Abstract
Circulating exosomes in bodily fluids such as blood are being actively studied as a rich source of chemical biomarkers for cancer diagnosis and monitoring. Although nucleic acid analysis is a primary tool for the discovery of circulating biomarkers in exosomes, metabolomics holds the potential of expanding the chemical diversity of biomarkers that may be easy and rapid to detect. However, only trace amounts of exosomes can be isolated from a small volume of patient blood, and thus a very sensitive technique is required to analyze the metabolome of exosomes. In this report, we present a workflow that involves multiple cycles of ultracentrifugation for exosome isolation using a starting material of 2 mL of human serum, freeze – thaw-cycles in 50% methanol/water for exosome lysis and metabolite extraction, differential chemical isotope labeling (CIL) of metabolites for enhancing liquid chromatography (LC) separation and improving mass spectrometry (MS) detection, and nanoflow LC-MS (nLC-MS) with captivespray for analysis. As a proof-of-principle, we used dansylation labeling to analyze the amine- and phenol-submetabolomes in two sets of exosome samples isolated from the blood samples of five pancreatic cancer patients before and after chemotherapy treatment. The average number of peak pairs or metabolites detected was 1964 ± 60 per sample for a total of 2446 peak pairs (n = 10) in the first set and 1948 ± 117 per sample for a total of 2511 peak pairs (n = 10) in the second set. There were 101 and 94 metabolites positively identified in the first and second set, respectively, and 1580 and 1590 peak pairs with accurate masses matching those of metabolites in the MyCompoundID metabolome database. Analyzing the mixtures of 12C-labeled individual exosome samples spiked with a 13C-labeled pooled sample which served as an internal standard allowed relative quantification of metabolomic changes of exosomes of blood samples collected before and after treatment.
Graphical Abstract

Exosomes are small (30 – 120 nm) extracellular vesicles that play an important role in intercellular communication and transmission of macromolecules between cells.1,2 For example, blood exosomes contain miRNAs,3,4 mRNAs,5 proteins,6 –9 and small molecules, which are secreted into the bloodstream to travel throughout the body and transmit their cargo to other cells.10 Consequently, exosomes are important contributing factors in the development of several diseases, including cancer.11 – 13 Blood exosomes can be easily obtained by routine blood draws for liquid biopsies. Therefore, they are one of the most promising sources for discovering cancer biomarkers for early detection and diagnosis as well as therapeutic monitoring.14 – 16
Most blood-exosome-related studies to date have focused on the analysis of RNA17 and proteins.18 – 22 Because of the possibility of scale-up in culturing cells, lipids23 – 26 and metabolites27 – 29 have been analyzed in exosomes isolated from cancer cell lines. However, to our knowledge, there is no report of an untargeted metabolomics study of blood exosomes with high coverage. The main issue lies in the sensitivity of most analytical platforms, which is not sufficient to realize high-coverage profiling of the exosome metabolome from the limited amount of material generally available after isolation. The total amount of exosomes is generally less than 1 fig in several milliliters of a patient-serum sample. Most commonly used metabolomics platforms such as microflow LC-MS, GC-MS, and NMR cannot provide sufficient sensitivity for comprehensive metabolomic profiling of exosomes.
In a previous study, we developed a chemical isotope labeling nanoflow-liquid-chromatography technique coupled with captivespray-ionization mass spectrometry (CIL nLC-MS) to profile the metabolome of small numbers of breast cancer cells.30 Even with the use of 100 cells as the starting material, we were able to detect thousands of metabolites. Encouraged by this high performance of analyzing trace amounts of sample, we set out to develop a metabolomics workflow for analyzing the small amount of exosome material obtained from patient serum. In this report, we describe the workflow tailored to exosome isolation and analysis using ultracentrifugation and CIL nLC-MS, demonstrate the possibility of profiling exosome metabolites with unprecedented coverage, and show a comparative analysis of pancreatic cancer patient samples obtained before and after chemotherapy.
EXPERIMENTAL SECTION
Workflow
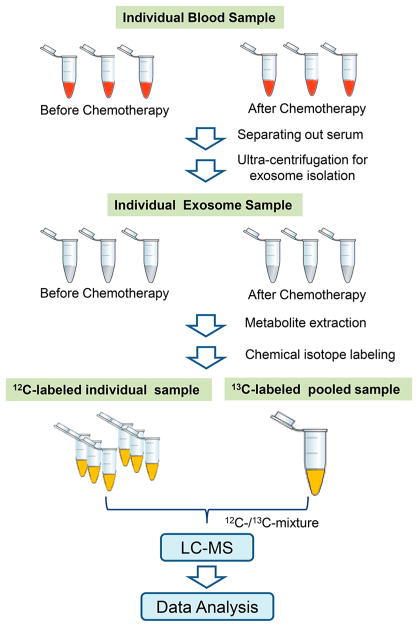
The overall workflow for exosome isolation and metabolomic profiling is shown in Figure 1. Two sets of whole blood samples from pancreatic cancer patients were collected before and after chemotherapy (n = 10 for each set). The serum was separated out by centrifugation. After dilution, multiple cycles of ultracentrifugation were performed to pellet the exosomes from serum. The pellets were cleaned by PBS to remove serum metabolites. The exosomes were then lysed and extracted with 50% MeOH and freeze – thaw cycles (five times). Each sample was divided into two aliquots: one was the sample and the other was for generating a pooled sample. Each individual sample was labeled with 12C-dansyl chloride (DnsCl, the light tag), and the pooled sample was labeled with 13C-DnsCl (the heavy tag), which served as an internal standard. The 12C-dansyl-labeled individual sample and 13C-dansyl-labeled pool were mixed together. The mixture was injected onto nanoflow LC-MS. The light- and heavy-labeled metabolites showed up as peak pairs with a 2.0067 Da difference in the mass spectra for single-tag-labeled metabolites. The relative ratio of metabolites could be determined by using the heavy peak as reference. Univariate and multivariate analyses were performed, and metabolites were identified or matched by searching against different libraries in MyCom-poundID (MCID, www.mycompoundid.org).31
Figure 1.
Workflow for exosome metabolomics based on CIL nLC-MS.
Serum Samples
Whole blood samples were obtained at the University of Michigan Hospital, Ann Arbor, Michigan, with Institutional Review Board approval. These included samples from 10 patients with locally advanced pancreatic cancer prior to treatment and after 3 weeks of chemotherapy with gemcitabine in combination with Wee1 inhibitor (AZD1775). Intravenous administration of gemcitabine at a dose of 1000 mg/m2 was done on days 1 and 8 of the 3 week cycle. AZD1775 was taken orally on days 1, 2, 8, and 9 of each three-week cycle. Blood samples were centrifuged at 500g for 10 min to separate out serum. All serum samples were stored at −80 °C until analysis.
Serum Exosome Isolation
To isolate the exosomes, 2 mL of serum from each patient was used. PBS (2 mL, AppliChem, St. Louis, MO) was added to each serum sample to decrease the viscosity; this was followed by centrifugation at 2000g for 10 min and then at 10 000g for 30 min at 4 °C to remove cell debris and large extracellular vesicles. The supernatant was transferred into an Ultra-Clear tube (Beckman Coulter, Indianapolis, IN) and centrifuged at 100 000g for 120 min at 4 °C using a Beckman Optima XL-70 Ultracentrifuge. Part of the supernatant was removed using a pipet, leaving 2 mL of supernatant remaining above the pellets. To clean the exosomes, 4 mL of PBS was added to the pellets containing exosomes, which were then centrifuged at 100 000g for 70 min at 4 °C; this was followed by partial removal of the supernatant. This step was repeated four more times to thoroughly clean the exosomes.
Metabolite Extraction and Chemical Isotope Labeling
The exosomes were extracted with 50% MeOH, and freeze – thaw cycles were carried out to assist the releasing of metabolites from exosomes during extraction. MeOH (50%, 200 μL) was added into the vials that contained the exosome pellets. The vials were placed in liquid nitrogen for 1 min and then thawed in an ice-bath for 1 min. This procedure was repeated five times. After extraction, the lysates were dried in a SpeedVac. The lysates were redissolved in 17 μL of Na2CO3/NaHCO3 buffer (250 mM; pH 9.4). Each lysate (7.5 μL) was aliquoted into a 0.6 mL vial for 12C-dansyl chloride labeling. Another aliquot of 7.5 μL was taken to generate a pooled sample for 13C-dansyl chloride labeling. For labeling, 7.5 μL of dansyl chloride dissolved in ACN at a concentration of 0.25 mg/mL was added to the lysate vial, followed by incubation for 1 h at 40 °C. NaOH (250 mM, 1 μL) was added to quench the excess DnsCl, and 5 μL of 425 mM formic acid was added to acidify the reaction mixture.
LC-MS
For the nanoflow LC-MS (nLC-MS) setup, a Waters NanoAcquity ultraperformance liquid chromatograph (UPLC; Milford, MA) was connected to a Bruker Impact quadruple time-of-flight mass spectrometer (Q-TOF; Billerica, MA) with a captivespray ion source.30 An Acclaim PepMap 100 trap column (75 μm × 20 mm, 3 μm; Thermo Scientific, Sunnyvale, CA) was used for trapping the labeled metabolites prior to injection into an Acclaim PepMap RSLC C18 analytical column (75 μm × 150 mm, 2 μm) for analytical separation. Mobile phase A was 0.1% formic acid in water, and mobile phase B was 0.1% formic acid in acetonitrile. Before injection, the 12C- and 13C-labeled extracts were combined, dried down, and reconstituted by 9:1 H2O/ACN. The injection volume was 5 μL. A 2 min trapping procedure was performed prior to sample loading onto the analytical column. The trapping solvent was 99% mobile phase A. The trapping flow rate was 7 μL/min. The chromatographic conditions were t = 0 min, 15% B; t = 2.0 min, 15% B; t = 4.0 min, 25% B; t = 24 min, 60% B; t = 28 min, 90% B; and t = 45 min, 90% B. The flow rate was 350 nL/min. The captivespray operation conditions were: a dry temperature of 200 °C, a dry gas rate of 3 L/min, a capillary voltage of 1400 V, and a nanoBooster pressure of 0.2 bar. The dopant gas was pure ACN.
RESULTS AND DISCUSSION
Exosome Isolation
In order to eliminate serum contamination, five rounds of ultracentrifugation (UC) were applied to purify the exosomes as described previously.32 We chose this UC method because we know from prior work that it is a robust and reliable method for eliminating high-abundance proteins and other contaminants from serum for proteomics research.32 Other methods, including antibody-based and size-based isolation techniques,33 are not evaluated in this study; however, it will be useful, in the future, to compare different exosome-isolation methods tailored to metabolomic profiling. In this work, the isolated exosomes were verified by transmission electron microscopy (TEM) and protein markers. The TEM images showed that the diameters of more than 90% of the vesicles were between 30 and 100 nm, and the median diameter was around 70 nm.34 Exosome markers, including CD9, CD63, CD81, and TSG101 were identified in the proteomics mass spectrometry data.18
Metabolite Extraction
As in cellular metabolomics, a lysis step is required before extracting metabolites from exosomes. In previous work, we investigated the efficiency and performance of different methods for lysing different types of cells.35 – 38 We found that for mammalian cells, the freeze – thaw-cycle lysis method was efficient. Considering that exosomes are membrane-bound phospholipid vesicles, this method was therefore selected for exosome lysis in this study. We also investigated the selection of extraction solvents for cellular metabolomics in previous studies.30,35,36 For the extraction of prokaryotic microbes (e.g., Escherichia coli), eukaryotic microbes (e.g., Saccharomyces cerevisiae), or mammalian cells (e.g., MCF-7 breast cancer cells), we found that 50% MeOH in water always provided the best extraction efficiency, whereas 50% ACN resulted in a lower extraction efficiency than 50% MeOH, and 1:1:1 ACN/MeOH/H2O resulted in the lowest efficiency. Therefore, 50% MeOH was selected as the extraction solvent in this work.
It should be noted that the presence of residual proteins in a sample does not cause a problem in CIL LC-MS, as residual proteins precipitate out during the labeling reaction. After centrifugation of a labeled exosome lysate, the supernatant was used for mixing with a labeled pool, and the mixture was then injected into LC-MS. We did not encounter the problem of column clogging in CIL nLC-MS. In this work, there is no desalting step before sample injection. This is because we already used a very low concentration of the labeling reagent (0.25 mg/mL), and the amount of the buffer used was also small for the labeling reaction. There is a trapping process before the sample is loaded onto the analytical column for separation; during trapping, the mobile phase, which contains mainly water, can remove most of the salts. Overall, the nLC-MS system is robust (we use a captivespray source) and the column lifetime is often more than 2000 injections per column.
nLC-MS
Figure 2A shows the total ion chromatogram of dansyl-labeled exosomes obtained by nLC-MS. A sodium formate peak appears at the very beginning of the chromatogram. Sodium formate was produced when formic acid was used to consume excess NaOH during the labeling step. The cluster peaks of sodium formate were used for mass calibration in each LC run. After the sodium formate peak, a small dansyl hydroxyl (Dns-OH) peak is observed. Dns-OH was produced from the labeling-reaction quenching step. Most of the dansyl labeled metabolites eluted between 7 and 32 min, where the 45 min gradient was sufficient for CIL nLC-MS. As the ion chromatogram shows, many peaks from labeled metabolites are detected across the entire gradient-elution time window.
Figure 2.

(A) Total ion chromatogram of a representative 12C- and 13C-labeled exosome sample. (B) Number of peak pairs identified or matched in three libraries. (C) Venn diagram showing the commonly detected peak pairs from the first sample set and the second sample set.
Exosome Metabolome
In CIL nLC-MS, the 12C- and 13C-labeled metabolites are detected as peak pairs, which are different from the singlet peaks arising from background chemical noises. IsoMS39 was used to pick the peak pairs, remove redundant pairs (e.g., adduct ions, dimers, etc.), align peak pairs from different samples, and determine the intensity ratios of peak pairs for relative quantification. For multiply labeled metabolites, IsoMS can determine the charge number and tag number (e.g., two tags with one charge) and filter out the redundant peak pairs if any. In general, only one peak pair, [M + H]+, was retained for one metabolite, and thus the number of peak pairs detected reflects the number of metabolites detected. We analyzed two separate sets of exosome samples to illustrate the technical reproducibility and robustness in terms of the number of peak pairs detectable. The average number of peak pairs detected from the first set and second set of exosomes was 1964 ± 60 (n = 10) and 1948 ± 117 (n = 10), respectively, with many peak pairs commonly detected in the two sets (see the Venn diagram in Figure 2C; only the peak pairs that showed up in more than 50% of the LC-MS runs were included for comparison). The total numbers of peak pairs detected were 2446 in the first sample set (n = 10) and 2511 in the second sample set (n = 10). These numbers from the two sets of samples are very consistent, indicating the robustness of the workflow. The large number of metabolites detected from exosomes reflects the complexity of the exosome metabolome.
Figure 2B shows a summary of the number of metabolites identified or matched using different metabolite databases. To identify the metabolite, the peak-pair mass and retention time were searched against a dansyl-standard library comprising 304 human endogenous amine and phenol metabolites (the retention-time tolerance was set at 1 min, and the mass tolerance was set at 10 ppm). There were 101 metabolites identified from the first sample set (Supplemental Table S1), and 94 metabolites were identified from the second set (Supplemental Table S4); many of them are in common in the two sets. They include most of the common amino acids, some dipeptides, and other metabolites. We also searched the MCID zero-reaction library (8021 known human endogenous metabolites) for putative structure assignments based on accurate-mass matches; 632 metabolites were matched in the first sample set (Supplemental Table S2), and 694 metabolites were matched in the second sample set (Supplemental Table S5). Lastly, we searched the masses against the MCID one-reaction library (i.e., 375 809 predicted human metabolites from one metabolic reaction of known metabolites). In the first sample set, 948 additional metabolites were matched with the predicted-metabolites library (Supplemental Table S3), and in the second sample set, 896 metabolites were matched with the predicted-metabolites library (Supplemental Table S6). In total, 1681 out of 2446 peak pairs (69%) were identified or matched in the first sample set, and 1675 out of 2511 peak pairs (68%) were identified or matched in the second sample set.
The above results indicate that the serum exosome metabolome contains many metabolites with diverse structures, and CIL nLC-MS has the sensitivity required to detect a large number of metabolites. To avoid serum-metabolite contamination in the exosome metabolome, we used extensive washing (five rounds), in combination with ultracentrifugation, to isolate and purify the exosomes from serum. In addition, metabolites were detected after lysis of exosomes using five freeze – thaw cycles. Because of the very small amounts of purified samples available for analysis, nLC-MS with rationally designed chemical isotope labeling of metabolites is needed to achieve high metabolome coverage. We note a report of metabolome analysis of exosomes-like vesicles (ELVs) isolated from human plasma,40 where conventional LC-MS using a 2.1 mm column was used for metabolite detection. Out of the 840 features detected in negative-ion mode and 2194 features detected in positive-ion mode, only 6 features were identified (mainly lipids). It was shown that the total ion chromatograms of the ELVs isolated from different volumes of human plasma (0.5, 1, and 2 mL) were almost the same,40 indicating the saturation of the MS signals. If the MS signals were from metabolites, the concentrations of these metabolites must be very high in the injected sample. Thus, the ELVs isolated in their work were very different from the low amounts of serum exosomes isolated in our work. Alternatively, the MS signals detected in their work were from background chemicals. In our work of using differential-isotope-labeling nLC-MS for metabolite detection, background signals can be readily differentiated from those of metabolites; MS signals from the background are shown as a singlet peaks, whereas the labeled metabolites are shown as peak pairs.
Comparative Metabolomics of Exosomes
In CIL nLC-MS, the same 13C-labeled pool prepared from a sample set was spiked into the individual 12C-labeled exosome samples. Thus, the peak ratio of a 12C-labeled metabolite in a sample versus that of the 13C-labeled version of the same metabolite in the pool reflects its relative concentration to that of the pool. Different ratio values from different samples mixed with the same pool can be used to measure the relative concentration differences among different samples. IsoMS calculates the chromatographic-peak area of the 12C- and 13C-labeled metabolite in a peak pair and then determines the peak-area ratios for all the peak pairs detected. The final metabolite-intensity table in a CSV file can be uploaded to statistical tools for analysis. Figure 3A,B shows the multivariate PCA analysis plots of the first and second data sets, respectively, whereas Figure 3C,D shows the corresponding PLS-DA plots. In both cases, the two groups before and after treatment show some separation. Univariate analysis using a volcano plot was performed. In the first data set, 54 metabolites were up-regulated (fold change, FC, >1.5 with p < 0.05), and 36 metabolites were down-regulated (FC < 0.67 with p < 0.05) (Supplemental Table S7). In the second data set, 34 metabolites were up-regulated, and 36 metabolites were down-regulated (Supplemental Table S8). Some of the significantly changed metabolites can be positively identified or matched to the MCID libraries. Supplemental Figure S1 shows the box plots of selected metabolites with significant changes (i.e., alanylhistidine, 6-dimethylaminopurine, leucylproline, and methionine sulfoxide).
Figure 3.
(A,B) PCA plots, (C,D) PLS-DA plots, and (E,F) volcano plots of exosome metabolomes from the first sample set (n = 10) and the second sample set (n = 10).
Although the sample size used in this study is too small to draw any biological significance from the changed metabolites, the above results demonstrate that CIL nLC-MS can be used to perform relative quantification of metabolites in the metabolomes of exosomes.
CONCLUSIONS
We have developed a CIL nLC-MS workflow for metabolomic analysis of exosomes isolated from serum that allows the detection of ~1950 metabolites per sample. To our knowledge, this level of detectability is unprecedented. In addition, accurate relative quantification of metabolites using a differentially labeled pooled sample as a control or internal standard can be carried out using CIL nLC-MS. As a proof-of-principle, we demonstrated the application of this workflow to detect significant changes of some metabolites before and after chemotherapy in exosomes isolated from serum of cancer patients. We expect that, after a large number of exosome samples are analyzed in the future, some potential biomarkers for therapeutic monitoring might be discovered. We are also trying to further optimize the coverage of the metabolome by applying other chemical isotope labeling techniques to profile the carbonyl, alcohol, and carboxyl acid submetabolomes of exosomes.
Supplementary Material
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (L.L.), the Canada Research Chairs program (L.L.), Genome Canada (L.L.), Genome Alberta (L.L.), Canada Foundation for Innovation (L.L.), and Alberta Innovates (L.L.). This work was also supported by the National Cancer Institute under grant R21CA189775 (D.M.L.) and the National Institutes of Health under grant R01GM49500 (D.M.L.).
Footnotes
ORCID
David M. Lubman: 0000-0001-7731-0232
Liang Li: 0000-0002-9347-2108
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.anal-chem.8b01726.
Metabolite lists and box plots of the four identified metabolites (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.van Niel G, D’Angelo G, Raposo G. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 2.Cocucci E, Meldolesi J. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. Cancer Lett. 2017;393:86–93. doi: 10.1016/j.canlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malla B, Zaugg K, Vassella E, Aebersold DM, Dal Pra A. Int J Radiat Oncol, Biol, Phys. 2017;98:982–995. doi: 10.1016/j.ijrobp.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Mathivanan S, Simpson RJ. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 6.Simpson RJ, Jensen SS, Lim JW. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 7.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Xie Y, Xu L, Zhan S, Xiao Y, Gao Y, Wu B, Ge W. Int J Cancer. 2017;140:900–913. doi: 10.1002/ijc.30496. [DOI] [PubMed] [Google Scholar]
- 9.Fontana S, Saieva L, Taverna S, Alessandro R. Proteomics. 2013;13:1581–1594. doi: 10.1002/pmic.201200398. [DOI] [PubMed] [Google Scholar]
- 10.Franzen CA, Blackwell RH, Foreman KE, Kuo PC, Flanigan RC, Gupta GN. J Urol. 2016;195:1331–1339. doi: 10.1016/j.juro.2015.08.115. [DOI] [PubMed] [Google Scholar]
- 11.Azmi AS, Bao B, Sarkar FH. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake RR, Kislinger T. Expert Rev Proteomics. 2014;11:167–177. doi: 10.1586/14789450.2014.890894. [DOI] [PubMed] [Google Scholar]
- 13.Henderson MC, Azorsa DO. Front Oncol. 2012;2:38. doi: 10.3389/fonc.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Risch HA. Future Oncol. 2016;12:1081–1090. doi: 10.2217/fon-2015-0005. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A. Br J Cancer. 2009;100:1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteside TL. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Philos Trans R Soc, B. 2014;369:20130502. doi: 10.1098/rstb.2013.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An M, Lohse I, Tan Z, Zhu J, Wu J, Kurapati H, Morgan MA, Lawrence TS, Cuneo KC, Lubman DM. J Proteome Res. 2017;16:1763–1772. doi: 10.1021/acs.jproteome.7b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Liu S, Qiao Z, Shang Z, Xia Z, Niu X, Qian L, Zhang Y, Fan L, Cao CX, Xiao H. Anal Chim Acta. 2017;982:84–95. doi: 10.1016/j.aca.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. J Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olver C, Vidal M. Subcell Biochem. 2007;43:99–131. doi: 10.1007/978-1-4020-5943-8_7. [DOI] [PubMed] [Google Scholar]
- 22.Principe S, Jones EE, Kim Y, Sinha A, Nyalwidhe JO, Brooks J, Semmes OJ, Troyer DA, Lance RS, Kislinger T, Drake RR. Proteomics. 2013;13:1667–1671. doi: 10.1002/pmic.201200561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, Vattulainen I, Ekroos K, Sandvig K. Biochim Biophys Acta, Mol Cell Biol Lipids. 2013;1831:1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Buratta S, Urbanelli L, Sagini K, Giovagnoli S, Caponi S, Fioretto D, Mitro N, Caruso D, Emiliani C. PLoS One. 2017;12:e0188840. doi: 10.1371/journal.pone.0188840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberg-Larsen H, Lund K, Seterdal KE, Solheim S, Vehus T, Solberg N, Krauss S, Lundanes E, Wilson SR. J Steroid Biochem Mol Biol. 2017;169:22–28. doi: 10.1016/j.jsbmb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Skotland T, Sandvig K, Llorente A. Prog Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. eLife. 2016;5(e10250) doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puhka M, Takatalo M, Nordberg M-E, Valkonen S, Nandania J, Aatonen M, Yliperttula M, Laitinen S, Velagapudi V, Mirtti T, Kallioniemi O, Rannikko A, Siljander PRM, af Hällström TM. Theranostics. 2017;7:3824–3841. doi: 10.7150/thno.19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iraci N, Gaude E, Leonardi T, Costa ASH, Cossetti C, Peruzzotti-Jametti L, Bernstock JD, Saini HK, Gelati M, Vescovi AL, Bastos C, Faria N, Occhipinti LG, Enright AJ, Frezza C, Pluchino S. Nat Chem Biol. 2017;13:951. doi: 10.1038/nchembio.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo X, Li L. Anal Chem. 2017;89:11664–11671. doi: 10.1021/acs.analchem.7b03100. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Li RH, Zhou JJ, Zuniga A, Stanislaus AE, Wu YM, Huan T, Zheng JM, Shi Y, Wishart DS, Lin GH. Anal Chem. 2013;85:3401–3408. doi: 10.1021/ac400099b. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Tan Z, Lubman DM. Electrophoresis. 2015;36:2017–2026. doi: 10.1002/elps.201500131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko J, Carpenter E, Issadore D. Analyst. 2016;141:450–460. doi: 10.1039/c5an01610j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An M, Zhu J, Wu J, Cuneo KC, Lubman DM. J Proteome Res. 2018;17:1690–1699. doi: 10.1021/acs.jproteome.8b00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Li L. Anal Chem. 2013;85:5755–5763. doi: 10.1021/ac400330z. [DOI] [PubMed] [Google Scholar]
- 36.Luo X, Zhao S, Huan T, Sun D, Friis RMN, Schultz MC, Li L. J Proteome Res. 2016;15:1602–1612. doi: 10.1021/acs.jproteome.6b00070. [DOI] [PubMed] [Google Scholar]
- 37.Schultz MC, Zhang J, Luo X, Savchenko O, Li L, Deyholos M, Chen J. J Proteome Res. 2017;16:2975–2982. doi: 10.1021/acs.jproteome.7b00273. [DOI] [PubMed] [Google Scholar]
- 38.Luo X, Gu X, Li L. Anal Chim Acta. 2018 doi: 10.1016/j.aca.2017.11.054. in press. [DOI] [PubMed] [Google Scholar]
- 39.Huan T, Wu YM, Tang CQ, Lin GH, Li L. Anal Chem. 2015;87:9838–9845. doi: 10.1021/acs.analchem.5b02282. [DOI] [PubMed] [Google Scholar]
- 40.Altadill T, Campoy I, Lanau L, Gill K, Rigau M, Gil-Moreno A, Reventos J, Byers S, Colas E, Cheema AK. PLoS One. 2016;11:e0151339. doi: 10.1371/journal.pone.0151339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.