Graphical abstract
Highlights
-
•
Echocardiography is used to diagnose hypertrophic obstructive cardiomyopathy (HOCM) and Takotsubo cardiomyopathy (TCM).
-
•
Hypotension in a patient with TCM should be evaluated for left ventricular outflow tract obstruction (LVOTO).
-
•
Management of TCM is challenging in patients with HOCM with severe LVOTO.
-
•
Hypotension in LVOTO may paradoxically worsen with standard intravenous inotropes.
-
•
Fluid resuscitation, beta-blockers, alpha agonists, and intra-aortic balloon pump are the treatment options in LVOTO.
Introduction
Takotsubo cardiomyopathy (TCM) is a condition that leads to transient left ventricular (LV) dysfunction. Hypotension in a patient with TCM should be carefully evaluated for LV outflow tract obstruction (LVOTO) with an echocardiogram. Here we present a case of TCM in a patient with known LVOTO secondary to hypertrophic obstructive cardiomyopathy (HOCM).
Case Presentation
An 81-year-old woman presented to our emergency department (ED) with a one-day history of worsening shortness of breath at rest associated with mild substernal chest pressure and one episode of nonbloody vomiting. She had a medical history of HOCM status after implantable cardioverter defibrillator (ICD) placement (due to syncopal episodes), lung cancer status post lobectomy, pulmonary embolism, paroxysmal atrial fibrillation, gastrointestinal bleeding due to arteriovenous malformations (AVMs), and chronic anemia. Two days prior to this admission, the patient was admitted to the ED with acute anemia (hemoglobin, 7.3 g/dL) due to possible bleeding AVM and was discharged after two units of packed red blood cell transfusion.
Her physical exam was significant for bilateral rales, a harsh systolic crescendo-decrescendo murmur along the left lower sternal border, and a grade 2 lower extremity pitting edema. In the ED, the patient was hemodynamically stable with mild tachypnea. An electrocardiogram and chest x-ray were performed and showed sinus tachycardia with intermittent ventricular pacing with new ST-T wave abnormalities in anterolateral leads (Figure 1) and bilateral infiltrates consistent with pulmonary edema, respectively (Figure 2). Her blood work performed in the ED revealed a hemoglobin of 10 g/dL, troponin I of 1.26 ng/mL (normal <0.05 ng/mL), creatine kinase-MB of 13.3 ng/mL (normal <6.3 ng/mL), and B-type natriuretic peptide of 3,472 pg/mL (normal <99 pg/mL). The basic metabolic panel and liver function tests were within normal limits. Based on her presentation and blood work, a working diagnosis of acute congestive heart failure with non-ST-segment elevation myocardial infarction was made, and she was initiated on medical therapy with dual antiplatelet therapy and intravenous heparin infusion.
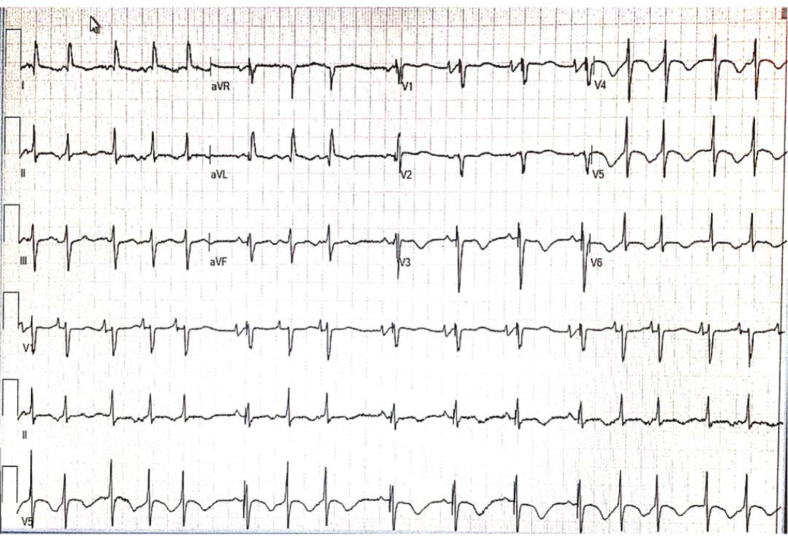
Figure 1.
The initial electrocardiogram showing sinus tachycardia with premature atrial contractions and intermittent ventricular pacing with ST-T wave abnormalities in anterolateral leads.
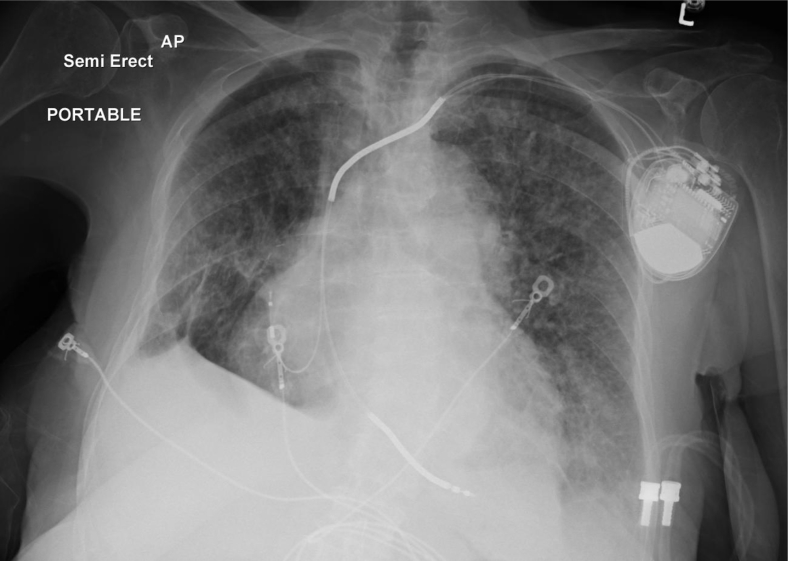
Figure 2.
The initial chest x-ray demonstrates cardiomegaly with interstitial and alveolar opacities consistent with pulmonary edema and ICD leads positioned in right atrial appendage and right ventricular apex.
She underwent urgent coronary angiography on the same day (Videos 1–3), which did not reveal any significant coronary artery disease and was diagnosed with TCM. Post-coronary angiogram she developed cardiogenic shock (blood pressure, 60/40 mm Hg) with worsened congestive heart failure and atrial fibrillation with rapid ventricular response. An emergent transthoracic echocardiogram performed bedside showed an ejection fraction of 20%–25% (significant hypokinesis of apical, apical lateral, and apical septal walls with a hyperdynamic basal inferoseptal and anterolateral walls), systolic anterior motion of mitral valve causing a dynamic LVOTO (peak gradient >90 mm Hg; Videos 4 and 5, Figure 3, Figure 4, Figure 5), and severe mitral regurgitation with a posteriorly directed jet (Figure 6, Video 6). Due to the severe LVOTO and heart failure, she was started on phenylephrine and amiodarone infusion. Her anticoagulation was discontinued, and she was transferred to the coronary care unit for closer monitoring.
Figure 3.
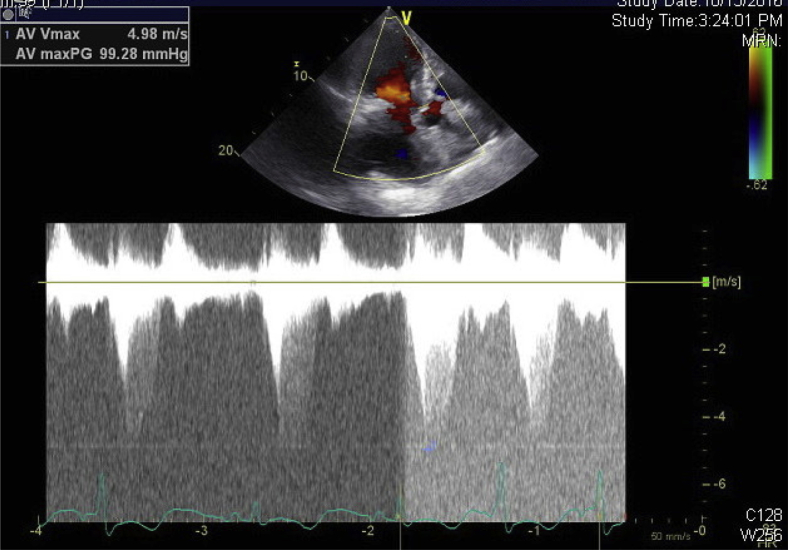
CW Doppler imaging in the apical four-chamber view demonstrating late peaking dynamic LVOTO gradient of over 90 mm Hg (peak velocity 5 m/sec).
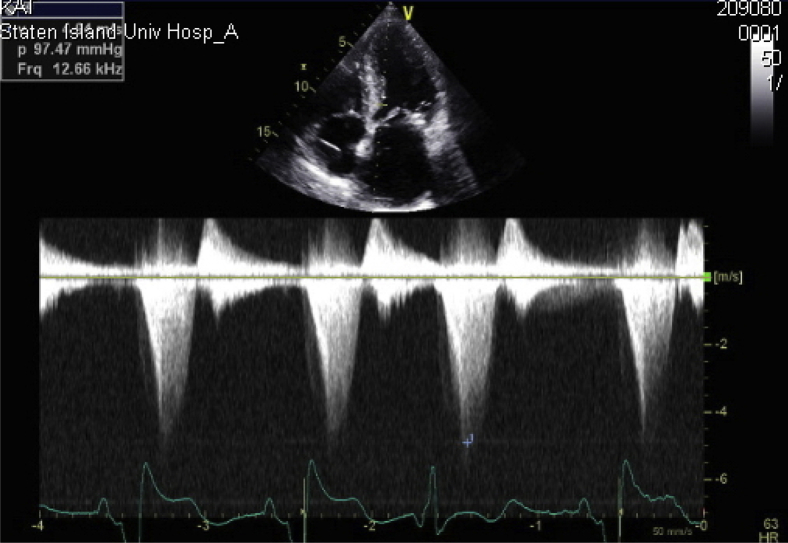
Figure 4.
CW Doppler in the apical long-axis view showing a gradient of 99.28 mm Hg.
Figure 5.
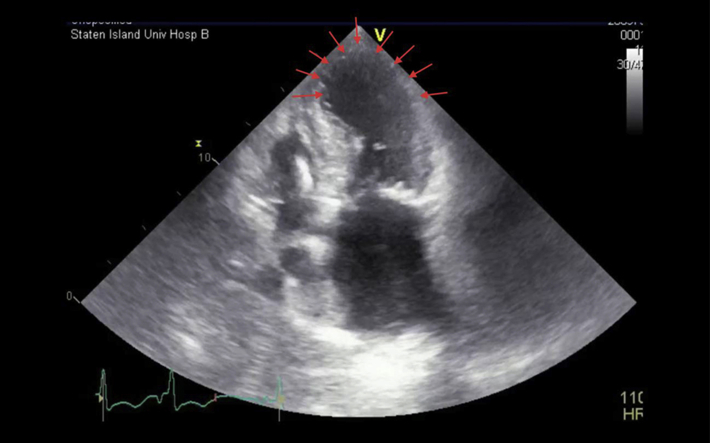
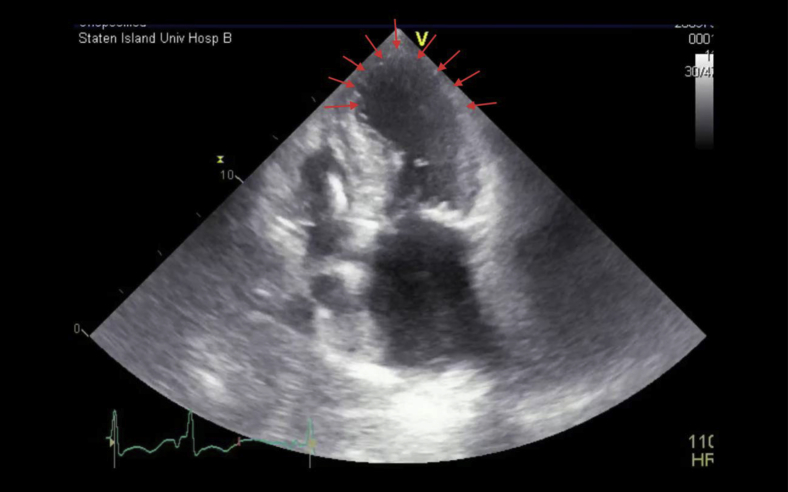
Apical four-chamber view showing hypokinesis of the apical interventricular septum, apex, and apical lateral walls (red arrows).
Figure 6.
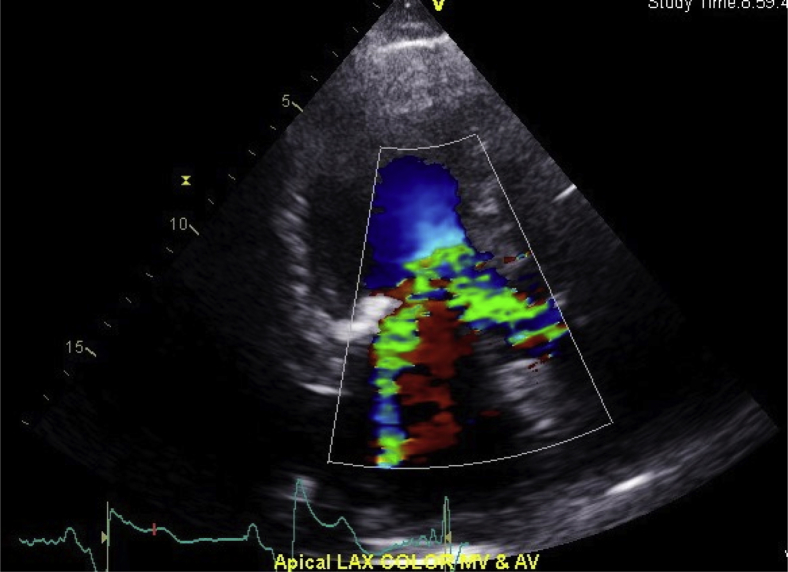
Apical long-axis (LAX) view showing flow acceleration and the posteriorly directed mitral regurgitation jet.
The patient's home dose of metoprolol was continued along with low-dose diuretics. Over a period of three days, her condition improved significantly. She was weaned off the phenylephrine drip and switched to oral amiodarone. The repeat echocardiogram performed on the fifth day showed significant improvement in LV function (estimated ejection fraction of 74% by biplane Simpson's method; Figure 7) with mild hypokinesis of the apical and apical septal walls (Videos 7 and 8), and the peak LV outflow tract (LVOT) gradient was reduced to 22 mm Hg (Figure 8). She was subsequently discharged to home in stable condition with a discharge diagnosis of cardiogenic shock secondary to TCM.
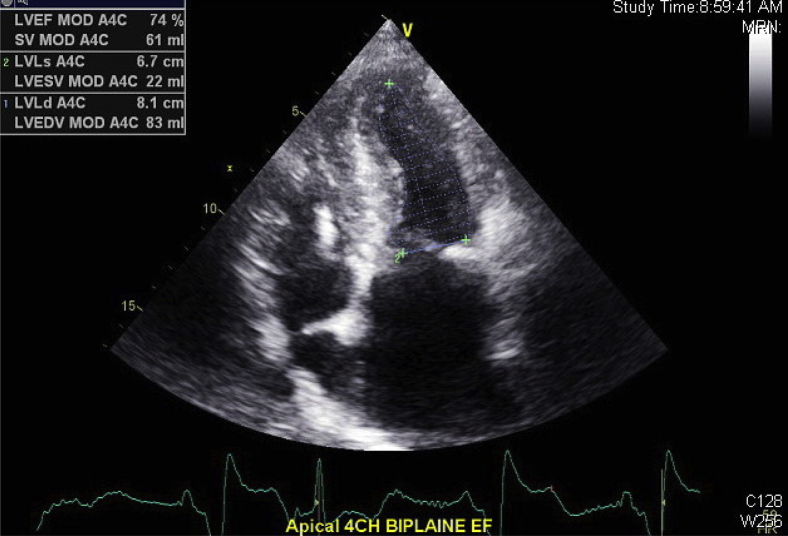
Figure 7.
Biplane Simpson's method of disks performed in the apical four-chamber (4CH) view showing improved ejection fraction (EF) of 74%.
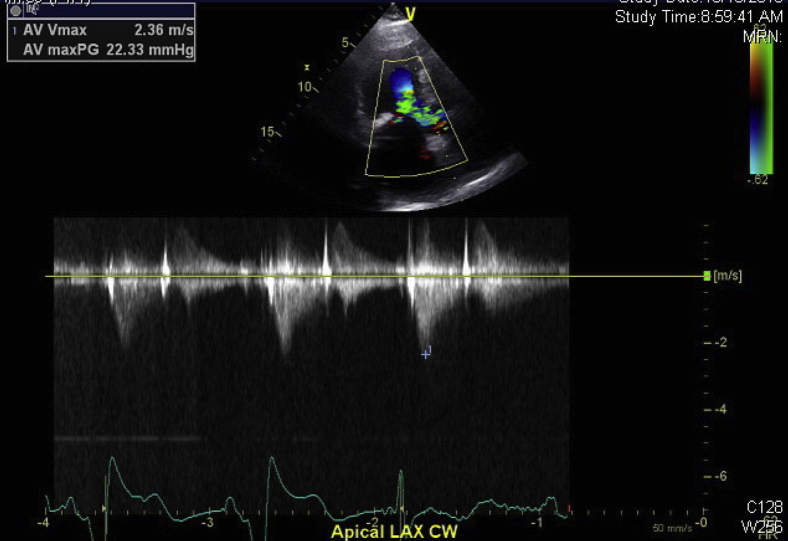
Figure 8.
The follow-up echocardiogram with CW Doppler in the apical long-axis (LAX) view showing a resolved gradient of 22.33 mm Hg.
Discussion
TCM is also known as stress cardiomyopathy, apical ballooning syndrome, and broken heart syndrome. TCM typically mimics acute myocardial infarction and is usually preceded by an emotional or physical trigger. TCM usually occurs in postmenopausal women and leads to transient regional LV systolic dysfunction, electrocardiographic abnormalities, and elevated serum troponin levels. Approximately 1%–2% of patients presenting with suspected acute coronary syndrome have TCM.1 The pathogenesis of TCM is not well understood, and suggested mechanisms are catecholamine surge, diffuse coronary artery spasm, microvascular dysfunction, and neurogenic stunned myocardium. Hence a diagnosis of TCM requires coronary angiography and serial assessment of LV function.2 In many patients, complete recovery of LV function occurs in 4-8 weeks. The risk of severe in-hospital complications including cardiogenic shock and death is similar when compared with those of patients with acute coronary syndrome. Approximately 10% of TCM patients develop cardiogenic shock.3 Although shock can be secondary to systolic dysfunction, it can also be caused by LVOTO, which was described in 10%–25% of TCM patients. A possible hypothesis was hyperdynamic basal segments causing intraventricular pressure gradient, which is associated with systolic anterior motion of mitral leaflet (SAM) and mitral regurgitation leading to further hemodynamic collapse.4
HOCM is an autosomal dominant genetic disorder with hypertrophy mainly involving the ventricular septum, and most patients have dynamic LVOTO. Echocardiography plays a vital role in diagnosing both HOCM and TCM separately. Any patient with a history of HOCM presenting with hypotension should be evaluated thoroughly by a proper physical exam including an echocardiogram. A comprehensive transthoracic echocardiogram would not only diagnose TCM but also help in identifying the relative contribution of the various factors leading to hypotension in each individual situation. When the LVOTO is significant, a late-peaking systolic ejection murmur can be appreciated maximally in the left third intercostal space. The two-dimensional imaging in the apical four-chamber and five-chamber views helps to identify the severity and duration of SAM (Videos 1 and 2).5 M-mode at the mitral valve level from the parasternal long-axis view will show us an adequate temporal resolution of the severity and duration of anterior mitral leaflet-septal proximity.6 A pulse wave color Doppler in the apical long-axis and five-chamber views can help localize the level of obstruction, and a continuous wave (CW) Doppler will quantify the severity of SAM. The characteristic late peaking dagger- shaped CW Doppler envelope is seen in dynamic LVOTO (Figure 3). When milder peak CW Doppler gradients are recorded, Valsalva maneuver can accentuate classical LVOTO by reducing LV chamber size.6
Therefore, hypotension in TCM patients should be carefully evaluated for LVOTO, and it may paradoxically worsen with standard intravenous inotropes. Fluid resuscitation (in the absence of significant pulmonary congestion) and beta-blockers are the initial therapeutic options. Alpha-agonists such as phenylephrine can be added to increase the blood pressure, which helps in decreasing gradient by increasing afterload.4 Intra-aortic balloon pump may be used if the shock is refractory to medical management.
In very few reported cases, TCM occurred in patients with HOCM. In a few of them TCM occurred in previously diagnosed patients with HOCM,7, 8, 9, 10, 11 and in others, HOCM was undiagnosed and was masked by TCM.12, 13, 14 We believe there is a wide spectrum of the TCM-LVOTO presentations, and at least in some situations LVOTO precipitates TCM due to the sudden increase in wall stress or reduction in cardiac output.5
Although it is a very rare scenario, management of TCM will be extremely challenging in HOCM patients when a severe LVOTO is contributed by both HOCM and TCM. Our patient had a history of HOCM and underwent ICD placement for syncopal episodes. Two days prior to presentation, the patient had acute anemia, which could also be a possible trigger leading to TCM. Unlike the previously reported cases, TCM in our patient led to cardiogenic shock, which was successfully treated with careful titration of beta-blockers and phenylephrine infusion under close hemodynamic monitoring. Cardiogenic shock resolved after three days without requiring intra-aortic balloon pump placement.
Conclusions
TCM mimics acute myocardial infarction and leads to transient LV dysfunction. While TCM itself can cause LVOTO, its management in patients with HOCM with preexisting LVOTO will be extremely challenging. Careful hemodynamic monitoring and echocardiographic surveillance along with judicious use of beta-blockers and vasopressors are the cornerstone of treatment.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.case.2017.09.008.
Supplementary Data
Right anterior oblique view of LCA. A JL3.5 catheter is used to engage and inject dye into the LCA, which showed no evidence of blockages in left circumflex artery and left anterior descending artery.
A left anterior oblique view of LCA.
Left anterior oblique view of the right coronary artery showing no evidence of disease. A JR4 catheter is used to engage and inject dye into the right coronary artery.
Initial apical four-chamber view displays significant hypokinesis of the apical, apical lateral, and apical septal walls with a hyperdynamic basal inferoseptal and anterolateral walls. The systolic anterior motion of mitral valve causing a dynamic LVOTO along with an enlarged left atrium and significant mitral annular calcification are also seen.
Apical five-chamber view showing severe hypokinesis of the apical, apical septal, and apical lateral walls along with basal asymmetric hypertrophy and the LVOTO caused by systolic anterior motion of the mitral leaflet.
Turbulence of blood flow through the LVOT associated with posteriorly directed mitral regurgitation due to LVOTO from systolic anterior motion of the mitral valve.
Follow-up echocardiogram in the four-chamber view shows the recovery of the apical hypokinesis.
Follow-up echocardiogram in the five-chamber view shows decreased turbulence in the LVOT and a decreased mitral regurgitation jet.
References
- 1.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Akashi Y.J., Goldstein D.S., Barbaro G., Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Templin C., Ghadri J.R., Diekmann J., Napp L.C., Bataiosu D.R., Jagzuweski M. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 4.Fefer P., Chelvanathan A., Dick A.J., Teitelbaum E.J., Strauss B.H., Cohen E.A. Takotsubo cardiomyopathy and left ventricular outflow tract obstruction. J Interv Cardiol. 2009;22:444–452. doi: 10.1111/j.1540-8183.2009.00488.x. [DOI] [PubMed] [Google Scholar]
- 5.Chockalingam A., Tejwani L., Aggarwal K., Dellsperger K.C. Dynamic left ventricular outflow tract obstruction in acute myocardial infarction with shock: cause, effect, and coincidence. Circulation. 2007;116:e110–e113. doi: 10.1161/CIRCULATIONAHA.107.711697. [DOI] [PubMed] [Google Scholar]
- 6.Chockalingam A., Dorairajan S., Bhalla M., Dellsperger K.C. Unexplained hypotension: the spectrum of dynamic left ventricular outflow tract obstruction in critical care settings. Crit Care Med. 2009;37:729–734. doi: 10.1097/CCM.0b013e3181958710. [DOI] [PubMed] [Google Scholar]
- 7.Akita K., Maekawa Y., Tsuruta H., Okuda S., Yanagisawa R., Kageyama T. “Moving left ventricular obstruction” due to stress cardiomyopathy in a patient with hypertrophic obstructive cardiomyopathy treated with percutaneous transluminal septal myocardial ablation. Int J Cardiol. 2016;202:194–195. doi: 10.1016/j.ijcard.2015.08.145. [DOI] [PubMed] [Google Scholar]
- 8.Ochiumi Y., Ikeda S., Hamada M. Reappearance of the left ventricular pressure gradient in a patient with hypertrophic obstructive cardiomyopathy. Intern Med. 2015;54:805–806. doi: 10.2169/internalmedicine.54.3868. [DOI] [PubMed] [Google Scholar]
- 9.Singh N.K., Rehman A., Hansalia S.J. Transient apical ballooning in hypertrophic obstructive cardiomyopathy. Tex Heart Inst J. 2008;35:483–484. [PMC free article] [PubMed] [Google Scholar]
- 10.Jaber W.A., Wright S.R., Murphy J. A patient with hypertrophic obstructive cardiomyopathy presenting with left ventricular apical ballooning syndrome. J Invasive Cardiol. 2006;18:510–512. [PubMed] [Google Scholar]
- 11.Patrianakos A.P., Nyktari E., Parthenakis F.I., Vardas P.E. Reversible left ventricular apical ballooning after heavy alcohol consumption in a patient with hypertrophic cardiomyopathy. Int J Cardiol. 2013;164:e29–31. doi: 10.1016/j.ijcard.2012.09.163. [DOI] [PubMed] [Google Scholar]
- 12.Roy R.R., Hakim F.A., Hurst R.T., Simper D., Appleton C.P. Two cases of apical ballooning syndrome masking apical hypertrophic cardiomyopathy. Tex Heart Inst J. 2014;41:179–183. doi: 10.14503/THIJ-13-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daralammori Y., El Garhy M., Gayed M.R., Farah A., Lauer B., Secknus M.A. Hypertrophic obstructive cardiomyopathy masked by Tako-tsubo syndrome: a case report. Case Rep Cardiol. 2012;2012:486427. doi: 10.1155/2012/486427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brabham W.W., Lewis G.F., Bonnema D.D., Nielsen C.D., O’Brien T.X. Takotsubo cardiomyopathy in a patient with previously undiagnosed hypertrophic cardiomyopathy with obstruction. Cardiovasc Revasc Med. 2011;12:70.e71–70.e75. doi: 10.1016/j.carrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Right anterior oblique view of LCA. A JL3.5 catheter is used to engage and inject dye into the LCA, which showed no evidence of blockages in left circumflex artery and left anterior descending artery.
A left anterior oblique view of LCA.
Left anterior oblique view of the right coronary artery showing no evidence of disease. A JR4 catheter is used to engage and inject dye into the right coronary artery.
Initial apical four-chamber view displays significant hypokinesis of the apical, apical lateral, and apical septal walls with a hyperdynamic basal inferoseptal and anterolateral walls. The systolic anterior motion of mitral valve causing a dynamic LVOTO along with an enlarged left atrium and significant mitral annular calcification are also seen.
Apical five-chamber view showing severe hypokinesis of the apical, apical septal, and apical lateral walls along with basal asymmetric hypertrophy and the LVOTO caused by systolic anterior motion of the mitral leaflet.
Turbulence of blood flow through the LVOT associated with posteriorly directed mitral regurgitation due to LVOTO from systolic anterior motion of the mitral valve.
Follow-up echocardiogram in the four-chamber view shows the recovery of the apical hypokinesis.
Follow-up echocardiogram in the five-chamber view shows decreased turbulence in the LVOT and a decreased mitral regurgitation jet.