Immunotherapy has now been established as a new treatment paradigm for multiple myeloma (MM), due to its ability to induce deep and durable responses, even in patients refractory to multiple classes of novel agents, without adding the cost of relevant toxicities.
Monoclonal antibodies (mAbs) targeting cell surface antigens expressed on MM cells represent a novel way of overcoming resistance to treatment. Daratumumab (dara) is a first-in-class human immunoglobulin (Ig)G1 mAb, with high affinity to CD38, a cell surface glycoprotein prominently, but not exclusively, expressed on MM cells and involved in regulation of cell adhesion, intracellular calcium signaling, apoptosis, survival, and proliferation.1 Dara induces cellular death through various mechanisms, including complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis and the induction of apoptosis via cross-linking.1 In addition, dara plays a role in immunomodulation by means of the depletion of regulatory B cells and CD38-positive immunosuppressive T-regulatory cells, which in turn leads to a greater expansion of T cells in responsive as compared with non-responsive patients.1 The efficacy and safety of dara, either alone or combined with other novel agents, have been explored in several phase I-III studies.2–7 Based on the results of these trials, dara monotherapy or dara in combination with the immunoderivatives (IMiDs) lenalidomide or pomalidomide or with the proteasome inhibitor (PI) bortezomib has been granted approval for the treatment of relapsed/refractory MM. Notably, patients with a creatinine clearance (CrCl) lower than 20–30 ml/min were excluded from these studies, and as such no data are available in the literature on the safety profile of dara in patients with end-stage renal impairment (RI). To the best of our knowledge, we report herein the first case of a patient with refractory (R) MM and end-stage RI requiring dialysis, who was successfully and safely treated with single agent dara.
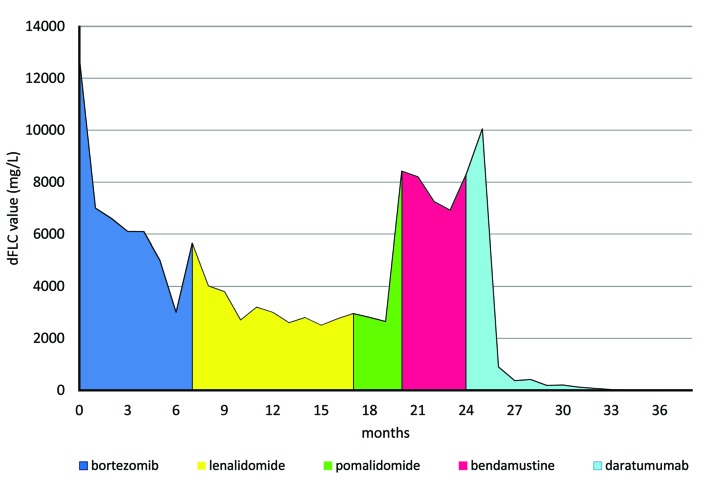
A 68-year-old man was diagnosed with International Staging System (ISS) stage III, oligo-secretory κ MM in October 2014. Main laboratory findings at presentation were the following: hemoglobin (Hb), 8.7 g/dL; creatinine (Cr), 13.4 mg/dL secondary to acute kidney injury (AKI), stage F using RIFLE criteria;8 biopsy-proven myeloma kidney; κ serum-free light chain (sFLC), 12.800 mg/L; difference between involved and uninvolved sFLCs (dFLC), 12.790 mg/L; absence of measurable serum and urine M-protein; bone marrow plasma cells (BMPCs), 30–40%; presence of del(17p) (cut-off value: >10%) in CD138+ PCs, by means of fluorescence in situ hybridization (FISH), 89%; presence of multiple osteolytic lesions at fluorine fluorodeoxyglucose positron emission tomography integrated with computed tomography (PET-CT). Most relevant comorbidities of the patient included post-acute myocardial infarction cardiomyopathy and type 2 diabetes mellitus. The patient received first-line therapy with bortezomib-dexamethasone (Vd) for five 42-day cycles in combination with high cut-off hemodialysis, followed by two additional cycles of Vd plus melphalan (VMP). During the second VMP cycle, progressive disease (PD)9 and lack of renal response were assessed (Figure 1). Second-line therapy with lenalidomide, at doses adjusted according to the estimated CrCl, in combination with dexamethasone (Rd) was started in May 2015. After nine 28-day cycles of Rd, assessment of stable disease and persistence of dialysis dependence provided the basis for switching to therapy with pomalidomide at 4 mg/day on days one to 21 and dexamethasone (Pom-d),10 which was, however, discontinued after two cycles due to aggressive PD with a rapid increase of dFLC of up to 8425 mg/L (Figure 1). Concerns about the safe administration of carfilzomib-based regimens due to patient’s prior cardiovascular disease, led to a fourth-line therapy with bendamustine at 70 mg/m2 and dexamethasone (Bd). After three months, disease reassessment was performed and the main results were as follows: dFLC, 8299.5 mg/L; estimated glomerular filtration rate (eGFR; as calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation),8 5.6 mL/min/1.73 m2; Hb, 7.9 g/dL requiring the transfusion of one unit of red blood cells (RBC) every two weeks; FISH, no additional abnormalities other than del(17p), and PET-CT, persistence of multiple focal hypermetabolic lesions. While on Bd therapy, patient’s clinical conditions progressively worsened and ultimately led to prolonged bed rest. At that time, dara monotherapy for the treatment of RMM had been approved by the European Medicines Agency (EMA), but the process of the drug’s reimbursement by the Italian National Health Service has not yet been completed. Based on the notion that dara is not metabolized by the kidney and there are no contraindications for its use in renal impairment (RI),11,12 the patient was thus enrolled in a Pre-Approval Access Program of dara. Treatment with single agent dara at the standard dosing schedule of 16 mg/kg was started in January 2017. Standard prophylaxis with dexamethasone, paracetamol and clorfenamina was administered. Dara was infused the day following the dialytic session and the frequency of dialysis remained unchanged. Dilution volumes (1000 ml for the first infusion and 500 ml thereafter) and infusion rates were those previously recommended,3 with the exception being that of the first infusion being given at an initial rate of 50 ml in the first two hours, and subsequent increases at the rate of 50 ml every two and a half hours in order to avoid fluid overload. The patient maintained a good cardiac compensation, with the exception of a mild weight gain requiring diuretic therapy. No infusion-related reactions were observed during the initial and subsequent infusions. After the first cycle, comprising four weekly dara administrations, a dramatic reduction in dFLC from 10.051 mg/L to 893 mg/L was observed. Response to treatment continued to improve throughout the subsequent cycles, as shown by a progressive decrease in dFLC down to the normal range (Figure 1) and a progressive rise in Hb concentration up to a value of 12.0 g/dL. A stringent complete remission (sCR) was assessed after the ninth cycle. Consistent with hematologic response, renal function improved, as revealed by a decrease in dialysis from thrice to twice a week, whilst maintaining stable urine output (average 1300 ml/daily), dialytic urea clearance and metabolic parameters. Patient’s clinical conditions and daily activities progressively improved throughout treatment, with an ultimate return to a good quality of life. At the time of writing, the patient has started the thirteenth cycle, while maintaining sCR.
Figure 1.
Trend of dFLC from first to fifth line of treatment. The main vertical axis shows the dFLC values (mg/L) at diagnosis and during treatment. The horizontal axis shows the sequential lines of treatment over time (months). dFLC: difference between involved and uninvolved serum-free light chains.
This report supports the good safety profile and dramatic activity of dara in a patient with heavily pretreated RMM and end-stage RI on dialysis.
Over the past 20 years the frequency of severe RI (eGFR<30 ml/min/1.73 m2) at the onset of MM was reported in approximately one fifth of patients8 and dialysis was required in about 1–13% of cases.13 Although a significant improvement in median overall survival (OS) was seen with the introduction of novel agents, RI is still associated with a worst prognosis and an increased risk of early mortality, particularly for those patients failing treatment.8 Based on the remarkable and fast anti-myeloma activity of bortezomib, its mechanisms of action, which also include inhibition of activated nuclear transcription factor (NF)-κB in renal tubular cells, ultimately leading to reduced inflammation and fibrosis in the kidney, and the half-life of the drug which is independent of renal clearance, full-dose bortezomib-containing regimens are actually considered the gold-standard therapy for MM patients with RI.8 Preliminary data on the pharmacokinetics (PK) and safety of the second generation PIs carfilzomib and ixazomib in MM-related end-stage RI support the administration of these drugs without dose modifications, although results from studies designed to explore their activity in this setting are still lacking. With the exception of lenalidomide, which needs dose adjustments according to CrCl, thalidomide, pomalidomide and the anti-signaling lymphocytic activation molecule (SLAM)F7 mAb elotuzumab can be safely administered in patients with RI.8,14
The management of MM patients refractory to PIs and IMiDs represents a special clinical challenge. Immune-based approaches, including mAbs, have provided remarkable advances in the treatment of patients who have exhausted all available therapeutic options. Although data on the use of dara in daily clinical practice have been very limited thus far, recent reports of real-world experiences have shown safety profiles and survival benefits from this drug comparable to those reported in controlled clinical trials.15 Nevertheless, data from the literature on the PK, pharmacodynamic and toxicity profile of dara in patients with RI are still lacking.
In our patient, information on the potential impact of end-stage RI on PK of dara were unfortunately unavailable, representing a limitation of the case report. However, despite end-stage RI, no drug-related adverse events emerged during the first infusion of dara and later on, confirming the favorable safety profile even in the setting of dialysis-dependence. Remarkably, fifth-line therapy with single agent dara for MM refractory to bortezomib, lenalidomide, pomalidomide and bendamustine offered the chance to get sCR, a finding reported in only 3% of patients enrolled in the SIRIUS trial.3 However, in this study, overall response rates of 30% were noted in pre-specified subgroups, regardless of prior lines of therapy and refractory status, including refractoriness to bortezomib, lenalidomide, carfilzomib or pomalidomide. In our case, and consistent with the progressive depth of response afforded by dara, the frequency of dialysis decreased from three times to twice a week, suggesting a late improvement in renal function. A longer follow up is needed to establish the duration of the response and confirm the OS benefit seen for responders to dara monotherapy in the registrative study.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015; 373(13):1207–1219. [DOI] [PubMed] [Google Scholar]
- 3.Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560. [DOI] [PubMed] [Google Scholar]
- 4.Usmani SZ, Weiss BM, Plesner T, et al. Clinical Efficacy of Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. [DOI] [PubMed] [Google Scholar]
- 7.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Sonnelveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34(13):1544–1557. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos M, Weisel K, van de Donk NWCJ, et al. Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and renal impairment: results from a phase II trial. J Clin Oncol. 2018. February 2 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Moreau P, van de Donk NW, San Miguel J, et al. Practical considerations for the use of daratumumab, a novel CD38 monoclonal antibody, in myeloma. Drugs. 2016;76(8):853–867. [DOI] [PubMed] [Google Scholar]
- 12.Gran C, Gahrton G, Alici E, Nahi H. Case report: treatment of light-chain amyloidosis with daratumumab monotherapy in two patients. Eur J Haematol. 2018;100(4):386–388. [DOI] [PubMed] [Google Scholar]
- 13.Chanan-Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA. Novel therapeutic agents for the management of patients with multiple myeloma and renal Impairment. Clin Cancer Res. 2012;18(8):2145–2163. [DOI] [PubMed] [Google Scholar]
- 14.Berdeja J, Jagannath S, Zonder J, et al. Pharmacokinetics and safety of elotuzumab combined with lenalidomide and dexamethasone in patients with multiple myeloma and various levels of renal impairment: results of a phase Ib study. Clin Lymphoma Myeloma Leuk. 2016;16(3):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakshman A, Abeykoon JP, Kumar SK, et al. Efficacy of daratumumab-based therapies in patients with relapsed, refractory multiple myeloma treated outside of clinical trials. Am J Hematol. 2017; 92(11):1146–1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.