Graphical abstract
Keywords: Truncus arteriosus, Congenital heart disease, Semilunar valves
Highlights
-
•
Truncus arteriosus is generally considered a failure of septation in the ventricular outflow tracts, the semilunar valves, and the aorta and pulmonary arteries.
-
•
This case involved the unusual finding of a partially septated truncal valve.
-
•
This case highlights the embryologic aberrations resulting in this disease.
Introduction
In truncus arteriosus, a common arterial trunk arises from the heart and gives rise to the ascending aorta, pulmonary arteries, and coronary arteries. Proximal to the arterial trunk is typically a single truncal valve, the morphology of which varies. Highlighting the embryologic aberration resulting in this disease, we present the unusual finding of a partially septated truncal valve, giving the appearance of two distinct semilunar valves arrested in development, in a neonate with truncus arteriosus and aortic arch hypoplasia.
Case Presentation
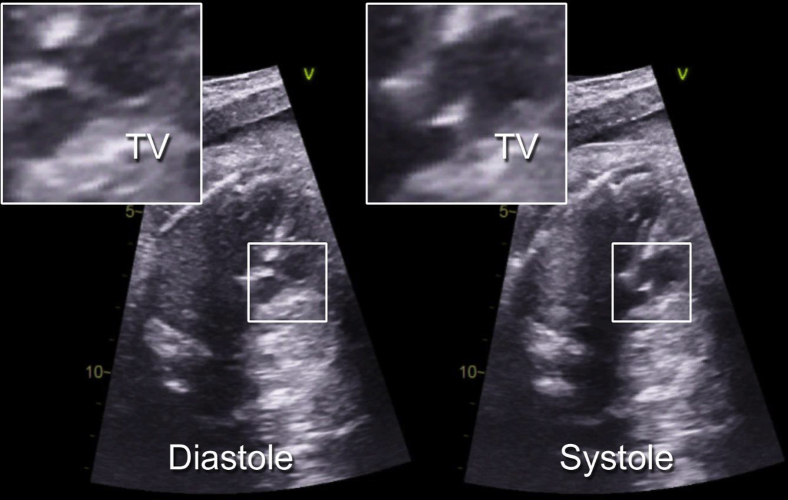
The patient's mother was initially referred to our Fetal Heart Program because of suspicion of congenital heart disease on screening obstetric fetal ultrasound. The mother was in good health, but her history was notable for oral isotretinoin use during the early weeks of pregnancy. Pre- and postnatal genetic testing was normal. Fetal echocardiography at 20 weeks' gestation revealed truncus arteriosus with aortic arch hypoplasia. The truncal valve was enlarged, and some images suggested an unusual central division therein (Figure 1, Video 1). The valve had no stenosis and only trivial insufficiency.
Figure 1.
Fetal echocardiogram showing an enlarged truncal valve (TV), with an unusual central division most apparent in systole.
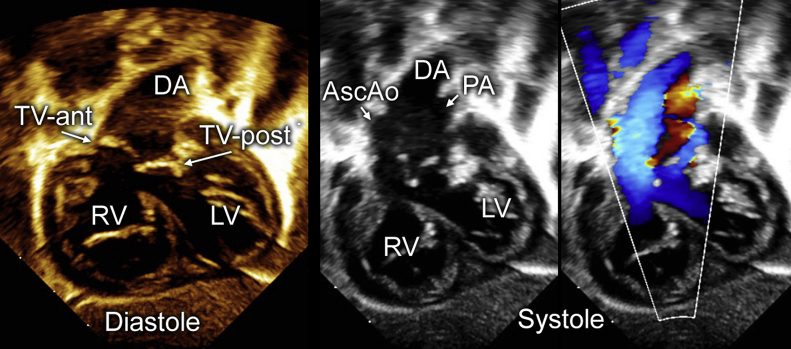
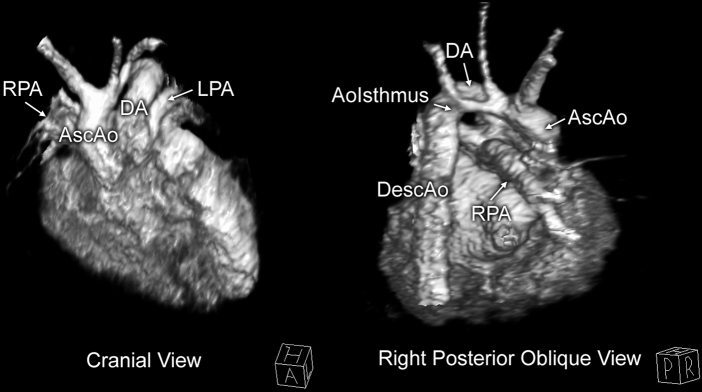
Labor was induced, and the patient delivered at 39 weeks’ gestation. The infant was started on prostaglandin E1 to maintain ductal patency and systemic blood flow. Thereafter the infant was admitted to the cardiac intensive care unit. Transthoracic echocardiography confirmed the anatomy of truncus arteriosus, comprising a large conoventricular septal defect and a single arterial vessel giving rise to the ascending aorta, pulmonary arteries, and coronary arteries (Figure 2, Video 2). The particular anatomic variant was most consistent with Van Praagh type A4, in that there was a large main pulmonary artery segment with a small ascending aorta, aortic arch hypoplasia, and near interruption at the aortic isthmus1, 2 (Video 3, Video 4, Video 5, Video 6). A large patent ductus arteriosus was in continuity with the descending aorta. Cardiac magnetic resonance imaging performed for preoperative planning confirmed the great vessel anatomy as described (Figure 3).
Figure 2.
From the subcostal sagittal views, the systolic motion of the valve leaflets and color Doppler flow patterns created the appearance of two separate valve orifices. AscAo, Ascending aorta; DA, ductal arch; LV, left ventricle; PA, pulmonary artery; RV, right ventricle; TV-ant, anterior aspect of truncal valve; TV-post, posterior aspect of truncal valve.
Figure 3.
Anatomy of the common trunk via cardiac magnetic resonance imaging. Volume rendering of the magnetic resonance angiogram shows that the common arterial trunk branches off into the ascending aorta (AscAo) and atretic aortic arch, the ductal arch (DA), which supplies the descending aorta (DescAo), and the pulmonary arteries. AoIsthmus, Atretic aortic isthmus; LPA, left pulmonary artery; RPA, right pulmonary artery.
The truncal valve was extraordinary. In most cases of truncus arteriosus, the truncal valve is tricuspid, bicuspid, or quadricuspid,3 and all valve leaflets are in the same plane. In this case, the valve consisted of two distinct valve orifices separated by a central fibrous raphe. Five leaflets were identified in total: three grouped posteriorly, with the remaining two grouped anteriorly. From the parasternal short-axis plane, this arrangement gave the appearance of a smaller posterior tricuspid aortic valve partially fused with a larger anterior bicuspid pulmonary valve (Figure 4, Video 7). Although all the leaflets were in continuity, the two groups of leaflets were clearly oriented in separate planes (Video 8, Video 9), with the central raphe dividing the two planes (Figure 2). The systolic motion of the valve leaflets and color Doppler flow patterns also suggested two separate distinct valve orifices (Figure 2, Video 10). The coronary arteries also appeared to originate from the posterior tricuspid aortic valve (Video 11). The truncal valve was surrounded by infundibular muscle, such that there was mitral-truncal discontinuity.
Figure 4.
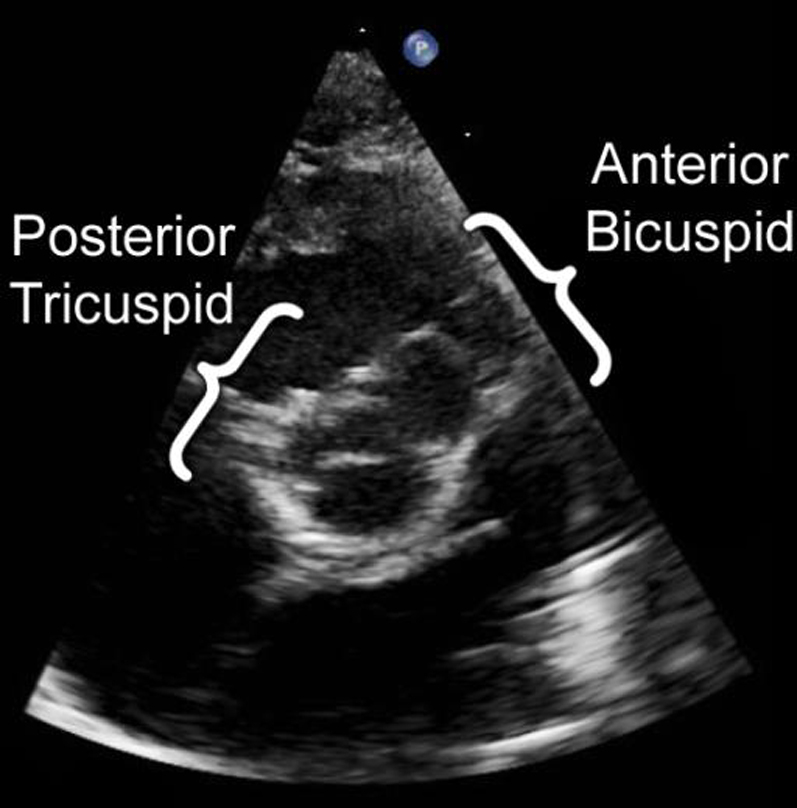
From the parasternal short-axis plane, the angulation of the anterior and posterior aspects of the truncal valve prevented an en face visualization of the entirety of the valve. In this image, the posterior tricuspid aspect of the valve is shown en face, whereas the anterior bicuspid aspect of the valve is directed superiorly and is not well visualized. A sweep directed superiorly was required to fully visualize the valve.
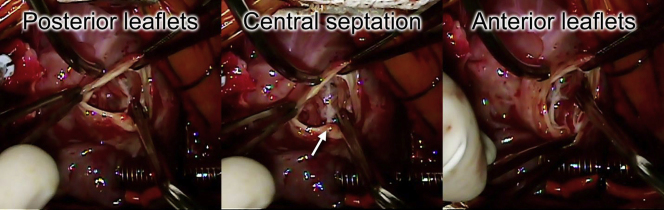
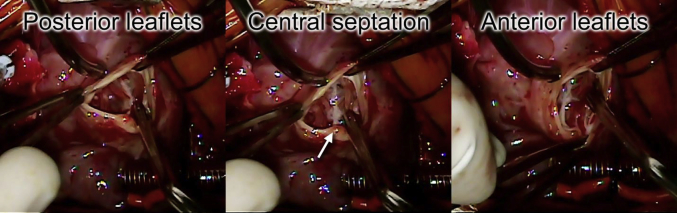
At 4 days of life, the patient underwent surgical repair. This consisted of closure of the ventricular septal defect to the truncal valve, division of the pulmonary arteries, direct anastomosis of the common trunk with the ascending aorta, placement of a femoral vein homograft from the right ventricle to the pulmonary artery branches, division of the nearly atretic aortic isthmus, and arch reconstruction by direct anastomosis. Intraoperative transesophageal echocardiography confirmed the findings of a truncal valve with two effective orifices at the annular level, but no true aortopulmonary septum was identified (Video 12). Intraoperative inspection of the truncal valve confirmed the unusual central thickening that appeared to separate the truncal valve into the anterior and posterior groups (Figure 5, Video 13).
Figure 5.
Surgeon's view of the truncal valve (bottom of screen is patient's right). The truncal valve is divided into a group of anterior leaflets and posterior leaflets by a central fibrous septation (arrow).
Discussion
From an embryologic standpoint, truncus arteriosus is conventionally thought to be caused by a failure in the development of the fetal conus cordis and truncus arteriosus. This results in failed septation of (1) the right and left ventricular outflow tracts, (2) the semilunar valves, and (3) the aorta and pulmonary arteries. More recently promulgated theories support accompanying abnormal infundibular development as being responsible for the abnormalities seen in conotruncal abnormalities such as truncus arteriosus.4 The finding of a partially septated truncal valve—or, depending on one's point of view, two separate semilunar valves—posed a diagnostic conundrum: was the disease a very large aortopulmonary window with only a small remnant of aortopulmonary septum and an accompanying conoventricular septal defect, or was it true truncus arteriosus with an arrested attempt at semilunar valve development?5 The presence of truncal-mitral discontinuity is also very unusual for truncus arteriosus,2 although it has been described in one case in an autopsy series.6 Interestingly, the presence of two semilunar valves has been reported in truncus arteriosus, albeit in the presence of an intact ventricular septum (Van Praagh type B).2 The central thickening identified in the truncal valve in this case suggested a potential line of developmental division between an aortic and a pulmonary valve. This particular case, therefore, appears to represent an intermediate embryologic step between what is typically considered to be truncus arteriosus and aortopulmonary window. Ultimately, the two abnormalities fall on an embryologic spectrum driven by failure of neural crest cell migration and associated abnormal infundibular development.3, 4
The unusual nature of the truncal valve did not change the overall surgical approach for the patient, as surgical septation of the arterial trunk into a true aorta and main pulmonary artery was not feasible. Prognostically speaking, there was no significant valve regurgitation, which has been identified as an important factor affecting surgical outcomes in truncus arteriosus.7 The patient tolerated the procedure well, was extubated by the third postoperative day, and was transferred out of the cardiac intensive care unit by the seventh postoperative day in stable condition. Follow-up echocardiography at 5 months of age demonstrated an excellent surgical result and only trivial truncal valve insufficiency (Video 14, Video 15). The patient is now a happy and playful child.
Conclusion
This fascinating case of a partially septated truncal valve highlights the embryologic perturbations responsible for truncus arteriosus and many other conotruncal abnormalities. Ultimately, what is traditionally thought of as truncus arteriosus falls on a spectrum of abnormalities of infundibuloarterial development. Regardless of its moniker, meticulous attention to anatomic detail is of paramount importance in surgical planning and resulted in an excellent outcome for this patient.
Acknowledgment
We wish to gratefully acknowledge the thoughtful review of this case by Dr Richard Van Praagh, whose insights continue to add to our understanding of congenital heart disease.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2017.09.006.
Supplementary Data
Fetal echocardiogram showing an enlarged truncal valve, with an unusual central division most apparent in systole.
From the subcostal sagittal views, the systolic motion of the valve leaflets and color Doppler flow patterns created the appearance of two separate valve orifices.
Parasternal short-axis view of the common trunk, showing a main pulmonary artery segment branching into two branch pulmonary arteries.
Suprasternal arch long-axis imaging demonstrating transverse arch hypoplasia.
Suprasternal arch short-axis imaging demonstrating an ascending aorta branching off into a right-sided innominate artery, left common carotid artery, and left subclavian artery.
Suprasternal arch short-axis sweep with color Doppler, demonstrating the aortic arch hypoplasia and aortic isthmus narrowing at the junction with ductal arch (located to the left).
Parasternal short-axis sweep demonstrating the orientations of the anterior and posterior portions of the truncal valve. Each portion was in a different plane, prohibiting en face visualization of the entirety of the valve. The posterior tricuspid aspect of the valve is initially shown en face, and the anterior bicuspid aspect of the valve is then demonstrated at the end of a sweep directed superiorly.
Subcostal coronal view of the posterior aspect of the valve.
Subcostal coronal view of the anterior aspect of the valve, which is slightly superior compared with the posterior aspect.
Parasternal long-axis view of the truncal valve. The systolic motion of the valve leaflets and color Doppler flow patterns suggested two separate distinct valve orifices.
Parasternal short-axis view of the truncal valve. Two coronary arteries are seen originating from the posterior aspect of the truncal valve, with the right coronary artery originating from the right cusp and left coronary artery originating from the leftward aspect of the posterior cusp.
Intraoperative transesophageal echocardiogram. A truncal valve with two effective orifices was seen at the annular level, but no true aortopulmonary septum was identified.
Intraoperative inspection of the truncal valve, showing an unusual central thickening separating the truncal valve into the anterior and posterior groups.
Postoperative study of truncal valve from parasternal long axis, showing the truncal valve continuing to have two distinct valve orifices.
Postoperative study of truncal valve from parasternal short axis, showing that the anterior aspect of the truncal valve has trivial valve insufficiency.
References
- 1.Calder L., Van Praagh R., Van Praagh S., Sears W.P., Corwin R., Levy A. Truncus arteriosus communis. Clinical, angiocardiographic, and pathologic findings in 100 patients. Am Heart J. 1976;92:23–38. doi: 10.1016/s0002-8703(76)80400-0. [DOI] [PubMed] [Google Scholar]
- 2.Van Praagh R., Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol. 1965;16:406–425. doi: 10.1016/0002-9149(65)90732-0. [DOI] [PubMed] [Google Scholar]
- 3.Lai W.W., Mertens L.L., Cohen M.S., Geva T. 2nd ed. Wiley-Blackwell; Hoboken, NJ: 2016. Echocardiography in pediatric and congenital heart disease: from fetus to adult. [Google Scholar]
- 4.Van Praagh R. What determines whether the great arteries are normally or abnormally related? Am J Cardiol. 2016;118:1390–1398. doi: 10.1016/j.amjcard.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Mori K., Ando M., Takao A., Ishikawa S., Imai Y. Distal type of aortopulmonary window. Report of 4 cases. Br Heart J. 1978;40:681–689. doi: 10.1136/hrt.40.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adachi I., Seale A., Uemura H., Mccarthy K.P., Kimberley P., Ho S.Y. Morphologic spectrum of truncal valvar origin relative to the ventricular septum: correlation with the size of ventricular septal defect. J Thorac Cardiovasc Surg. 2009;138:1283–1289. doi: 10.1016/j.jtcvs.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Russel H.M., Pasquali S.K., Jacobs J.P., Jacobs M.L., O’Brien S.M., Mavroudis C. Outcomes of repair of common arterial trunk with truncal valve surgery: a review of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;93:164–169. doi: 10.1016/j.athoracsur.2011.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fetal echocardiogram showing an enlarged truncal valve, with an unusual central division most apparent in systole.
From the subcostal sagittal views, the systolic motion of the valve leaflets and color Doppler flow patterns created the appearance of two separate valve orifices.
Parasternal short-axis view of the common trunk, showing a main pulmonary artery segment branching into two branch pulmonary arteries.
Suprasternal arch long-axis imaging demonstrating transverse arch hypoplasia.
Suprasternal arch short-axis imaging demonstrating an ascending aorta branching off into a right-sided innominate artery, left common carotid artery, and left subclavian artery.
Suprasternal arch short-axis sweep with color Doppler, demonstrating the aortic arch hypoplasia and aortic isthmus narrowing at the junction with ductal arch (located to the left).
Parasternal short-axis sweep demonstrating the orientations of the anterior and posterior portions of the truncal valve. Each portion was in a different plane, prohibiting en face visualization of the entirety of the valve. The posterior tricuspid aspect of the valve is initially shown en face, and the anterior bicuspid aspect of the valve is then demonstrated at the end of a sweep directed superiorly.
Subcostal coronal view of the posterior aspect of the valve.
Subcostal coronal view of the anterior aspect of the valve, which is slightly superior compared with the posterior aspect.
Parasternal long-axis view of the truncal valve. The systolic motion of the valve leaflets and color Doppler flow patterns suggested two separate distinct valve orifices.
Parasternal short-axis view of the truncal valve. Two coronary arteries are seen originating from the posterior aspect of the truncal valve, with the right coronary artery originating from the right cusp and left coronary artery originating from the leftward aspect of the posterior cusp.
Intraoperative transesophageal echocardiogram. A truncal valve with two effective orifices was seen at the annular level, but no true aortopulmonary septum was identified.
Intraoperative inspection of the truncal valve, showing an unusual central thickening separating the truncal valve into the anterior and posterior groups.
Postoperative study of truncal valve from parasternal long axis, showing the truncal valve continuing to have two distinct valve orifices.
Postoperative study of truncal valve from parasternal short axis, showing that the anterior aspect of the truncal valve has trivial valve insufficiency.