaic cellular pattern of the olfactory epithelium. J Cell Biol. 2016;212:561–575. doi: 10.1083/jcb.201509020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desai RA, Gao L., Raghavan S., Liu WF, Chen CS. Cell polarity triggered by cell-cell adhesion via E-cadherin. J Cell Sci. 2009;122:905–911. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun BO, Fang Y., Li Z., Chen Z., Xiang J. Role of cellular cytoskeleton in epithelial-mesenchymal transition process during cancer progression. Biomed Rep. 2015;3:603–610. doi: 10.3892/br.2015.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai JH, Yang J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin M., Ma W., An L. Cortactin in cancer cell migration and invasion. Oncotarget. 2017;8:88232–88243. doi: 10.18632/oncotarget.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W., Liu Y., Liao K. Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 2011;12:49. doi: 10.1186/1471-2121-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ambati J., Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong WL, Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 52.Krstić J., Trivanović D., Mojsilović S., Santibanez JF. Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. 2015;2015:654594. doi: 10.1155/2015/654594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Z., Zhao J., Xue J., Zhao X., Liu P. Autophagy inhibition promotes epithelial-mesenchymal transition through ROS/HO-1 pathway in ovarian cancer cells. Am J Cancer Res. 2016;6:2162–2177. [PMC free article] [PubMed] [Google Scholar]
- 54.Li G., Li CX, Xia M., et al. Enhanced epithelial-to-mesenchymal transition associated with lysosome dysfunction in podocytes: role of p62/Sequestosome 1 as a signaling hub. Cell Physiol Biochem. 2015;35:1773–1786. doi: 10.1159/000373989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitter SK, Song C., Qi X., et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iannaccone A., Giorgianni F., New DD, et al. Circulating autoantibodies in age-related macular degeneration recognize human macular tissue antigens implicated in autophagy, immunomodulation, and protection from oxidative stress and apoptosis. PLoS One. 2015;10:e0145323. doi: 10.1371/journal.pone.0145323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catalano M., D'Alessandro G., Lepore F., et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol Oncol. 2015;9:1612–1625. doi: 10.1016/j.molonc.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grassi G., Di Caprio G., Santangelo L., et al. Autophagy regulates hepatocyte identity and epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions promoting Snail degradation. Cell Death Dis. 2015;6:e1880. doi: 10.1038/cddis.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JY, Yao TP. Quality control autophagy: a joint effort of ubiquitin, protein deacetylase and actin cytoskeleton. Autophagy. 2010;6:555–557. doi: 10.4161/auto.6.4.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao TP. The role of ubiquitin in autophagy-dependent protein aggregate processing. Genes Cancer. 2010;1:779–786. doi: 10.1177/1947601910383277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanus J., Anderson C., Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 2015;24:286–298. doi: 10.1016/j.arr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaarniranta K., Tokarz P., Koskela A., Paterno J., Blasiak J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol Toxicol. 2017;33:113–128. doi: 10.1007/s10565-016-9371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.