Graphical abstract
Keywords: Pseudoaneurysm, Mitral-aortic intervalvular fibrosa, Echocardiography
Highlights
-
•
P-MAIVF is usually a complication of endocarditis or aortic valve surgery.
-
•
It is promptly recognized on transesophageal echocardiography.
-
•
It may cause symptoms and complications that warrant surgical intervention.
-
•
Surgical intervention remains the recommended management for P-MAIVF.
Introduction
Pseudoaneurysm of the mitral-aortic intervalvular fibrosa (P-MAIVF) was traditionally considered a rare, life-threatening sequela of endocarditis or valve surgery.
Pseudoaneurysm is an outpouching where the aortic and mitral valves meet and forms between the aorta and the left atrium with superior and posterior extension. It is contiguous with the left ventricular outflow tract, which differentiates it from an abscess.1, 2 In this article we present three consecutive cases and one highly suspicious case of P-MAIVF diagnosed at our institution using transesophageal echocardiography and discusses the causes, presentation, diagnosis, and management of that entity.
Case 1 Presentation
A 56-year-old white man presented with fevers and blood cultures positive for β-hemolytic Streptococcus group B.
Ten months prior, he was diagnosed with aortic valve endocarditis and an aortic root abscess, due to Streptococcus agalactiae infection. He was managed with aortic valve replacement with a 19-mm St. Jude mechanical prosthesis and patch reconstruction of the aortic root abscess. Transthoracic echocardiography upon discharge demonstrated a well-seated mechanical valve and a normal aortic root.
On his current presentation, initial transthoracic echocardiography showed mild aortic prosthesis regurgitation with no vegetation or evidence of perivalvular infection. Subsequently, transesophageal echocardiography revealed the presence of P-MAIVF, approximately 1.2 cm at its largest dimension (Figure 1). The pseudoaneurysm extended superiorly and posteriorly between the aorta, the left atrium, and the pulmonary artery and exhibited systolic expansion and diastolic collapse (Video 1, Video 2). There was no communication with the left atrium or the aorta and no signs of prosthetic valve dysfunction. Because of hemodynamic and electrical stability, the patient was treated with penicillin G and gentamycin for 1 month and subsequently underwent repair of the pseudoaneurysm with a bovine patch and received a new 19-mm mechanical aortic valve. He had an uneventful recovery and was discharged home 1 week later.
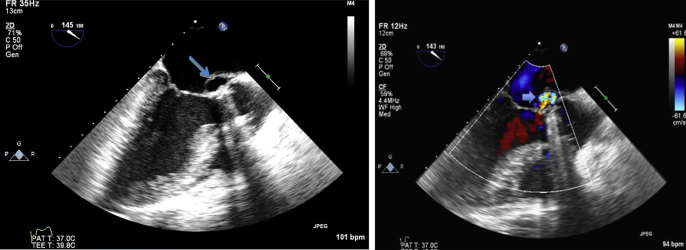
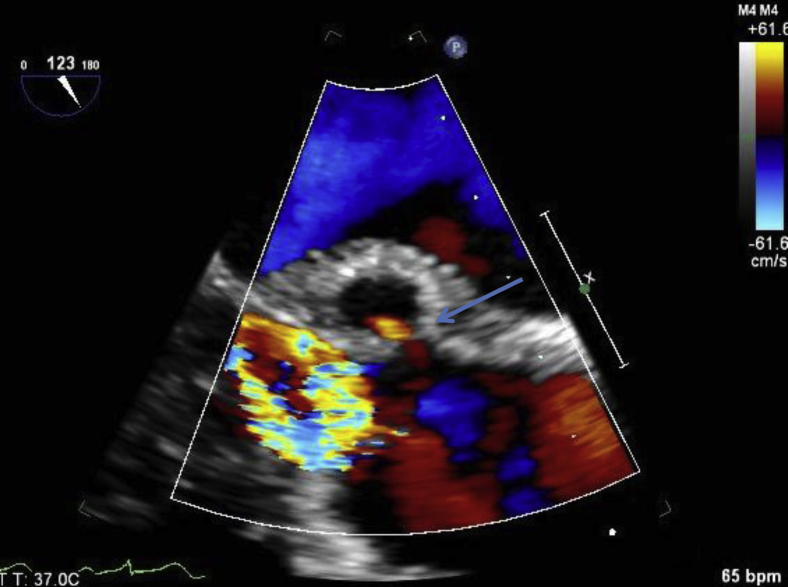
Figure 1.
Transesophageal echocardiogram, midesophageal long-axis views without color (left) and with color (right), demonstrating P-MAIVF. Both images were obtained during systole and show the characteristic systolic expansion of the pseudoaneurysm (arrows). The color Doppler image demonstrates the pseudoaneurysm's communication with the left ventricular outflow tract (thick arrow). A mechanical prosthesis in the aortic position is present.
Case 2 Presentation
An 84-year-old Caucasian man with diabetes and chronic stage IV kidney disease was referred for transesophageal echocardiography for evaluation of endocarditis.
Eighteen months previously, he had undergone single vessel (left internal mammary artery to left anterior descending coronary artery) coronary artery bypass graft surgery combined with aortic valve replacement with a 23-mm Magna bovine pericardial valve for severe aortic stenosis. Results of postoperative transthoracic echocardiography were unremarkable.
One month before his current presentation, he developed fevers and malaise. He was found to have transient staphylococcal bacteremia and was treated with 2 weeks of antibiotics. The fevers resolved, but the patient developed dyspnea on exertion and lower extremity edema. He was then referred for transesophageal echocardiography, which showed a well-seated aortic bioprosthesis with markedly thickened cusps, no vegetation, and mild transvalvular regurgitation. The images were highly suggestive of P-MAIVF without fistula formation or compression of adjacent structures (Figures 2 and 3, Video 3, Video 4, Video 5, Video 6). After discussion of the risks and benefits of complex surgical intervention in an elderly patient, he and his family opted against it. He was managed with chronic suppressive intravenous antibiotics with close clinical and imaging follow-up. He was alive 15 months after the diagnosis.
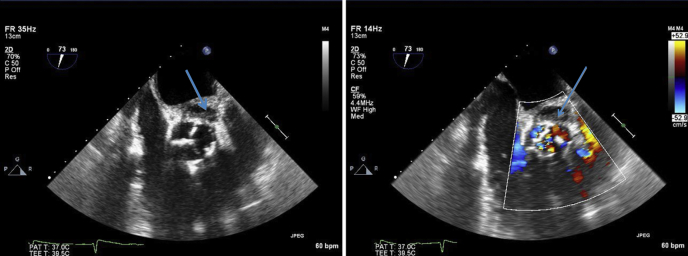
Figure 2.
Transesophageal echocardiogram, midesophageal short-axis views showing the pseudoaneurysm (arrows) at the level of the aortic valve without color (left) and with color (right).
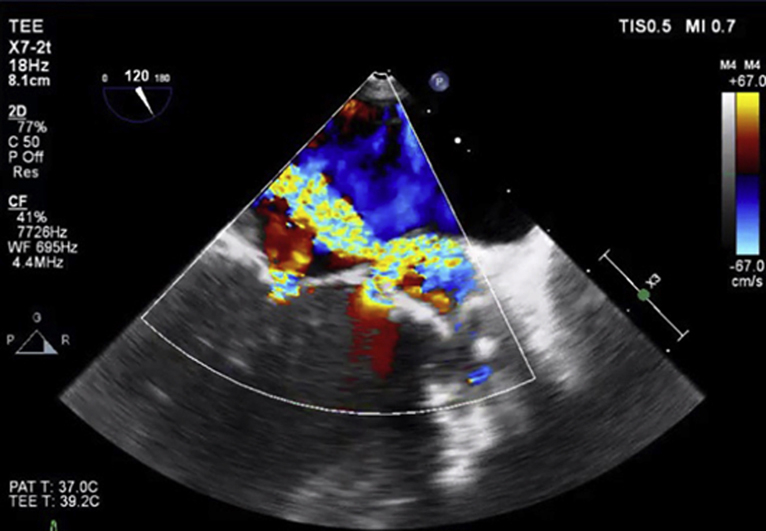
Figure 3.
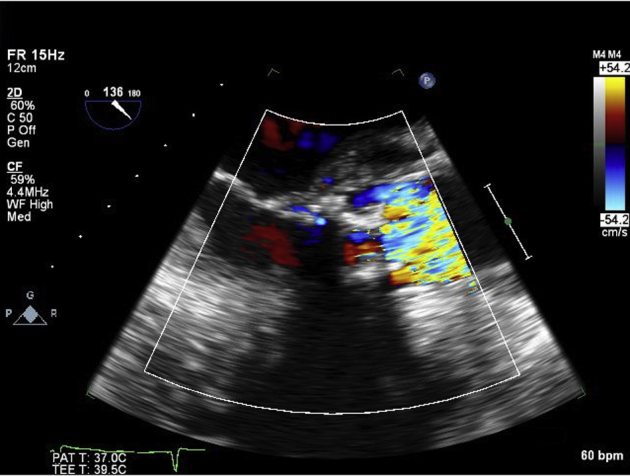
Image was obtained at the end of systole and demonstrates a thickened bioprosthesis in the aortic position with a possible pseudoaneurysm between the left atrium, the aorta, and the pulmonary artery.
Case 3 Presentation
A 67-year-old Caucasian man was referred for evaluation of dyspnea on exertion and weight gain of 1 week’s duration. He had a history of two-vessel bypass surgery in 2007.
Five months before the current presentation, he was admitted with fevers and disorientation. His blood cultures grew β-hemolytic Streptococcus group G. Transesophageal echocardiography at that time revealed a large vegetation on the noncoronary cusp of the aortic valve (1.7 × 0.2 cm) and an aortic root abscess. He underwent aortic valve replacement with a 23-mm bovine pericardial valve and patch repair of the aortic abscess. He was discharged 1 week later and completed a 2-week course of intravenous ceftriaxone.
On his current presentation, transesophageal echocardiography revealed P-MAIVF with characteristic systolic expansion and diastolic collapse, which measured 3.5 cm at its largest dimension. The pseudoaneurysm demonstrated a fistula to the left atrium (Figure 4, Figure 5, Figure 6, Video 7, Video 8, Video 9, Video 10, Video 11). Multiple blood cultures at that time were negative, and the patient had no fevers, but he was restarted prophylactically on ceftriaxone. He underwent a third cardiac surgical procedure at an outside institution per the family's wishes, which by report involved repeat aortic valve replacement with a tissue valve and repair of the pseudoaneurysm with an unremarkable postoperative course. After discharge, the patient had alleviation of his heart failure symptoms and was enrolled in cardiac rehabilitation. A follow-up transesophageal study 4 months later showed persistent P-MAIVF. Because of his disease complexity and multiple prior sternotomies, he was deemed at very high risk for a repeat intervention. He was managed with close clinical and echocardiographic follow-up. He has remained symptom free to date.
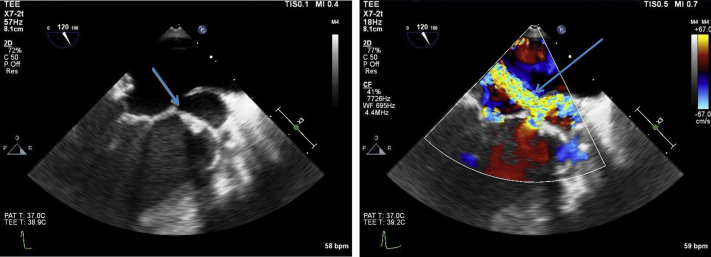
Figure 4.
Transesophageal echocardiogram, midesophageal zoomed long-axis views showing the pseudoaneurysm (arrows) without color (left) and with color (right) in midsystole. The image on the left is a two-dimensional picture of the large P-MAIVF. On color Doppler, a fistula between the pseudoaneurysm and the left atrium was demonstrated (arrow). During systole, the blood moved from the left ventricle into the pseudoaneurysm and then the left atrium. There was no diastolic flow, as the pseudoaneurysm was not pressurized in diastole.
Figure 5.
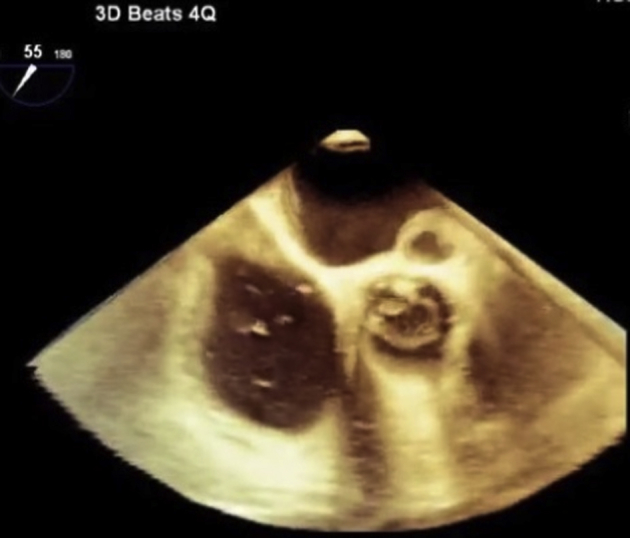
Three-dimensional representation of the pseudoaneurysm in systole in the short-axis view.
Figure 6.
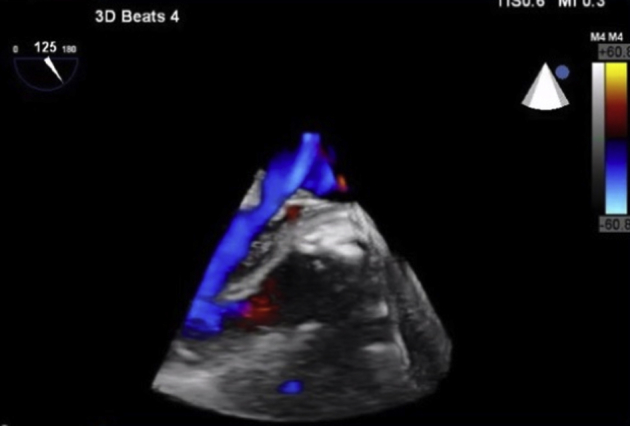
Three-dimensional long-axis view with color demonstrating the communication of the pseudoaneurysm with the left ventricular outflow tract obstruction.
Case 4 Presentation
A 43-year-old African American woman presented with right upper extremity pain and was found to have a thrombus in the right axillary/brachial artery, for which she underwent thrombectomy. The patient had a history significant for end-stage renal disease requiring hemodialysis three times per week through an arteriovenous fistula in the right arm and two failed renal transplantations. Upon presentation she was afebrile, and blood cultures grew Streptococcus epidermidis. The same bacterium grew from the excised right upper arm thrombus. Transthoracic echocardiography showed normal biventricular systolic function and an 11.7 × 8 mm vegetation in the aortic valve with associated severe aortic regurgitation. Electrocardiography showed new first-degree atrioventricular block. Transesophageal echocardiography confirmed the presence of a large vegetation in the aortic valve with significant regurgitation. The mitral-aortic intervalvular fibrosa appeared thickened, consistent with an aortic root abscess, and demonstrated what appeared to be the beginning of pseudoaneurysm formation (Figure 7), which had a small communication to the aorta (Figure 8). The pseudoaneurysm demonstrated minimal pulsatility and minimal systolic expansion and diastolic collapse (Video 12, Video 13). The patient underwent surgery, which involved extensive debridement of the intervalvular fibrosa and aortic valve replacement with a 19-mm St. Jude’s mechanical valve. After separation from cardioplegia, severe mitral regurgitation was noted, which was managed with a 26-mm Cosgrove annuloplasty ring, but moderate to severe mitral regurgitation persisted at the conclusion of the case. Unfortunately, 24 hours after surgery, the patient became unresponsive and apneic and developed pulseless electrical activity arrest. Despite prolonged resuscitation efforts, the patient expired.
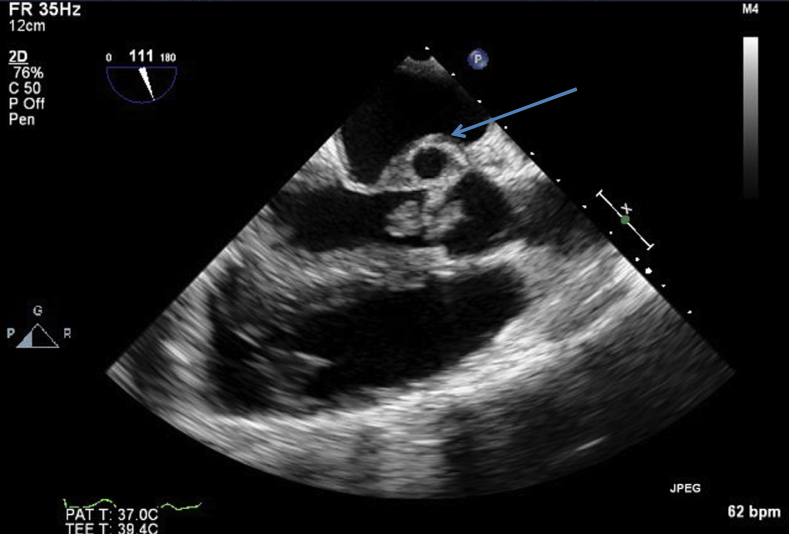
Figure 7.
Transesophageal echocardiogram, midesophageal zoomed long-axis view without color. A large vegetation in the aortic valve prolapsing in the left ventricular outflow tract and P-MAIVF are visualized (arrow).
Figure 8.
Transesophageal echocardiogram, midesophageal zoomed long-axis view with color demonstrating the pseudoaneurysm communicating with the aorta (arrow).
Discussion
Definitions
The mitral-aortic intervalvular fibrosa or aortomitral curtain is the fibrous, avascular region between the anterior leaflet of the mitral valve and the noncoronary cusp of the aortic valve.3 It is prone to infection and injury, and as a result a pseudoaneurysm may develop. Eighty-nine cases were reported in the literature from 1966 to 2009.1 Advances in imaging techniques and the widespread use of transesophageal echocardiography have led to its prompt recognition. As a result, 166 cases were reported by 2014, almost double the number in a span of 5 years.4
Etiology
Infection and surgical trauma are the most common causes of P-MAIVF.1, 2, 5 History or active endocarditis, most commonly involving the aortic valve, is the number one association with pseudoaneurysm formation.1, 2 Staphylococcus and Streptococcus spp are the most common causative microorganisms.1, 2, 5 Trauma from valve surgery without evidence of infection is the second most common cause of pseudoaneurysm formation.1, 2, 5 That is usually a sequela of aortic valve replacement6 but has been reported after ablation, repair of ventricular septal defects, and even cardiac catheterization.5 The presence of aortic regurgitation appears to contribute to pseudoaneurysm formation.7 A few cases of idiopathic pseudoaneurysms, with no identifiable causes, have been reported.8
Our four cases are in line with the discussed etiology of pseudoaneurysms. The first patient had active endocarditis of the aortic valve. Patients 2 and 4 had bacteremia without valve vegetation or destruction visualized by echocardiogram. Patient 3 initially presented with endocarditis of the aortic valve requiring aortic valve replacement. The pseudoaneurysm was found 3 months later with repeated negative blood cultures, but it was unclear if the pseudoaneurysm was present at the time of surgery or formed later secondary to infection. Streptococcus infection was present in two patients and Staphylococcus infection in one patient (Table 1).
Table 1.
Summary of clinical and imaging characteristics and management strategies for the four patients
| Patient | Gender | Age (y) | Diagnostic method | Aortic valve | Vegetation | Causative organism | Associated AR | Fistula | Surgery |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 56 | TEE | AVR, mechanical prosthesis | No vegetation | Streptococcus group G | Mild | — | AVR + P-MAIVF repair with a patch |
| 2 | Male | 84 | TEE | AVR, bioprosthesis | No vegetation | Reported Staphylococcus bacteremia | Mild | — | Refused surgery |
| 3 | Male | 67 | TEE | AVR, bioprosthesis | No vegetation | — | — | LA | AVR + P-MAIVF repair |
| 4 | Female | 43 | TEE | Native | Vegetation of the aortic valve | Streptococcus epidermidis | Severe | Aorta | AVR + extensive debridement of MAIVF |
AR, Aortic regurgitation; AVR, aortic valve replacement; LA, left atrium; MAIVF, mitral-aortic interventricular fibrosa; TEE, transesophageal echocardiography.
Clinical Presentation
In the current case series, two patients presented with symptoms of endocarditis (fevers, embolic events, positive blood cultures), and two presented with heart failure symptoms (Table 1).
Symptoms and signs of active infection are the most common manifestation of that entity (40%), followed by heart failure symptoms (16%).1 Cerebrovascular accidents and embolic events occur in 12% of cases and chest pain in 10%.1 Approximately 10% of cases are asymptomatic, especially in the absence of complications and are incidentally discovered on routine imaging. Rare presentations include the development of a chest wall mass1, 2 and palpitations due to ventricular ectopy.9
Complications
Compression of Adjacent Structures
The pseudoaneurysm can compress the coronary arteries, most commonly the left circumflex artery, causing angina.1, 2, 5 It may compress the pulmonary artery, causing pulmonary hypertension.1, 2 Rarely, compression of the anterior mitral valve leaflet leads to mitral regurgitation.1, 2, 5
Fistula Formation
A fistula can develop between the left ventricular outflow tract and the left atrium or the aorta.10 Patient 3 in our series demonstrated a fistula to the left atrium, and patient 4 demonstrated a communication to the aorta.
Rupture
The pseudoaneurysm may rupture into the pericardium, which is usually a fatal complication because of tamponade.11 Rupture in the anterior wall has been reported and presented with a pulsatile chest wall mass.1, 2
Thromboembolic Events
Cerebrovascular accidents and systemic embolization may follow clot formation in the pseudoaneurysm cavity.1, 2
Diagnosis
Echocardiography is the major imaging modality for diagnosis and evaluation of complications of P-MAIVF.12 Transesophageal echocardiography has superior sensitivity in the diagnosis of that entity compared with transthoracic echocardiography.10
Pseudoaneurysm presents as an echo-free pouch located between the anterior mitral valve leaflet and the posterior aortic root, exhibits systolic expansion and diastolic collapse, and communicates with the left ventricular outflow tract.7, 10 The blood during systole moves from the left ventricle into the pseudoaneurysm. There is no diastolic flow in the pseudoaneurysm, because during diastole, the blood flows back into the left ventricular outflow tract. Color Doppler demonstrates the to-and-fro (pulsatile) flow in the pseudoaneurysm and may also demonstrate the development of complications.10, 12 When a fistula develops between the left ventricular outflow tract and the aorta, the pulsatility of the pseudoaneurysm is less pronounced.10 Pericardial effusion with or without tamponade may be demonstrated in case of pseudoaneurysm rupture into the pericardium. Three-dimensional echocardiography can clearly demonstrate the exact location and size of the pseudoaneurysm, its relationship to adjacent structures, and the presence of complications.13 Cardiac computed tomography and magnetic resonance imaging appear to be useful complementary imaging modalities to determine the pseudoaneurysm extent and its relationship with neighboring structures.1, 4 Transesophageal echocardiography diagnosed the pathologic condition in all four patients in this series (Table 1) because of technical limitations of transthoracic imaging.
Natural History
The natural history of uncomplicated P-MAIVF is not well known, because the diagnosis of the entity usually leads to surgery for treatment of complications or in asymptomatic patients to prevent complications.1 Over the past few years, some cases of uncomplicated pseudoaneurysms were managed with watchful surveillance. Gin et al.14 described three patients who had minimal change in pseudoaneurysm dimensions and were alive without surgery at the end of a 5-year follow-up period, except one patient who died at the age of 92 years of noncardiac causes. Similarly, Hasin et al.15 presented two cases of large, uncomplicated pseudoaneurysms that were managed with watchful follow-up and had benign long-term clinical courses.
Management
Aortic valve replacement is performed in the majority of patients in conjunction with some type of pseudoaneurysm repair, which varies from simple closure of the aneurysm neck to closure using pericardial or synthetic graft material and even complete removal of the pseudoaneurysm from the left ventricular outflow tract.1, 2, 5 Some centers advocate extensive debridement and replacement of the aortic valve, aortic root, and mitral-aortic intervalvular fibrosa with a homograft. In our case series, three patients underwent aortic valve replacement with pseudoaneurysm repair. Homografts were not used, because of surgeon's preference.
The presence of a complication and the rapid expansion of the pseudoaneurysm mandate surgical intervention. In our series, three patients underwent surgery, and one was managed conservatively because of advanced age and multiple comorbidities (Table 1).
Successful percutaneous and transapical closure cases have been reported, but this should be considered only when patients are not operative candidates given the risks of introducing foreign material at the site of a current infection.2
Clinical and echocardiographic monitoring of uncomplicated P-MAIVF may be an alternative in the management of high-risk surgical patients.14, 15
Conclusion
P-MAIVF usually follows endocarditis or aortic valve surgery, and it has been increasingly recognized with the use of echocardiography, especially transesophageal echocardiography. It may cause symptoms or important complications that warrant timely diagnosis and surgical intervention. In this case series, two of four cases of asymptomatic P-MAIVF were managed without surgery, but surgical intervention is still the recommended management.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2017.07.001.
Supplementary Data
(Case 1): Long-axis midesophageal 2D view without color demonstrating the P-MAIVF with its characteristic systolic expansion and diastolic collapse.
(Case 1): Long-axis midesophageal view with color demonstrating the P-MAIVF and its communication with the left ventricular outflow tract.
(Case 2): Short-axis midesophageal view at the level of the aortic valve. A bioprosthetic aortic valve with thickened leaflets without vegetation is shown. The P-MAIVF is noted between the left atrium, the aorta, and the pulmonary artery.
(Case 2): Short-axis midesophageal view at the level of the aortic valve with color demonstrating the P-MAIVF.
(Case 2): Long-axis midesophageal view with color highly suspicious for communication between the pseudoaneurysm and the left ventricular outflow tract.
(Case 2): Long-axis midesophageal view without color highly suspicious for communication between the pseudoaneurysm and the left ventricular outflow tract.
(Case 3): Midesophageal zoomed, long-axis view demonstrating the P-MAIVF with systolic expansion and diastolic collapse. The drop-out seen in 2D imaging is the fistula between the pseudoaneurysm and the left atrium.
(Case 3): Midesophageal long-axis view with color Doppler demonstrating systolic flow from the pseudoaneurysm into the left atrium.
(Case 3): Three-dimensional midesophageal short-axis view demonstrating the pseudoaneurysm and its association with the adjacent structures.
(Case 3): Zoomed 3D view of the fistula between the pseudoaneurysm and the left atrium with flow from the pseudoaneurysm to the left atrium during systole.
(Case 3): Short-axis midesophageal view without color demonstrating the persistence of the pseudoaneurysm (arrow) after the second surgical intervention.
(Case 4): Two-dimensional x-plane image through the aortic valve demonstrating a native aortic valve with attached vegetation. Thickened intravalvular fibrosa and the formation of a pseudoaneurysm with minimal systolic expansion, usually seen when the pseudoaneurysm communicates with the aorta.
(Case 4): Long-axis midesophageal view with color demonstrating severe aortic regurgitation and the fistula between the aorta and the pseudoaneurysm.
References
- 1.Sudhakar S., Sewani A., Agrawal M., Uretsky B.F. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF): A comprehensive review. J Am Soc Echocardiogr. 2010;23:1009–1018. doi: 10.1016/j.echo.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Turiel M., Gottardi B., Muzzupappa S. A case of subannular aortic aneurysm detected by transesophageal echocardiography. Acta Cardiol. 2001;56:395–397. doi: 10.2143/AC.56.6.2005704. [DOI] [PubMed] [Google Scholar]
- 3.Tak T. Pseudoaneurysm of mitral-aortic intervalvular fibrosa. Clin Med Res. 2003;1:49–52. doi: 10.3121/cmr.1.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahan E., Gul M., Sahan S., Sokmen E., Guray Y.A., Tufekçioglu O. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: a new comprehensive review. Herz. 2015;40:182–189. doi: 10.1007/s00059-014-4185-z. [DOI] [PubMed] [Google Scholar]
- 5.Xie M., Li Y., Cheng T., Wang X., Lu Q., He L. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa. Int J Cardiol. 2013;166:2–7. doi: 10.1016/j.ijcard.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Entrikin D.W., Shroff G.S., Kon N.D., Carr J.J. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: a delayed complication of aortic root replacement. J Cardiovasc Comput Tomogr. 2011;5:333–335. doi: 10.1016/j.jcct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Bansal R.C., Graham B.M., Jutzy K.R., Shakudo M., Shah P.M. Left ventricular outflow tract to left atrial communication secondary to rupture of mitral-aortic intervalvular fibrosa in infective endocarditis: diagnosis by transesophageal echocardiography and color flow imaging. J Am Coll Cardiol. 1990;15:499–504. doi: 10.1016/s0735-1097(10)80082-8. [DOI] [PubMed] [Google Scholar]
- 8.Sabnis G.R., Phadke M.S., Patil D.V., Lanjewar C.P., Kerkar P.G. Idiopathic pseudoaneurysm of mitral-aortic intervalvular fibrosa with rupture into the left atrium. Eur Heart J Cardiovasc Imaging. 2015;16:456. doi: 10.1093/ehjci/jeu278. [DOI] [PubMed] [Google Scholar]
- 9.Bonou M., Papadimitraki E., Vaina S., Kelepeshis G., Tsakalis K., Alexopoulos N. Mitral-aortic intervalvular fibrosa pseudoaneurysm. J Cardiovasc Ultrasound. 2015;23:257–261. doi: 10.4250/jcu.2015.23.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afridi I., Apostolidou M.A., Saad R.M., Zoghbi W.A. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: dynamic characterization using transesophageal echocardiographic and Doppler techniques. J Am Coll Cardiol. 1995;25:137–145. doi: 10.1016/0735-1097(94)00326-l. [DOI] [PubMed] [Google Scholar]
- 11.Qizilbash A.H., Schwartz C.J. False aneurysm of the left ventricle due to perforation of mitral-aortic intervalvular fibrosa with rupture and cardiac tamponade. Rare complication of infective endocarditis. Am J Cardiol. 1973;32:110–113. doi: 10.1016/s0002-9149(73)80095-5. [DOI] [PubMed] [Google Scholar]
- 12.Karalis D.G., Bansal R.C., Hauck A.J., Ross J.J., Applegate P.M., Jutzy K.R. Transesophageal echocardiographic recognition of subaortic complications in aortic valve endocarditis: clinical and surgical implications. Circulation. 1992;86:353–362. doi: 10.1161/01.cir.86.2.353. [DOI] [PubMed] [Google Scholar]
- 13.Casselli S., Mazzesi G., Tritapepe L., Barretta A., Pandian N.G., Agati L. 3D echocardiographic delineation of mitral- aortic intervalvular fibrosa pseudoaneurysm caused by bicuspid aortic valve endocarditis. Echocardiography. 2011;28:E1–E4. doi: 10.1111/j.1540-8175.2010.01229.x. [DOI] [PubMed] [Google Scholar]
- 14.Gin A., Hong H., Rosenblatt A., Black M., Ristow B., Popper R. Pseudoaneurysms of the mitral-aortic intervalvular fibrosa: survival without reoperation. Am Heart J. 2011;161:130.e1–130.e5. doi: 10.1016/j.ahj.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Hasin T., Reisner S., Agmon Y. Large pseudoaneurysms of the mitral-aortic intervalvular fibrosa: long- term natural history without surgery in two patients. Eur J Echocardiogr. 2011;12:E24. doi: 10.1093/ejechocard/jeq183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Case 1): Long-axis midesophageal 2D view without color demonstrating the P-MAIVF with its characteristic systolic expansion and diastolic collapse.
(Case 1): Long-axis midesophageal view with color demonstrating the P-MAIVF and its communication with the left ventricular outflow tract.
(Case 2): Short-axis midesophageal view at the level of the aortic valve. A bioprosthetic aortic valve with thickened leaflets without vegetation is shown. The P-MAIVF is noted between the left atrium, the aorta, and the pulmonary artery.
(Case 2): Short-axis midesophageal view at the level of the aortic valve with color demonstrating the P-MAIVF.
(Case 2): Long-axis midesophageal view with color highly suspicious for communication between the pseudoaneurysm and the left ventricular outflow tract.
(Case 2): Long-axis midesophageal view without color highly suspicious for communication between the pseudoaneurysm and the left ventricular outflow tract.
(Case 3): Midesophageal zoomed, long-axis view demonstrating the P-MAIVF with systolic expansion and diastolic collapse. The drop-out seen in 2D imaging is the fistula between the pseudoaneurysm and the left atrium.
(Case 3): Midesophageal long-axis view with color Doppler demonstrating systolic flow from the pseudoaneurysm into the left atrium.
(Case 3): Three-dimensional midesophageal short-axis view demonstrating the pseudoaneurysm and its association with the adjacent structures.
(Case 3): Zoomed 3D view of the fistula between the pseudoaneurysm and the left atrium with flow from the pseudoaneurysm to the left atrium during systole.
(Case 3): Short-axis midesophageal view without color demonstrating the persistence of the pseudoaneurysm (arrow) after the second surgical intervention.
(Case 4): Two-dimensional x-plane image through the aortic valve demonstrating a native aortic valve with attached vegetation. Thickened intravalvular fibrosa and the formation of a pseudoaneurysm with minimal systolic expansion, usually seen when the pseudoaneurysm communicates with the aorta.
(Case 4): Long-axis midesophageal view with color demonstrating severe aortic regurgitation and the fistula between the aorta and the pseudoaneurysm.