Abstract
Cytogenetic damage is among the few radiobiological end points that allow a precise distinction to be made between misrepaired damage, represented by exchange-type aberrations such as dicentrics and translocations, and unrepaired damage that leads to “open breaks’’. This latter category includes both terminal deletions and incomplete exchanges, whose different mechanisms of formation can be recognized by multicolor fluorescence in situ hybridization (mFISH). mFISH was used to examine the yields of chromosome aberrations at the first postirradiation mitosis in human fibroblasts and lymphocytes irradiated with 137Cs γ rays, a radiation of low-linear energy transfer (LET), and two sources of high-LET radiation: α particles from 238Pu and 1 GeV/amu 56Fe ions. In agreement with previous studies, our results show that irrespective of radiation quality, the overall level of misrepaired damage exceeds that of unrepaired damage by a large margin. The unrepaired component of damage produced by γ rays and α particles was remarkably similar, about 5%. On that basis it is difficult to justify the popular notion that the strong LET-dependence for aberration formation is due to unrepaired DNA double-strand breaks (DSBs) that, by virtue of their complexity at the nanometer scale, are qualitatively different in nature. In marked contrast, this unrejoined component rose to about 14% after exposure to Fe ions. A closer look at the unrepaired component revealed that most of this roughly threefold difference was derived from incomplete exchanges. Despite vast differences in LET, unrejoined breaks from incomplete exchanges were far more likely to occur among exchanges that involved more than two breakpoints. We attempted to reconcile these observations in the form of a hypothesis that predicts that exchanges, irrespective of LET, should exhibit an increasing tendency for incompleteness as the number of initial breaks destined to take part in the exchange increases. This effect, we argue is not caused by the number of initial breaks per se, but instead reflects the maximum distance over which proximate breaks can interact. This adds a spatial aspect to multi-break interactions that we call “A Break Too Far”.
INTRODUCTION
Ionizing radiations (IR) of high-linear energy transfer (LET) are more biologically effective per unit dose than are low-LET radiations. This manifests itself in the classical RBE-LET relationship found in textbooks (1) and applies to several higher-order cellular end points, most notably cell killing and chromosome aberrations. It follows that viable explanations of this phenomenon require consistency with cytogenetic observations.
Of the various cellular lesions produced by IR, it is the DNA double-strand break (DSB) that is considered to be of paramount importance in clastogenesis. The dependency of DSB induction on LET is a topic of some controversy and conclusions depend largely on methods of analysis. Some studies show a slight decline in DSBs with increasing LET, other studies show a modest increase in DSB yields with increasing LET. But, to the extent that there is consensus on this topic, it is clear that the initial yield of DSBs fails to explain the strong LET dependence of RBE on final cellular effects (2, 3). The same can be said of initial chromosome breaks as visualized by the technique of premature chromosome condensation (4–6).
Since the LET dependence for chromosome aberrations derives from a source other than the absolute yield of DSBs, there must be something else about the breaks that differentially affects the way that high-LET and low-LET induced damage is processed by the cell. The classical explanation for this difference relies on inveterate models of lesion interaction, which assert that exchange-type aberrations result from the illegitimate pairwise rejoining of initial chromosome breaks (discontinuity in the chromonema), more specifically the rejoining of broken chromosome ends that such breaks generate (7–11). Modern interpretations consider that a subset of DSBs produces the obligatory break pairs, the rejoining of which is mediated by the bimolecular process of nonhomologous end joining (NHEJ). Otherwise, the basic concept remains the same (12–15). An additional biophysical requirement for such illegitimate recombination is that the breaks be proximate, meaning close in both time and space. For both high doses and dose rates of sparsely ionizing radiation, this requirement is typically met through the cooperation of breaks produced by independent multiple tracks (inter-track action). For high-LET IR, lesion interaction takes place almost exclusively through the rejoining of breaks created along the trajectory of single-charged-particle tracks (intratrack action). Seen in this way, high-LET IR is more effective per unit dose than its low-LET counterpart simply because the former ‘‘packages its ionizations” in a way that spatially favors the production of proximate initial breaks. In that sense, the foregoing explanation for LET dependence might be considered quantitative in nature. By contemporary analogy, it derives from DSB-DSB interactions capable of taking place over large (e.g., submicron) distances.
Besides DSBs, IR produces in DNA a rather wide spectrum of molecular damage of varying complexity. In deliberating alternative explanations for RBE-LET relationships, it must be appreciated that the passage of individual high-LET tracks leads to localized energy deposition within subcellular volumes whose high ionization densities are seldom (if ever) achieved by low-LET IR, irrespective of dose (16). This has given rise to the notion of ‘‘clustered damage’’ (17), whose origins can be traced to the locally multiply damaged sites (LMDS) concept of Ward (18). It serves to buttress the idea that DSBs resulting from high-LET IR differ from those produced by low-LET IR in a qualitative sense (19, 20). Indeed, various studies have shown an LET dependence for the formation of what are termed complex DSBs, often defined as base lesions, abasic sites, or single-strand breaks in close proximity to the break termini (21–25). In contrast to the submicron distances implied by the scenario described in the preceding paragraph, proximity in this case involves nanometer dimensions.
The obvious implication is that radiogenic lesions produced by high-LET (e.g., complex DSBs) are inherently more difficult for the cell to correctly process. Of course, it matters little what types of lesions are envisioned to be problematic in that regard. Rather, it is how the cell actually “interprets” and responds to such damage during subsequent processing that is of primary importance (26). Certainly, LET-dependent changes in the kinetics of DSB repair have been reported, including studies showing that the rejoining of DSBs following high-LET exposure is delayed or diminished compared to that observed following low-LET irradiation (27–30). These sorts of ‘‘qualitative’’ arguments have undeniable intuitive appeal. If they fall short of being compelling, it is because of the experimental difficulties involved in defining at the molecular level the types of complex DSBs quintessential to high-LET damage, while providing direct correspondence to their diminished repair.
The broad focus of this article relates to the LET dependence of chromosome-type aberrations observed at the first mitosis after irradiation, a higher-order cellular end point. In that context, structural aberrations may be viewed as residual radiogenic damage that the cell—irrespective of underlying molecular processes—has dealt with ‘‘unsuccessfully’’. By analogy to potential outcomes of DSB repair by NHEJ, microscopically visible damage in metaphase chromosomes may be defined as follows. The general term ‘‘eurepair’’ equates with the cytogenetic concept of restitution, whereby both broken ends of an initial chromosome break rejoin back together again. Neither restitution nor its equivalent counterpart resulting from NHEJ guarantee perfect repair at the DNA base pair level, but rather restoration of continuity of the chromonema or corresponding DNA macromolecule. As defined here, eurepair is by far the most likely outcome of radiation damage. The next most likely outcome involves the category of ‘‘misrepair’’ which, at the cytogenetic level, is associated with the familiar exchange-type aberrations, such as dicentrics, translocations and rings. Cytogenetics excels at detecting these events, which would involve DSB-DSB rejoinings that take place over large distances, although on a smaller dimensional scale, they are certainly detectable by molecular approaches (31–33).
The narrower focus of this paper involves what is ostensibly the least likely outcome of radiogenic lesion processing, namely unrepaired damage, which chromosome analysis also excels at detecting. From a cytogenetic perspective, unrepaired damage takes two forms. The simplest instance results from a chromosome break whose associated broken ends fail to rejoin at all, the consequence of which is visible at metaphase as a terminal deletion. The second source of unrepaired damage is manifest when, during the formation of an exchange aberration, at least one broken end fails to find a partner with which to rejoin in an otherwise complete exchange. This leads to incomplete exchanges, which, as with terminal deletions, result in the truncation of one or more chromosomal elements. The relationship between exchanges and incompleteness was recognized many years ago and became the focus of much debate (34–37).
The distinction between the two sources of unrepaired damage has also been the cause of some confusion in nomenclature, which is understandable because it is strictly operational (mechanistic) in nature, and both processes lead to the production of linear acentric fragments. In the context of the present discussion, it is sometimes the case that no such distinction need be made. In that situation, the term ‘‘open breaks’’ can be used to express the totality of unrepaired damage. Making an LET-dependent connection between open breaks (or unrepaired DSBs) and chromosome exchanges requires additional assumptions. For example, because complex DSBs are said to be less effectively repaired, it would be reasonable to assume that they would remain in some type of transitional unrepaired state long enough to serve as active substrates for subsequent misrejoining mediated by a similar or different process. We will return to this point later.
We reasoned that if some qualitative aspect of DSBs associated with densely ionizing radiation renders them less amenable to repair compared to DSBs from sparsely ionizing radiation, this difference should translate into an LET-dependent increase in the level of open breaks at the cytogenetic level. To investigate this prediction, we conducted an in-depth analysis of microscopically visible chromosome-type aberrations using 24-color mFISH data from both lymphocytes and fibroblasts that were exposed to graded doses of 137Cs γ rays, 238Pu α particles or 1 GeV/amu 56Fe ions. Results are discussed in the context of a model that explains unrepaired breaks in terms of spatial hindrances imposed during the attempted misrejoining of large initial proximate break clusters.
MATERIALS AND METHODS
The current study draws on a compilation of experiments conducted over the years at various institutions using a variety of radiation sources, the results of which have recently been published where, unlike the current study, emphasis was placed on exchange-type aberrations (38). Given the retrospective nature of the data set, it should be appreciated that ideal comparisons among different cell types and radiations were not always possible.
Irradiations and Culture Conditions
56Fe ions.
Lymphocytes from the blood of a healthy volunteer were suspended in RPMI-1640 (Gibco) medium supplemented with 20% fetal bovine serum. A volume of 2 ml (at a concentration of approximately 1 × 106 cells/ml) was loaded into specially constructed Lucite holders and irradiated at room temperature with graded doses (0.0, 0.2, 0.4, 0.7, 1.0, 1.5 Gy) of 1 GeV/amu 56Fe ions at the NASA Space Radiation Laboratory (NSRL) at Brookhaven National Laboratory (Upton, NY). The dose average LET of this beam was 147 keV/μm. Immediately after exposure, lymphocytes were aspirated from the holder and transferred into 25 cm2 tissue culture flasks containing 10 ml of RPMI-1640 medium supplemented with 1% phytohaemagglutinin (PHA; Gibco). Cultures were incubated at 37°C for 46 h before Colcemid (Gibco) was added (0.2 pg/ml final concentration) 2 h prior to the harvest of mitotic cells. Calyculin-A (50 mM final concentration) was added to Colcemid-blocked cultures to induce premature chromosome condensation (PCC) in G2-phase cells (39). As a result, chromosome spreads represented a mixture of cells, some containing mitotic chromosomes, and others containing G2-phase prematurely condensed chromosomes. Collected cells were fixed in a 3:1 mixture of methanol to acetic acid by standard cytogenetic procedures and transported to the University of Texas Medical Branch at Galveston for analysis.
Alpha particles.
The α-particle experiments were conducted at the Los Alamos National Laboratory using low passage AG1521 normal primary human fibroblasts. Cells were revived from low passage frozen stocks into Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin and streptomycin, and maintained at 37°C in a humidified atmosphere of 5% CO2 in air. Four days before irradiation 1 × 105 cells were plated onto 1.5 pm thick Mylar that formed the bottom growth surface of specially constructed 28 mm diameter dishes. Confluent monolayers of cells were irradiated through the Mylar membranes with graded doses (0.0, 0.1, 0.2, 0.4, 0.6 Gy) of 238Pu α particles at a dose rate of 3.5 cGy/s (5, 40). Under these conditions, the incident energy of the particles was calculated to be 3.5 MeV. This corresponded to a track segment LET of 116 keV/μm. Immediately after irradiation the cells were returned to the incubator where they remained for 24 h. Following incubation, cells from two Mylar flasks were trypsinized, pooled and subcultured into 75 cm2 tissue culture flasks that were then returned to the incubator. Each dose was represented by two T-flasks. Colcemid was added to the first flask 28 h post-subculture and was added to the second flask 4 h later. Mitotic cells were harvested after a 4 h Colcemid block and fixed in 3:1 methanol and acetic acid as above. Mitotic indices were determined and the collection period yielding the highest index for each dose point was used in further analyses.
Gamma rays.
Methods pertaining to the exposure of lymphocytes and fibroblasts have been detailed elsewhere (41, 42). Briefly, cells were exposed to 137Cs γ rays (0.0, 1.0, 2.0 and 4.0 Gy for lymphocytes, and 0.0, 0.9, 1.8 and 3.6 Gy for fibroblasts) at a dose rate of 1.3 Gy/min. For fibroblasts, exposures were to confluent cultures that were allowed a postirradiation recovery of 12 h at 37°C prior to release from the density-inhibited state. As before, two 4 h Colcemid collection intervals were established for each dose, 28–32 and 32–36 h following subculture, and subsequent analysis was performed on the sample with the highest mitotic index. For lymphocytes, exposures were to blood samples that were cultured in in RPMI-1640 (Gibco) medium, supplemented with 15% fetal bovine and containing 0.1 ml PHA (Murix, Dartford, UK). Colcemid to a final concentration of 0.1 μg/ml was added 45 h later, and cultures were harvested for metaphase analysis at 48 h.
mFISH Hybridization and Image Collection
Cells previously fixed in a 3:1 ratio of methanol to glacial acetic acid were spread onto glass microscope slides. Slides were then treated with acetone, RNase A and proteinase K before another fixation in 3.7% formaldehyde. Slides were dehydrated through an ethanol series (70, 85 and 100%) and air-dried. They were then incubated in 72°C formamide (70%) in 2× SSC (0.3 M NaCl, 0.03 M sodium citrate) for 2 min to denature chromosomal DNA. After dehydration through another ethanol series, 10 pl of denatured (10 min at 72°C) SpectraVision 24-color mFISH assay probe (Vysis) was applied to each slide. Slides were covered with a 22 × 22 mm glass cover slip sealed into position with rubber cement. Samples were allowed to hybridize for 48 h in a 37°C incubator. Following hybridization, cover slips were removed and the slides were washed for 2 min in 0.4× SSC containing IGEPAL (0.3%) nonionic detergent at 72°C. This was followed by a 30 s wash in 2× SSC (0.1% IGEPAL) at room temperature. Prior to image capture, 15 μl of DAPI (0.14 μg/ml) dissolved in Vectashield anti-fade mounting media (Vector Laboratories) was applied to each slide and covered with a 24 × 40 mm cover slip.
Images of chromosome spreads were captured using a Zeiss Axiophot epifluorescence microscope interfaced with a SensSys black and white CCD camera. Karyotypes were constructed from as many good-quality chromosome spreads as could be found on each slide using PowerGene image analysis software.
Aberration Analysis
Metaphase cells were analyzed by procedures previously established (41). First, mPAINT descriptors were assigned to chromosomes involved in aberrations. Next, each rearrangement was brought to ‘‘pattern closure’’ by grouping elements in the most conservative way possible, minimizing the number of breakpoints required to reconstruct the exchange (43). Reciprocal pairwise rejoinings between one chromosome (rings and interstitial deletions) or two chromosomes (translocations and dicentrics) were scored as simple exchanges. Exchanges involving three or more breakpoints were regarded as complex. This classification was also applied to incomplete exchanges where one or more elements failed to rejoin, as well as to so-called ‘‘one-way’’ exchanges where one or more translocated segments appeared to be missing, presumably because they were too small to be resolved by chromosome painting. Since the large majority of one-way staining patterns have been shown to be complete exchanges (44), and since we lacked the ability to simultaneously visualize telomere signals in mFISH preparations, they were treated as such for the purpose of achieving closure.
Unrejoined (open) breaks were classified as terminal deletions when a mono-colored acentric linear fragment was accompanied by a truncated centric element of the same color. Included in this category were less frequent instances where lone linear acentric mono-colored fragments and lone truncated mono-colored centric elements without acentric partners were observed. An exchange was classified as being incomplete when all elements of the exchange were observable and at least two such elements failed to rejoin.
For reasons important to this article, we desired to correlate incomplete breaks with the ‘‘size’’ of the exchange that contained them. As explained more fully in the sections that follow, this exercise becomes tantamount to limiting the assessment of incompleteness to exchanges with ‘‘full-cycle structures” (43). Most of the time this could be done by inspection, but for complex exchanges containing large numbers of breakpoints, occasionally we turned to computer-assisted approaches (45, 46).
RESULTS AND DISCUSSION
Quantitative and Qualitative Damage
Nearly hidden in a seemingly unrelated publication (47) are data that give the yields of ‘‘terminal deletions’’ produced in human fibroblasts exposed to 662 keV 137Cs γ rays and 3.5 MeV α particles. In retrospect, the noncommittal term ‘‘excess linear acentric fragments’’ should have been used instead to describe these aberration types. That is because conventional Giemsa staining that was used does not distinguish between bona fide terminal deletions and linear acentric fragments deriving from incomplete exchanges. Collectively, these unrepaired events were found to make up a small percentage of the overall chromosome damage after acute exposures. This is in apparent disagreement with earlier work that reported a somewhat higher frequency of excess linear fragments produced by IR of widely ranging ionization densities (48). Nevertheless, and more to the point of the present discussion, both studies showed that the unrepaired fraction of total damage is not systematically affected by immense differences in LET.
By the time the more recent of these studies was published (47), a number of investigators had already been using more sophisticated approaches to examine the issue of unrejoined breaks (49), most notably by using telomere staining in conjunction with various whole chromosome painting schemes (44, 50–52) or multicolor banding (53). All of these studies are in agreement insofar as concluding that unrejoined (open) breaks comprise a significant, but relatively small, percentage of overall damage. However, with respect to dependence on LET, there is room for debate.
In apparent disagreement with the aforementioned studies, the work of Deng and colleagues (54) found an excess of unrepaired breaks in lymphocytes after irradiation with high-LET neutrons compared to γ rays. This is somewhat surprising because, although the study used 3-color whole chromosome painting instead of Giemsa, the two methods, in principle, should have provided a similar measure of open breaks. The addition of telomere probes to whole chromosome painting seems not to have completely resolved the issue, perhaps because the relevant studies had slightly different experimental objectives regarding the assessment of unrepaired damage. For transmissible aberrations in human lymphocytes exposed to 1 GeV/amu 56Fe ions, presumptive terminal deletions were produced in significant excess compared to 137Cs γ rays (53). However, for incomplete exchanges no such increase was observed in other studies that looked at lymphocytes exposed to either neutrons (55) or 56Fe ions (51). It is probably fair to say that none of the approaches used in the studies cited above is ideally suited for the purpose of estimating the relative contributions of terminal deletions versus incomplete exchanges, especially in the likely presence of complex exchanges (43). Although also not perfect in that regard, 24-color combinatorial painting (56, 57) is arguably a better approach from an experimental standpoint.
The reason we place emphasis on the ability to distinguish open breaks deriving from terminal deletions compared to those deriving from incomplete exchanges will now be evident. Table 1 displays the results of mFISH analysis conducted on some 2,300 human karyotypes after exposure to the various radiations indicated. As is typically our practice, and for reasons given previously (38, 41), the data are presented in terms of breakpoints required to produce various aberration types. The purpose of the table is to highlight the contribution of the two sources of unrepaired chromosome damage. After pooling individual doses for each of the radiation types studied, two conspicuous results emerge.
TABLE 1.
Sources of Unrejoined (open) Chromosome Breaks in Human Cells after Exposure to Low- and High-LET Radiation
| Radiation source/cell type | Cells scored | Exchange-related | Terminal deletions | Total unrejoined breakpointsa | Total breakpoints | Fraction of total unrepaired | ||
|---|---|---|---|---|---|---|---|---|
| Total exchanges | Total exchange breakpoints | Incomplete open breaks | ||||||
| 137Cs γ rays | ||||||||
| Fibroblasts | 622 | 369 | 787 | 11 | 29 | 40 | 816 | 0.049 ± 0.008 |
| Lymphocytes | 438 | 774 | 1892 | 50 | 57 | 107 | 1949 | 0.055 ± 0.005 |
| 238Pu α particles | ||||||||
| Fibroblasts | 243 | 201 | 487 | 7 | 14 | 21 | 501 | 0.042 ± 0.009 |
| 1 GeV 56Fe ions | ||||||||
| Lymphocytes | 973 | 711 | 2280 | 185 | 142 | 327 | 2422 | 0.135 ± 0.007 |
| Grand sum Σ | 2276 | 2055 | 5446 | 253 | 242 | 495 | 5688 | |
Includes open breaks associated with incomplete exchanges and terminal deletions.
First, from the standpoint of overall unrepaired damage, there is rather good agreement between the results of the previously mentioned study (47) and the data of Table 1. This relates to ‘‘Total Unrejoined Breakpoints” (column 7), which totals the contributions of incompletes and terminals. As an overall fraction of total breakpoints (column 9), cytogenetic damage from unrejoined breaks makes up roughly 5% of the damage, irrespective of whether such damage was produced by low-LET γ photons or by high-LET α particles. With respect to unrepaired cytogenetic damage as it is defined in the previous section, together with corresponding arguments relating DSBs to chromosome aberrations, we conclude the following. The data for a particles are wholly inconsistent with their higher RBE for chromosome aberrations that are due to unrepaired DSBs. The strong causal connection between chromosome aberrations and lethality in human fibroblasts (58) allows one to extend this conclusion to include cell survival. Strictly speaking, therefore, it is most assuredly not a general feature of high-LET radiation that the type of damage it causes to DNA is fundamentally different than that produced by sparsely ionizing radiations in terms of its tendency to remain unrepaired by the cell.
Issues with Iron
A second noteworthy feature of Table 1 relates to incongruencies between the data for γ rays and α particles, compared to that for 1 GeV/amu 56Fe ions. The most striking of these is shown in the last column of the table. Whereas some 5% of the total breakpoints end up as either incomplete exchanges or terminal deletions after exposure to either γ rays or α particles, this value jumps to about 14% for the Fe ions. A closer look at columns 5 and 6 reveals another interesting aspect to the data. Considering only γ rays and α particle exposures, it can be seen that terminals outnumber incompletes in all three cases. Summed together, column 5 for these exposures yields a total of 68 incompletes versus a corresponding 100 terminals for column 6. Thus, about 40% of the total open breaks for these two sources of radiation derive from incomplete exchanges, the remainder are due to terminal deletions. Yet, the corresponding comparison for Fe ions leads to an opposite result: the majority (almost 60%) of the unrejoined damage comes from incompletes.
The data of Table 1 are graphically summarized in Fig. 1. From panels A and B it can be appreciated that the contribution of all ‘‘open breakpoints” (terminals plus incompletes) is small compared to overall damage, most of which takes the form of complete exchanges. Consequently, large numbers of mFISH karyotypes are needed to accurately assess the frequencies of open breaks. Still larger numbers of karyotypes are required to break down these frequencies in any meaningful way by the individual doses delivered. For reasons associated with experimental design and access to radiation sources, this was simply not feasible for fibroblasts. Therefore, the data of Fig. 1 are confined to lymphocytes. Panel C of Fig. 1 shows the fraction of open breakpoints as a function of dose. While these values are associated with relatively large errors, it is evident that they are not strongly influenced by dose. We mention this because it gives justification for pooling the doses, as shown in Table 1. The noticeable curvature in the overall dose response for 56Fe particles (panel B) has been previously reported and discussed in detail (38).
FIG. 1.
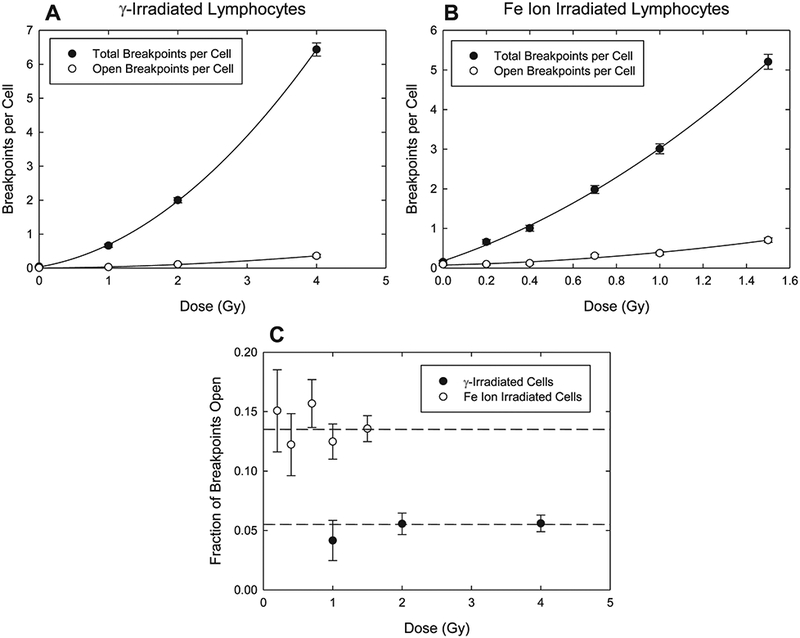
Dose response curves for chromosome breakpoints in lymphocytes exposed to either 137Cs γ-rays (panel A) or 1 GeV/amu 56Fe ions (Panel B). Solid symbols represent total breakpoints per cell; open symbols are for unrejoined or ‘‘open’’ breakpoints, which include both terminal deletions and incomplete exchanges. Panel C: Fraction of total breakpoints remaining open as a function of dose. Solid symbols represent γ-irradiated cells; open symbols represent cells exposed to 1 GeV/amu 56Fe ions. Dotted lines show the mean for each respective group. Note that open fraction is not systematically influenced by dose. Uncertainties about the mean are standard errors.
Beyond its capacity to distinguish terminal deletions from incompletes, another obvious advantage that mFISH brings to the analysis chromosome aberrations is its ability to discern and quantify complex exchanges. Defined as rearrangements involving the misrejoining of three or more chromosome breaks, complex exchanges have been shown to exhibit a strong dependency on radiation quality (38, 59–62). In seeking an explanation for the data of Table 1, it seemed reasonable to examine the influence of complex exchanges (CExs). More specifically, because a major difference between Fe ions and the other two radiations studied focused on the relative frequency of incomplete exchanges, we focused our attention there. Consequently, the date of Table 2’s data ignores altogether the contribution of terminal deletions and shows only the number of open breaks from incomplete exchanges (column 5). The fraction of total breakpoints remaining open is shown in the adjacent column 6. Breakpoints are classified as to whether they derived from simple exchanges: pairwise interactions, the outcomes of which led to reciprocal translocations or dicentrics, or whether instead they originated from CExs. Results shown in the last column convey the underlying purpose of this exercise, which is to show the ratio of complex-to-simple open breaks. This ratio for α particles represents a small number of events, and for that reason it is difficult to place much confidence in it. But for the remaining ratios the results are quite clear: incomplete exchange breakpoints that are part of CExs outnumber those deriving from simple exchanges by a large margin. In the discussion that follows we attempt to reconcile this result though classical cytogenetic principles: that is, without recourse to the idea that incomplete exchanges arise from DSBs that are inherently less repairable.
TABLE 2.
Open Breaks Deriving Only from Incomplete Exchanges
| Radiation | Cell type | Exchange type | Exchange breakpoints | Open breaksa | Fraction of breaks remaining open | Complex/simple open breaksa |
|---|---|---|---|---|---|---|
| Gamma | Fibroblasts | Simple | 664 | 6 | 0.009 ± 0.004 | 4.56 ± 2.85 |
| Gamma | Fibroblasts | Complex | 123 | 5 | 0.041 ± 0.018 | |
| Gamma | Lymphocytes | Simple | 1236 | 13 | 0.011 ± 0.003 | 5.09 ± 1.61 |
| Gamma | Lymphocytes | Complex | 656 | 37 | 0.056 ± 0.009 | |
| Alpha | Fibroblasts | Simple | 300 | 5 | 0.017 ± 0.007 | 0.65 ± 0.54 |
| Alpha | Fibroblasts | Complex | 187 | 2 | 0.011 ± 0.008 | |
| 56Fe | Lymphocytes | Simple | 838 | 31 | 0.037 ± 0.007 | 2.89 ± 0.60 |
| 56Fe | Lymphocytes | Complex | 1442 | 154 | 0.107 ± 0.009 |
Conservative estimate in which closure of one-way configurations is assumed (see Materials and Methods).
Errors represent standard deviations of the mean.
A Break Too Far?
The explanation we propose takes the form of the following hypothesis. When three or more proximate initial breaks are destined to form a complex exchange (CEx), a broken end of a given chromosome must rejoin with another from a second chromosome, which effectively eliminates both of these ends from participating in subsequent rejoinings. The remaining broken end from the second chromosome must then rejoin with another broken end, for example, of a third chromosome, and so on. Consider, for example, a CEx involving three breakpoints and three different chromosomes. [In the CAB system of nomenclature this is referred to as a 3/3/3 actual configuration, signifying that 3 chromosomes, 3 chromosome arms and 3 breaks are involved (63).] For it to be complete, the last break end from the third chromosome involved in the rejoining reaction must, by necessity, rejoin to the remaining break end from the first chromosome. Now consider the formation of CExs that involve larger numbers of breakpoints, as graphically illustrated in Fig. 2A. They also can be complete, but only if all broken ends within the constellation of proximate breakpoints find illegitimate partners with which to rejoin.
FIG. 2.
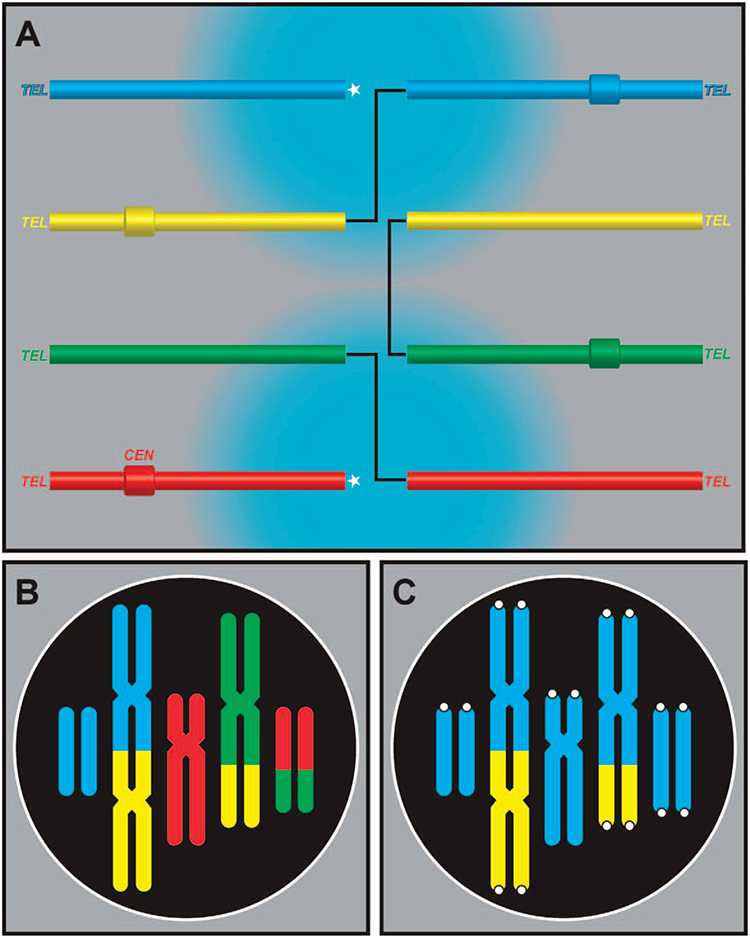
Schematic representation of the Break-Too-Far hypothesis. Panel A: This panel shows four unreplicated chromosomes in the nucleus of a G1 phase cell. Each is composed of a single centromere (CEN) and is capped at both ends by an unreactive telomere (TEL). Each has suffered one radiation-induced break, liberating eight reactive (broken) ends that all seek to rejoin with one another. In this scenario, the break-end on the centromeric segment of the blue chromosome, rather that rejoining with its partner, has initiated an interchange by joining with the broken end provided by the centromeric segment of the yellow chromosome. This is allowed because the two broken ends are well within the maximum distance over which such interaction can take place, as designated by the bluish halo centered on the break. (Each of the four beaks has its own halo; not shown.) If the break-end on the remaining yellow acentric fragment had joined back to the break-end of its acentric blue counterpart, a simple exchange (dicentric) would have resulted. Instead, it has joined to the break on the centromeric segment of the green chromosome. The exchange is now destined to become complex, which would have been complete if the green acentric fragment had joined to the original leftover break from the blue chromosome. But it did not. The nascent complex exchange became even larger (now involving four breakpoints) when the acentric green fragment instead joined up with the centric red chromosome segment. At this point the two once-reactive broken ends (marked by the white stars) remain unrejoined, without partners. Under the right conditions, they might have found each another, completing the four-way exchange. In this particular case, however, they are simply too far apart and an incomplete complex exchange results. Panel B: mFISH image produced by this exchange as it would appear at mitosis. While the bicolored dicentric and acentric fragment indicate the involvement of both the blue and red chromosomes in the exchange, the presence of the acentric fragment from the blue chromosome and the truncated red chromosome mark this exchange as incomplete. Panel C: Telomere staining together with whole chromosome painting would not always distinguish between terminals and incompletes when complex exchanges are involved. That would depend on which particular chromosome of the exchange was painted. In case of the yellow chromosome or the green chromosome, for that matter the resulting hybridization pattern would correctly identify the exchange as complex, involving (minimally) three breakpoints. However the observer would probably incorrectly conclude that the leftmost blue acentric fragment derived from a terminal deletion. Unpainted chromosomes are labeled blue to indicate nonspecific counterstaining. Traditional Giemsa staining of the same rearrangement at metaphase would reveal an excess linear acentric fragment of unknown origin, and would miss altogether the complex nature of the rearrangement.
At the heart of the proposed hypothesis is the assumption that, as the CEx begins to involve more and more breakpoints, there is a tendency for breaks in this otherwise proximate constellation to become isolated from the rejoining reaction due to diminished spatial proximity. This creates an ‘‘odd-man-out’’ scenario whereby the final break ends, because they are no longer proximate, will result in an incomplete exchange. We half-whimsically have dubbed this hypothesis, ‘‘A Break Too Far’’, a nickname used throughout the following discussion to describe the above scenario. To reiterate, this process makes no assumptions about the qualitative nature of broken ends regarding their inherent reparability from a molecular or biochemical standpoint. Otherwise, for example, in the case of NHEJ one can easily substitute DSBs for chromosome breaks without loss of meaning.
A testable aspect of the Break Too Far hypotheses is that incompletes show a preference for exchanges with large numbers of breakpoints. Before proceeding with this line of reasoning, it becomes necessary to introduce into the discussion some rudimentary concepts and definitions regarding the formation of CExs. (The casual reader may elect to skip over the next few paragraphs in this section and go directly to a discussion of Fig. 3, while still retaining some sense of the argument being made.)
FIG. 3.
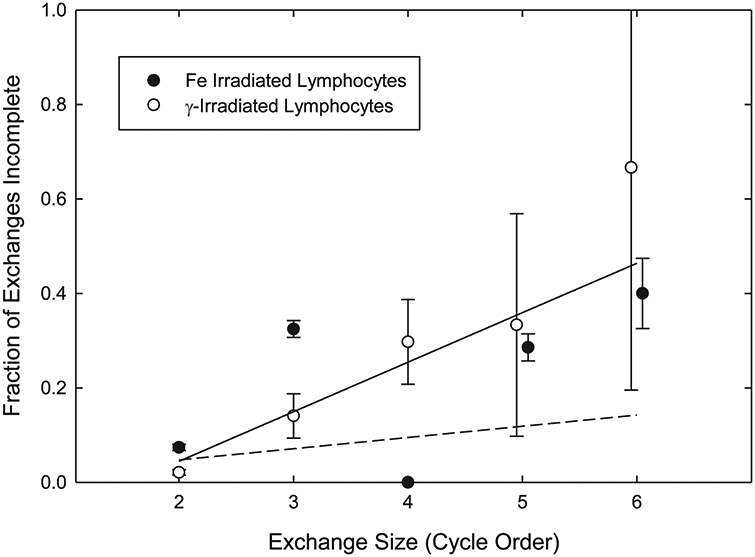
Fraction of incomplete exchanges found in lymphocytes, plotted against the discrete number of breakpoints they contain (i.e., their size). For reasons explained in the text, only exchanges with full cycle structures are considered. Solid symbols are for 1 GeV 56Fe ions; open symbols are for γ rays. The solid line depicts a combined linear fit through both sets of data, and includes the seemingly errant point associated with four-break category for 56Fe, which we attribute to the vagaries of sampling from a small number of observations. Otherwise, note the positive correlation between incomplete exchange breakpoints and the size of the exchange they belong to. The dashed line shows the response that would be predicted in the absence of the break-too-far effect (see text for a full explanation). Uncertainties are standard errors of the mean.
It is important to recognize that not all CExs, even those having the same number of breakpoints, take the same functional form regarding proximateness. In that vein, it is instructive to consider the formation of tricentric chromosomes, which require the misrejoining of 4 breaks distributed among three chromosomes (CAB 3/4/4). Although they are bona fide CExs, the 4 breaks do not need to belong to the same proximate break cluster. Instead, this aberration may be thought of as being formed by two simple pairwise exchanges that just happen to take place on opposite ends of the same chromosome. Seen in this way, a tricentric is nothing more than a concatamer of two separate simple exchanges (dicentrics) strung together by the involvement of a common chromosome. The term sequential exchange complex (SEC) is used to describe situations like this, in which a CEx can be reduced to a combination of rejoining reactions that are more simple. We will not directly concern ourselves with SECs here, except to say that there are other types of CExs in the same 3/4/4 family (and many other families) for which this is most certainly not the case. CExs of this latter type are called irreducible, meaning that during their formation all breakpoints were necessarily involved in the same rejoining reaction. Irreducible CExs are said to have “full-cycle” structures, and they figure prominently in the discussion below. The curious are directed to an in-depth discussion of these and related aspects of CEx formation (43). For our purposes, suffice it to say that the in situ hybridization patterns produced by mFISH can distinguish between CExs that take either SEC or full-cycle forms.
The astute reader may recognize the diagram of Fig. 2A as representing a 4/4/4 CEx of the full-cycle variety that has failed to come to closure via a Break Too Far scenario. By mFISH, it would be revealed at mitosis as an incomplete exchange (Fig. 2B). Following traditional Giemsa staining, it would appear as a single excess linear acentric fragment (excess means in addition to that accounted for by the dicentric element) and by convention would be unwittingly scored as a terminal deletion. It is instructive to imagine the staining patterns that would result from single-color painting combined with a pan telomeric probe. This, of course, would depend on which particular chromosome one imagines to have painted. But, overall the incomplete nature of this particular exchange would fail to be recognized half of the time, an example of which is shown in Fig. 2C.
We are now in a position to restate with more refinement the previously mentioned prediction attached to the Break Too Far hypothesis, namely that incompletes should be preferentially found in exchange aberrations with large numbers of breaks. To this prediction, we now add the stipulation (as implied by Fig. 2A) that the breaks under consideration be part of a constellation of proximate breaks, that is, only those breaks having the opportunity to interact with one another. In principle, this stipulation could be applied to individual rejoining reactions within SECs. Using the tricentric example above, each of the presumptively separate simple exchange reactions could be assessed with respect to incompleteness. However, SECs are frequently more complicated than in this example. Recognizing SEC configurations within reducible CExs that have more than four breaks can be tricky and do not always avail themselves to unique solutions (43). As a practical matter, the only reliable way to assess the outcome of incomplete-ness, as it pertains to proximate breaks, is by confining analysis to CExs of full-cycle structure.
For reasons given above, Fig. 3 is concerned only with full-cycle exchanges. These are binned by the discrete number of breaks they contain, which is referred to as their order. Exchanges of order 2 represent simple pairwise exchanges such as dicentrics and translocations, exchanges of higher order are complex, and exchanges within each order can be either complete or incomplete. Figure 3 shows the fraction of exchanges within each order that is incomplete. Individual data points attached to each order are relatively large and sometimes involve a significant degree of scatter about the best linear fit. Nevertheless, there is an overall positive correlation between cycle order and the relative fraction of incomplete exchanges, meaning that the likelihood of finding an incomplete configuration increases with the ‘‘size’’ of the exchange. Equally important to note is that this tendency seems to apply to both 56Fe ions and γ rays, suggesting that it is not dependent on LET. By satisfying these conditions, the data of Fig. 3 support the basic tenets of the Break Too Far hypothesis.
More formally, we can state that a necessary condition for the Break Too Far scenario is met when an initial constellation of otherwise proximate breaks encompasses at least two broken ends (usually from different chromosomes) of which physical distance apart precludes their rejoining. Moreover, since the maximum physical distance apart will, in some way, depend on the number of proximate breaks, and since the model makes no operational distinction concerning qualitative differences among the breaks themselves, it is a general feature of the model that complex exchanges exhibit an increasing tendency for incomplete exchange as the number of proximate breaks increases.
The ‘‘physical distance apart’’ aspect of the model deserves further inspection. It stands to reason that, quite apart from the spatial considerations affecting a Break Too Far, there would be an inherent tendency for exchanges with large numbers of breaks to spawn more incomplete elements compared to those with fewer breaks (44). This is a virtual truism since on the basis of random probability alone each additional break serves to incrementally increase the likelihood of incompleteness. To assess the magnitude of this ‘‘random confounding effect’’ we concentrated on incomplete simple exchanges (i.e., those of order 2). Ignoring terminal deletions for this exercise, of the 1,037 simple exchanges tallied, 44 were incomplete (data not shown). Since simple exchanges involve exactly two breakpoints, this translates into an ‘‘incompleteness rate’’ of 0.021 ± 0.003 per breakpoint. When multiplied by each of the respective cycle orders shown in Fig. 3, this rate gives the estimated magnitude of the confounding effect (dashed line). As shown in Fig. 3, although the effect is not altogether trivial, it is too small to explain the rising level of incompleteness associated with the ‘‘proximate size’’ of the exchange. Thus it is not, strictly speaking, the number of breaks that is responsible for the ‘‘size effect’’. In our minds, this fact lends credibility to the interpretation that the fundamental variable at play relates to the maximum distance between proximate breaks. Stated another way, even when adjusted for the absolute number of breaks contained within various full-cycle exchanges, the expectations of a Break Too Far are met.
Misrepair
The intent of this paper is to point out some noteworthy observations regarding the unrepaired component of cytogenetic lesions, which, by any reasonable cytogenetic measure, is small by comparison to overall residual damage. It should therefore be clear that any serious attempt to explain RBE-LET relationships will necessitate a consideration of misrepair events that cause complete exchanges as well. Returning to a comment made in the Introduction section with respect to unrepaired damage, we stated that making an LET-dependent connection between chromosome exchanges and unrepaired complex DSBs would require additional assumptions. If it is assumed that complex DSBs remain unrejoined because they are inefficiently repaired, then it also seems reasonable to assume that they would remain reactive, at least temporarily, during which time they may serve as substrates for subsequent lesion processing that ultimately leads to misrepair.
There is a least one molecular paradigm for which this situation would seem to apply. A relatively recent notion has emerged that there are two related aspects to NHEJ. The historically more familiar ‘‘canonical’’ NHEJ pathway (henceforth termed c-NHEJ) requires little or no overlapping sequence homology between breaks and is responsible for the majority of DSB repair in mammalian cells. This would include eurepair (restitution) at the cytogenetic level. In addition, alternate NHEJ (alt-NHEJ) pathways are now known to exist, the rejoining of which relies on limited (a few base pairs) DNA sequence homology, and which experimental evidence collectively suggests is principally responsible for translocations (64–69). Importantly, such micro homology-mediated alt-NHEJ reactions, which depend on the resection protein CtIP, are thought to participate in rejoining when the c-NHEJ process fails or is in some way bypassed (69). From a biophysical standpoint it is worth mentioning that the proposed dual roles for NHEJ makes it compatible with traditional models of aberration formation that have existed for decades (70) in the sense that misrejoining is defined as the interaction of two radiogenic lesions. We hazard to assume that the LET dependence of complex DSB formation has been established to the satisfaction of most investigators in that field of study. What remains to be seen is whether the types (or increased amounts of) complex DSBs produced by high-LET radiation and the types of breaks causing bypass of canonical NHEJ pathway are one in the same.
Nuclear Geometry
Again, given the retrospective nature of the data involved, ideal comparisons among different cell types and radiations were not always possible. That being said, we now turn the focus to unrejoined chromosome damage in the context of nuclear geometry, a parameter almost certain to affect an assessment of the Break Too Far hypothesis (71). The issue arises due to stark differences in shape between the nuclei of fibroblasts and lymphocytes. The former can be described as flattened ellipses with a mean thickness of 1.2 μm (72), while the latter are adequately modeled as 6 pm diameter spheres (61).
The last column of Table 2 shows the ratio of open breaks from CExs to open breaks from simple exchanges. In accordance with the predictions of the model, that ratio is significantly greatly than unity. The sole exception involves α particles delivered to fibroblasts. This seemingly discordant result may be explained by considering the flattened shape of fibroblast nuclei with respect to α-particle track structure. The absorbed doses used in these experiments are the result of relatively few orthogonal tracks traversing the thickness (z dimension) of the nucleus. The secondary ionizations associated with each such track (δ rays) are radially confined in the x-y dimensions to within a few nanometers of the trajectory of the track (73). For all practical purposes this means that damage produced by the interaction of multiple α particle tracks cannot occur. It also means that the narrow, densely ionizing-particle trail travels through only 1.2 pm of chromatin. To produce a complex exchange, this short segment must traverse multiple interphase chromosome domains (74), which seems somewhat unlikely to us (at least by comparison to the dimensions of a lymphocyte nucleus).
The situation for fibroblasts and γ rays is fundamentally different in the following way. For the same dose, a comparable number of ionizations is distributed among a huge number of tracks, which consequently deposit their collective energy in a more-or-less uniform pattern throughout the nuclear volume. This allows for damage interaction between independent tracks, as aptly confirmed by the upward curvature in the dose response for complex breakpoints produced in fibroblasts (38). This ‘‘3D aspect’’ of track interaction is further enhanced as one considers the spherical geometry of lymphocyte nuclei, and explains the associated large complex-to-simple ratio for open breaks shown in Table 2.
In theory, the ratio might be expected to exceed unity for α particles delivered to lymphocytes, not because of intertrack interaction, but because a particle’s mean path through a 6 μm sphere stands a better chance of depositing energy in multiple interphase chromosome domains than does the path through a 1.2 pm thick flattened ellipse. In other words, the predicted Break Too Far effect would be mediated solely by intra-track action. Unfortunately, the available data set does not include an α-particle lymphocyte combination. We recognize the potential value that such data may provide in furthering the testing of the hypothesis and hope to revisit the issue as resources allow.
Concluding Remarks
As for providing an explanation for RBE/LET relationships, we do not wish to leave the impression that we regard qualitative differences in DNA damage as lacking merit. Actually, there are data within Table 1 that suggests such a qualitative difference. For example, although most of the unrepaired damage following 56Fe ion exposure is due to incomplete exchanges, the relative frequency of damage from terminal deletions (5.9%) is roughly twofold higher than that for the corresponding values for any of the remaining three radiations. The proposed Break Too Far mechanism does nothing to explain this result, because it does not apply to terminal deletions. We have no tenable explanation for this observation other than to suggest that the difference derives in some way from dissimilarities in track structure. We prefer not to speculate as to how such differences might manifest to produce a qualitatively different response by the cell, and whether such differences, real or imagined, relate to targets of nanometer versus micrometer dimensions.
It was only after dissecting unrepaired damage into its individual components, terminal deletions and incomplete exchanges, that we discovered some curious correlations between the latter and radiation quality. The Break Too Far hypothesis was born from an attempt to make sense of these observations. We do not claim to have developed a new model suddenly capable of explaining all unresolved issues pertaining to RBE-LET relationships. Nor do we claim that unrepaired damage is paramount in that regard. On the contrary, we see our results as confirming a conclusion that has been either stated or strongly implied by a number of cytogeneticists: unrepaired chromosome breaks make a relatively minor contribution to residual chromosome damage seen at mitosis. It is only with respect to this contribution that the Break Too Far hypothesis offers a partial explanation of RBE-LET effects.
We do not discount the idea that LET-dependent differences in the unrepaired component may exist, but think these have more to do with track structure than with average ionization density. However, we want to be clear on one point: by the strict definition we apply, it is an inescapable conclusion that unrepaired damage fails to explain RBE-LET relationships that depend on qualitative differences in the nature of initial radiogenic DSBs. That much is not conjecture. However, we remain receptive to the possibility that LET-dependent, qualitative differences in DSBs can manifest themselves as changes in RBE if they are subject to additional processing that lead to misrepair.
Results of this work and the work of others (54) suggest it may be possible to distinguish cellular damage produced by radiations of different track structure, particularly if that distinction relies on the unrepaired component. The ultimate goal in that direction would be a “cytogenetic signature’’ of prior unknown exposure that could be used to reconstruct absorbed dose and radiation quality (75). From a practical standpoint, we are somewhat skeptical concerning the routine use of mFISH to screen large numbers of exposed individuals. Nevertheless, in principle, the results described here it may find theoretical application in the area of biodosimetry.
There is no particular reason that the basic premise of the hypothesis we propose needs to be restricted to recombinational events spanning large fractions of a micron. In fact, there are indications that other investigators have previously considered applying similar principles to the rejoining of DSBs separated instead by tens of nanometers [Johnston and Hill, unpublished; cited within ref. (28)]. Beyond that, our findings hopefully provide some insight into fundamental mechanisms governing the cellular processing of radiation damage. As the focus here is chromosome aberrations, and since this end point is causally implicated in cell killing, mutation and carcinogenesis, we hope that an enhanced understanding of their formation will lead to a greater appreciation of health risks associated with exposure to ionizing radiation.
DEDICATION
Having benefited from the tutelage provided by him over the years, and in recognition of the profound influence he has had on both our lives, the authors would like to dedicate this paper to Dr. Joel S. Bedford.
ACKNOWLEDGMENTS
The authors thank Drs. Dudley Goodhead (Medical Research Council, retired) and Peter O’Neill of the Gray Institute for provocative discussion. Thanks also to Drs. Susan Bailey (Colorado State University) and Edwin Goodwin (Los Alamos National Laboratory, retired) for help with the alpha particle experiments. Thanks to Dr. Marco Durante at GSI for blood irradiations conducted at the NASA Space Radiation Laboratory, and for the considerable expert help provided there by Adam Rusek and staff. Research was supported by the Department of Energy and NASA/OBPR grant number DE-FG03–02ER63442. Additional funding was provided by NASA (NNJ04HD83G and NNX08AB65G) and the National Institutes of Health, NIH/NIAID (R01080486–02).
REFERENCES
- 1.Barendsen GW. Responses of cultured cells, tumors and normal tissues to radiations of different linear energy transfer. Curr Top Radiat Res 1968; 4:293–356. [Google Scholar]
- 2.Prise KM, Folkard M, Newman HC, Michael BD. Effect of radiation quality on lesion complexity in cellular DNA. Int J Radiat Biol 1994; 66:537–42. [DOI] [PubMed] [Google Scholar]
- 3.Hill MA. Radiation damage to DNA: The importance of track structure. Radiat Meas 1999; 31:15–23. [DOI] [PubMed] [Google Scholar]
- 4.Bedford JS, Goodhead DT. Breakage of human interphase chromosomes by alpha particles and x-rays. Int J Radiat Biol 1989; 55:211–6. [DOI] [PubMed] [Google Scholar]
- 5.Cornforth MN, Goodwin EH. The dose-dependent fragmentation of chromatin in human fibroblasts by 3.5-MeV alpha particles from 238Pu: Experimental and theoretical considerations pertaining to single-track effects. Radiat Res 1991; 127:64–74. [PubMed] [Google Scholar]
- 6.Loucas BD, Geard CR. Initial damage in human interphase chromosomes from alpha particles with linear energy transfers relevant to radon exposures. Radiat Res 1994; 139:9–14. [PubMed] [Google Scholar]
- 7.Sax K Chromosome aberrations induced by x-rays. Genetics 1938; 23:494–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sax K Time factor in x-ray production of chromosome aberrations. Proc Natl Acad Sci U S A 1939; 25:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catcheside DG, Lea DE, Thoday JM. Types of chromosome structural change induced by the irradiation of Tradescantia microspores. J Genetics 1946; 47:113–36. [DOI] [PubMed] [Google Scholar]
- 10.Lea DE. Actions of radiations on living cells. Cambridge, England: Cambridge University Press; 1955. [Google Scholar]
- 11.Cornforth MN, Bedford JS. Ionizing radiation damage and its early development in chromosomes In: Lett JT, Sinclair WK, editors. Advances in radiation biology. San Diego: Academic Press; 1993. P. 423–96. [Google Scholar]
- 12.Cornforth MN. Radiation-induced damage and the formation of chromosomal aberrations In: Nickoloff JA, Hoekstra M, editors. DNA damage and repair. New Jersey: Humana Press; 1998. P. 559–85. [Google Scholar]
- 13.Sachs RK, Levy D, Chen AM, Simpson PJ, Cornforth MN, Ingerman EA, et al. Random breakage and reunion chromosome aberration formation model; an interaction-distance version based on chromatin geometry. Int J Radiat Biol 2000; 76:1579–88. [DOI] [PubMed] [Google Scholar]
- 14.Sachs RK, Levy D, Hahnfeldt P, Hlatky L. Quantitative analysis of radiation-induced chromosome aberrations. Cytogenet Genome Res 2004; 104:142–8. [DOI] [PubMed] [Google Scholar]
- 15.Cornforth MN. Perspectives on the formation of radiation-induced exchange aberrations. DNA Repair 2006; 5:1182–91. [DOI] [PubMed] [Google Scholar]
- 16.Goodhead DT. The initial physical damage produced by ionizing radiations. Int J Radiat Biol 1989; 56:623–34. [DOI] [PubMed] [Google Scholar]
- 17.Prise KM. Use of radiation quality as a probe for DNA lesion complexity. Int J Radiat Biol 1994; 65:43–8. [DOI] [PubMed] [Google Scholar]
- 18.Ward JF. The complexity of DNA damage: Relevance to biological consequences. Int J Radiat Biol 1994; 66:427–32. [DOI] [PubMed] [Google Scholar]
- 19.Goodhead DT. Saturable repair models of radiation action in mammalian cells. Radiat Res 1985; 8:S58–67. [PubMed] [Google Scholar]
- 20.Sutherland BM, Bennett PV, Schenk H, Sidorkina O, Laval J, Trunk J, et al. Clustered DNA damages induced by high and low LET radiation, including heavy ions. Physica Medica 2001; 17(Suppl 1):202–4. [PubMed] [Google Scholar]
- 21.Brenner DJ, Ward JF. Constraints on energy deposition and target size of multiple damaged sites associated with DNA double-strand breaks. Int J Radiat Biol 1992; 61:737–48. [DOI] [PubMed] [Google Scholar]
- 22.Nikjoo H, O’Neill P, Terrissol M, Goodhead DT. Quantitative modelling of DNA damage using Monte Carlo track structure method. Radiati Environ Bioph 1999; 38:31–8. [DOI] [PubMed] [Google Scholar]
- 23.Nikjoo H, O’Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res 2001; 156:577–83. [DOI] [PubMed] [Google Scholar]
- 24.Dobbs TA, Palmer P, Maniou Z, Lomax ME, O’Neill P. Interplay of two major repair pathways in the processing of complex doublestrand DNA breaks. DNA Repair 2008; 7:1372–83. [DOI] [PubMed] [Google Scholar]
- 25.Hada M, Georgakilas AG. Formation of clustered DNA damage after high-LET irradiation: A review. J Radiat Res 2008; 49:203–10. [DOI] [PubMed] [Google Scholar]
- 26.Olive PL. The role of DNA single- and double-strand breaks in cell killing by ionizing radiation. Radiat Res 1998; 150:S42–51. [PubMed] [Google Scholar]
- 27.Pastwa E, Neumann RD, Mezhevaya K, Winters TA. Repair of radiation-induced DNA double-strand breaks is dependent upon radiation quality and the structural complexity of double-strand breaks. Radiat Res 2003; 159:251–61. [DOI] [PubMed] [Google Scholar]
- 28.Pinto M, Prise KM, Michael BD. Evidence for complexity at the nanometer scale of radiation-induced DNA DSBs as a determinant of rejoining kinetics. Radiat Res 2005; 164:73–85. [DOI] [PubMed] [Google Scholar]
- 29.Asaithamby A, Chen DJ. Mechanism of cluster DNA damage repair in response to high-atomic number and energy particles radiation. Mutat Res 2011; 711:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asaithamby A, Hu B, Chen DJ. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc Natl Acad Sci USA 2011; 108:8293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobrich M, Kuhne M, Wetzel J, Rothkamm K. Joining of correct and incorrect DNA double-strand break ends in normal human and ataxia telangiectasia fibroblasts. Genes Chromos Cancer 2000; 27:59–68. [PubMed] [Google Scholar]
- 32.Kuhne M, Rothkamm K, Lobrich M. No dose-dependence of DNA double-strand break misrejoining following alpha-particle irradiation. Int J Radiat Biol 2000; 76:891–900. [DOI] [PubMed] [Google Scholar]
- 33.Rothkamm K, Kuhne M, Jeggo PA, Lobrich M. Radiation-induced genomic rearrangements formed by nonhomologous end-joining of DNA double-strand breaks. Cancer Res 2001; 61:3886–93. [PubMed] [Google Scholar]
- 34.Revell SH. Evidence for a dose squared term in the dose-response curve for real chromatid discontinuties induced by x-rays, and some theoretical consequences thereof. Mutat Res 1966; 3:34–53. [DOI] [PubMed] [Google Scholar]
- 35.Revell SH. The breakage-and-reunion theory and the exchange theory for chromosomal aberrations induced by ionizing radiations: A short history In: Lett JT, Alder H, Zelle M, editors. Advances in radiation biology. New York: Academic Press; 1974. P. 367–416. [Google Scholar]
- 36.Brewen JG, Brock RD. The exchange hypothesis and chromosome-type aberrations. Mutat Res 1968; 6:245–55. [Google Scholar]
- 37.Savage JRK. On the nature of visible chromosomal gaps and breaks. Cytogenet Genome Res 2004; 104:46–55. [DOI] [PubMed] [Google Scholar]
- 38.Loucas BD, Durante M, Bailey SM, Cornforth MN. Chromosome damage in human cells by γ rays, α particles and heavy ions: Track interactions in basic dose-response relationships. Radiat Res 2013; 179:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durante M, Furusawa Y, Gotoh E. A simple method for simultaneous interphase-metaphase chromosome analysis in biodosimetry. Int J Radiat Biol 1998; 74:457–62. [DOI] [PubMed] [Google Scholar]
- 40.Inkret WC, Eisen Y, Harvey WF, Koehler AM, Raju MR. Radiobiology of alpha particles. I. Exposure system and dosimetry. Radiat Res 1990; 123:304–10. [PubMed] [Google Scholar]
- 41.Loucas BD, Cornforth MN. Complex chromosome exchanges induced by gamma rays in human lymphocytes: An mFISH study. Radiat Res 2001; 155:660–71. [DOI] [PubMed] [Google Scholar]
- 42.Loucas BD, Eberle R, Bailey SM, Cornforth MN. Influence of dose rate on the induction of simple and complex chromosome exchanges by gamma rays. Radiat Res 2004; 162: 339–49. [DOI] [PubMed] [Google Scholar]
- 43.Cornforth MN. Analyzing radiation-induced complex chromosome rearrangements by combinatorial painting. Radiat Res 2001; 155:643–59. [DOI] [PubMed] [Google Scholar]
- 44.Fomina J, Darroudi F, Natarajan AT. Accurate detection of true incomplete exchanges in human lymphocytes exposed to neutron radiation using chromosome painting in combination with a telomeric PNA probe. Int J Radiat Biol 2001; 77:1175–83. [DOI] [PubMed] [Google Scholar]
- 45.Levy D, Vazquez M, Cornforth M, Loucas B, Sachs RK, Arsuaga J. Comparing DNA damage-processing pathways by computer analysis of chromosome painting data. J Comput Biol 2004; 11:626–41. [DOI] [PubMed] [Google Scholar]
- 46.Vives S, Loucas B, Vazquez M, Brenner DJ, Sachs RK, Hlatky L, et al. SCHIP: Statistics for chromosome interphase positioning based on interchange data. Bioinformatics 2005; 21:3181–2. [DOI] [PubMed] [Google Scholar]
- 47.Cornforth MN, Bailey SM, Goodwin EH. Dose responses for chromosome aberrations produced in noncycling primary human fibroblasts by alpha particles, and by gamma rays delivered at sublimiting low dose rates. Radiat Res 2002; 158:43–53. [DOI] [PubMed] [Google Scholar]
- 48.Bauchinger M, Schmid E. LET dependence of yield ratios of radiation-induced intra- and interchromosomal aberrations in human lymphocytes. Int J Radiat Biol 1998; 74:17–25. [DOI] [PubMed] [Google Scholar]
- 49.Wu H, Durante M, George K, Yang TC. Induction of chromosome aberrations in human cells by charged particles. Radiat Res 1997; 148:S102–7. [PubMed] [Google Scholar]
- 50.Wu H, George K, Yang TC. Estimate of true incomplete exchanges using fluorescence in situ hybridization with telomere probes. Int J Radiat Biol 1998; 73:521–7. [DOI] [PubMed] [Google Scholar]
- 51.Wu H, George K, Yang TC. Estimate of the frequency of true incomplete exchanges in human lymphocytes exposed to 1 GeV/μ Fe ions in vitro. Int J Radiat Biol 1999; 75: 593–9. [DOI] [PubMed] [Google Scholar]
- 52.Fomina J, Darroudi F, Boei JJ, Natarajan AT. Discrimination between complete and incomplete chromosome exchanges in x-irradiated human lymphocytes using FISH with pan-centromeric and chromosome specific DNA probes in combination with telomeric PNA probe. Int J Radiat Biol 2000; 76:807–13. [DOI] [PubMed] [Google Scholar]
- 53.Durante M, George K, Cucinotta FA. Chromosomes lacking telomeres are present in the progeny of human lymphocytes exposed to heavy ions. Radiat Res 2006; 165:51–8. [DOI] [PubMed] [Google Scholar]
- 54.Deng W, Morrison DP, Gale KL, Lucas JN. A comparative study on potential cytogenetic fingerprints for radiation LET in human lymphocytes. Int J Radiat Biol 2000; 76:1589–98. [DOI] [PubMed] [Google Scholar]
- 55.Fomina J, Darroudi F, Natarajan AT. Incomplete chromosome exchanges are not fingerprints of high-LET neutrons. Radiat Prot Dosim 2002; 99:215–6. [DOI] [PubMed] [Google Scholar]
- 56.Speicher MR, Gwyn Ballard S, Ward DC. Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nat Genet 1996; 12:368–75. [DOI] [PubMed] [Google Scholar]
- 57.Schrock E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA, et al. Multicolor spectral karyotyping of human chromosomes. Science 1996; 273:494–7. [DOI] [PubMed] [Google Scholar]
- 58.Cornforth MN, Bedford JS. A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat Res 1987; 111:385–405. [PubMed] [Google Scholar]
- 59.Sabatier L, Al Achkar W, Hoffschir F, Luccioni C, Dutrillaux B. Qualitative study of chromosomal lesions induced by neutrons and neon ions in human lymphocytes at G0 phase. Mutat Res 1987; 178:91–7. [DOI] [PubMed] [Google Scholar]
- 60.Griffin CS, Marsden SJ, Stevens DL, Simpson P, Savage JRK. Frequencies of complex chromosome exchange aberrations induced by 238Pu alpha-particles and detected by fluorescence in situ hybridization using single chromosome-specific probes. Int J Radiat Biol 1995; 67:431–9. [DOI] [PubMed] [Google Scholar]
- 61.Anderson RM, Marsden SJ, Wright EG, Kadhim MA, Goodhead DT, Griffin CS. Complex chromosome aberrations in peripheral blood lymphocytes as a potential biomarker of exposure to high-LET alpha-particles. Int J Radiat Biol 2000; 76:31–42. [DOI] [PubMed] [Google Scholar]
- 62.Wu H, Durante M, Furusawa Y, George K, Kawata T, Cucinotta FA. M-FISH analysis of chromosome aberrations in human fibroblasts exposed to energetic iron ions in vitro. Adv Space Res 2003; 31:1537–42. [DOI] [PubMed] [Google Scholar]
- 63.Savage JR, Simpson PJ. FISH ‘‘painting’’ patterns resulting from complex exchanges. Mutat Res 1994; 312:51–60. [DOI] [PubMed] [Google Scholar]
- 64.Iliakis G, Wang H, Perrault AR, Boecker W, Rosidi B, Windhofer F, et al. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogen Genome Res 2004; 104:14–20. [DOI] [PubMed] [Google Scholar]
- 65.Nussenzweig A, Nussenzweig MC. A backup DNA repair pathway moves to the forefront. Cell 2007; 131:223–5. [DOI] [PubMed] [Google Scholar]
- 66.Bennardo N, Cheng A, Huang N, Stark JM. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet 2008; 4: e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McVey M, Lee SE. MMEJ repair of double-strand breaks (director’s cut): deleted sequences and alternative endings. Trends Genet 2008; 24:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zha S, Boboila C, Alt FW. Mre11: Roles in DNA repair beyond homologous recombination. Nat Struct Mol Biol 2009; 16:798–800. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Jasin M. An essential role for CtIP in chromosomal translocation formation through an alternative end-joining pathway. Nat Struct Mol Biol 2011; 18:80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Savage JRK. A brief survey of aberration origin theories. Mutat Res 1998; 404:139–47. [DOI] [PubMed] [Google Scholar]
- 71.Durante M, Pignalosa D, Jansen JA, Walboomers XF, Ritter S. Influence of nuclear geometry on the formation of genetic rearrangements in human cells. Radiat Res 2010; 174:20–6. [DOI] [PubMed] [Google Scholar]
- 72.Cornforth MN, Schillaci ME, Goodhead DT, Carpenter SG, Wilder ME, Sebring RJ, et al. Radiobiology of ultrasoft x rays. III. Normal human fibroblasts and the significance of terminal track structure in cell inactivation. Radiat Res 1989; 119:511–22. [PubMed] [Google Scholar]
- 73.Katz R, Cucinotta FA, Zhang CX. The calculation of radial dose from heavy ions: Predictions of biological action cross sections. Nucl Instrum Methods Phys Res Sect B-Beam Interact Mater Atoms 1996; 107:287–91. [DOI] [PubMed] [Google Scholar]
- 74.Anderson RM, Stevens DL, Goodhead DT. M-FISH analysis shows that complex chromosome aberrations induced by alpha-particle tracks are cumulative products of localized rearrangements. Proc Natl Acad Sci U S A 2002; 99:12167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ritter S, Durante M. Heavy-ion induced chromosomal aberrations: A review. Mutat Res Genet Toxicol Environ Mutagen 2010; 701:38–46. [DOI] [PubMed] [Google Scholar]


