Abstract
Polycythemia vera is a chronic myeloproliferative neoplasm characterized by the JAK2V617F mutation, elevated blood cell counts and a high risk of thrombosis. Although the red cell lineage is primarily affected by JAK2V617F, the impact of mutated JAK2 on circulating red blood cells is poorly documented. Recently, we showed that in polycythemia vera, erythrocytes had abnormal expression of several proteins including Lu/BCAM adhesion molecule and proteins from the endoplasmic reticulum, mainly calreticulin and calnexin. Here we investigated the effects of hydroxycarbamide and interferon-α treatments on the expression of erythroid membrane proteins in a cohort of 53 patients. Surprisingly, while both drugs tended to normalize calreticulin expression, proteomics analysis showed that hydroxycarbamide deregulated the expression of 53 proteins in red cell ghosts, with overexpression and downregulation of 37 and 16 proteins, respectively. Within over-expressed proteins, hydroxycarbamide was found to enhance the expression of adhesion molecules such as Lu/BCAM and CD147, while interferon-α did not. In addition, we found that hydroxycarbamide increased Lu/BCAM phosphorylation and exacerbated red cell adhesion to its ligand laminin. Our study reveals unexpected adverse effects of hydroxycarbamide on red cell physiology in polycythemia vera and provides new insights into the effects of this molecule on gene regulation and protein recycling or maturation during erythroid differentiation. Furthermore, our study shows deregulation of Lu/BCAM and CD147 that are two ubiquitously expressed proteins linked to progression of solid tumors, paving the way for future studies to address the role of hydroxycarbamide in tissues other than blood cells in myeloproliferative neoplasms.
Introduction
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm (MPN) characterized by clonal expansion of an abnormal hematopoietic stem cell due in most cases to the V617F activating mutation in the tyrosine kinase JAK2.1–4 PV is marked by thrombohemorrhagic complications and a propensity to transform into myelofibrosis and acute leukemia.5
Reducing the vascular risk and preventing transformation are the main purpose of PV treatment. According to current guidelines, low-risk PV patients are managed with phlebotomy and aspirin, whereas high-risk patients receive cytoreduction with hydroxycarbamide (HC) or interferon-α (IFN).6 New therapeutic approaches aim at developing small molecules as specific inhibitors of JAK2 mutated forms.7 HC, also known as hydroxyurea, is commonly used to treat PV patients requiring cytoreductive therapy. HC is a cytostatic non-alkylating hydroxylated urea analog that inactivates ribonucleotide reductase, the enzyme that catalyses the conversion of ribonucleotides to deoxyribonucleotides during de novo DNA synthesis,8 leading to the interruption of DNA synthesis and cell death at the S-phase.9 HC does not target the malignant clone and therefore appears unable to eradicate it. The efficacy of HC in preventing thrombosis was suggested in several randomized clinical trials,10 but is still not proven. HC alone or in combination with other therapies may increase the risk of acute leukemia in MPN.11
Interferon-α is a non-leukemogenic drug that, in some cases, induces cytogenetic remissions or reversion from monoclonal to polyclonal patterns of hematopoiesis, and may specifically target the malignant clone.12 Its widespread use was hampered by its parenteral administration, cost and toxicity. The development of pegylated forms (PEG-IFN-α) increased tolerance and efficacy in IFN-treated patients.13
Increased thrombotic risk in PV patients is associated with high levels of hemoglobin, impaired rheology, and increased viscosity resulting from erythrocytosis.14–16 Targeting a hematocrit below 45% seems to decrease the thrombotic risk of these patients.10 An additional parameter that might contribute to this risk was brought to light by our work showing abnormal activation of adhesion proteins in PV red blood cells (RBC).17,18 More recently, using a proteomic approach, we also found that membranes of PV RBCs had abnormal expression of several proteins including the adhesion protein Lu/BCAM (Lutheran/Basal Cell Adhesion Molecule) and proteins from the endoplasmic reticulum, such as calreticulin (Calr) and calnexin.19 These findings indicate that JAK2V617F not only impacts cell proliferation, but also induces changes in the repertoire of erythroid proteins expressed in circulating RBCs, which might contribute to the circulatory complications described in PV.
In this study, we investigated the effects of HC and IFN treatments on the expression of membrane proteins in circulating RBCs of PV patients. We show that HC and IFN each have a different impact on the expression and function of erythroid membrane proteins. While both drugs seem to normalize Calr expression, HC (but not IFN) enhances the expression of several proteins, including Lu/BCAM and CD147 adhesion proteins, and further exacerbates RBC adhesion to laminin.
Methods
Patients and blood samples
The study obtained ethical approval from Comité de Protection des Personnes Ile de France VII (PP 14-035); blood samples from 53 PV patients, followed at Saint Louis Hospital, Paris, were obtained with informed consent. Patients are divided in 3 groups: 25 patients (16 men and 9 women; mean age: 63±14 years) were treated with phlebotomy in addition to low-dose aspirin; 20 patients (11 men and 9 women; mean age: 64±16 years) were treated with HC [mean dose 0.8 g/day (0.3–1.5g/day)]; 12 patients (9 men and 3 women; mean age: 50±13 years) were treated with PEG-IFN-α according to international and local guidelines20 [mean dose 115 μg/week (45–180 μg/week)]. The patient groups are small, and not necessarily matched for age or sex, partially due to the treatment guidelines of IFN and HC. Four patients from the first category were treated with HC during the study; blood samples from these 4 patients were collected before and during HC-treatment, at least six months after starting the treatment. All HC-treated patients were compliant to the treatment as they had complete hematologic response and increased mean corpuscular volume (MCV). JAK2V617F mutation was observed in all patients. Blood samples from 16 regular healthy donors at the Etablissement Français du Sang were also analyzed in this study (9 women and 7 men; mean age: 40±12 years, range 25–67 years). The 2 control (CT) blood samples used for the proteomic analysis, CT1 and CT2, were chosen to match as well as possible with the PV patient group of the study; they were from 67 and 57 year-old donors, respectively.
Blood samples and ghost preparation
Blood samples were collected on sodium heparin tubes. Buffy coat was removed and RBCs were cryopreserved at Centre National de Référence pour les Groupes Sanguins, Paris. RBC membranes were prepared by hypotonic lysis and washed with 5 mM sodium phosphate, pH 8.0 containing 0.2 mM phenylmethylsulfonylfluoride.
ITRAQ labeling and quantification by nano-liquid chromatography and mass spectrometry
Isobaric tag for relative and absolute quantitation (ITRAQ) multiplex analysis was carried out at the 3P5 proteomics facility as previously described,21 with some modifications (Online Supplementary File 1). Trypsin digested peptides from red cell ghosts of 3 PV patients (before and during HC treatment: total 6 samples) and 2 healthy donors (CT) were processed. (For further details see Online Supplementary File 1). Data were analyzed using Protein Pilot 4 with the Uniprot human database. The analysis yielded 12,459 peptide spectrum matches corresponding to 2664 non-redundant peptides assigned to 375 proteins. Proteins were considered over-expressed or down-regulated when they showed PV/CT or PVHC/PV ratios ≥1.3 or ≤0.7, respectively, in at least 2 out of 3 PV samples, with no ratios lower than 1.2 or higher than 0.8 for over-expressed or down-regulated proteins, respectively.
JAK2V617F allele burden quantification
The percentage of the JAK2V617F allele was determined in DNA extracted from whole blood using the Mutaquant® (Ipsogen) method as described.22
Flow adhesion assays
Red blood cell adhesion to laminin 521 was measured under flow conditions using a capillary flow chamber. Recombinant laminin 521 (BioLamina) at 5 ng/μL was immobilized in Vena8 Endothelial+™ biochips (internal channel dimensions: length 20 mm, width 0.8 mm, height 0.12 mm). RBCs were perfused at 5.107 RBCs/mL for 5 minutes (min) at 0.5 dyn/cm2 and 5-min washouts were performed at 0.5, 1, 2, 3, 5, 7 dyn/cm2 using the ExiGo™ pump (Cellix Ltd., Dublin, Republic of Ireland). After each wash, adherent RBCs were counted in 6 representative areas along the centerline of the biochip using the AxioObserver Z1 microscope (10X objective) and AxioVision 4 analysis software (Carl Zeiss). Images of the same 6 areas were obtained throughout each experiment using the “Mark and Find” module of AxioVision analysis software.
Flow cytometry
Cell surface expression of Lu/BCAM and CD147 and percentage of reticulocytes were determined using specific antibodies and Retic-Count™ (thiazole orange) reagent, respectively, using a BD FACScanto II flow cytometer (Becton-Dickinson), as described.23,24
Phosphorylation assays and western blot
Phosphorylation of Lu/BCAM was assessed in PV RBCs, as described.23,24 Briefly, RBCs were incubated in DMEM (GlutaMAX™ I, 4500 mg/L D-glucose) without sodium pyruvate for 2 hours at 37°C, 0.5% CO2, centrifuged at 1500 rpm for 5 min, and suspended in 1 mL of the same DMEM containing 32P (160 μCi) overnight at 37°C, 0.5% CO2. RBCs were lysed for 45 min at 4°C with lysis buffer containing: 20mM Tris, 150mM NaCl, 5mM EDTA, 0.002% NaN3, 1% Triton X-100, 0.2% BSA, phosphatase (Sigma-Aldrich), and protease inhibitor cocktails (Roche Diagnostics). Lu/BCAM was immunopurified with F241 mAb and protein A-sepharose CL4B beads (Roche Diagnostics) overnight at 4°C. After electrophoresis and protein transfer, phosphorylated proteins were detected and quantified with a FujiFilm BAS-1800 II PhosphorImager, using Image Reader BAS-1800 II v.1.8 and Multi Gauge, v.3.0 software, respectively (Fuji). Total Lu/BCAM was then revealed on the same membrane using biotinylated anti-Lu/BCAM antibody (R&D Systems) and ECL. Proteins were quantified using Chemidoc and Quantity One software.
Statistical analysis
Statistical analysis was performed with GraphPad Prism using Mann-Whitney test (Figures 1–3) and paired t-test (Figure 4): *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
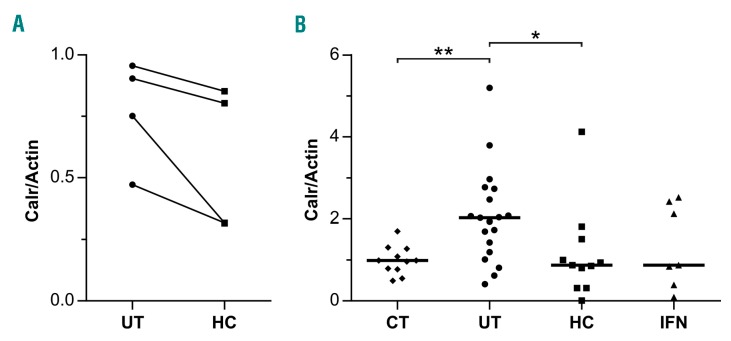
Figure 1.
Calreticulin expression is decreased in polycythemia vera (PV) patients under hydroxycarbamide (HC) and interferon-α (IFN) treatments. Quantification of Calr expression normalized by actin from (A) 4 PV patients before (UT) and after (HC) HC treatment and (B) 11 control (CT), 19 UT, 11 HC, and 7 IFN patients. Horizontal lines represent medians: 0.9883, 2.023, 0.8760 and 0.8760, respectively.
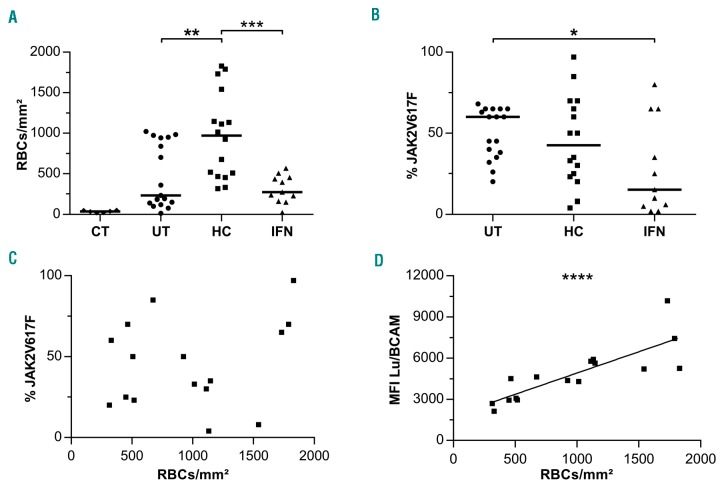
Figure 3.
Increased adhesion to laminin of red blood cells (RBCs) from hydroxycarbamide (HC)-treated patients. (A) Adhesion to laminin at 3 dyn/cm2 of RBCs from 11 control (CT), 17 untreated (UT), 16 HC-treated (HC) and 11 IFN-treated (IFN) polycythemia vera patients. Horizontal lines represent medians. (B) The JAK2V617F allele burden (%JAK2V617F). RBC adhesion as a function of (C) %JAK2V617F (R2=0.048) and (D) Lu/BCAM mean fluorescence intensity (MFI) (R2=0.67) for 16 HC-treated patients.
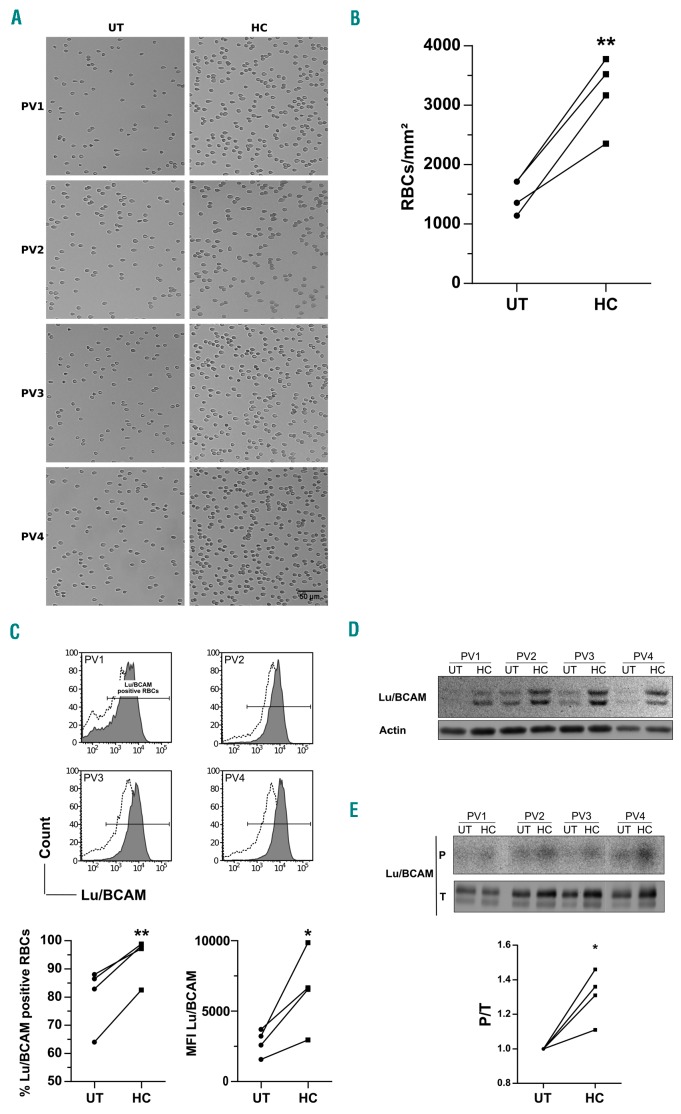
Figure 4.
Longitudinal analyses of red blood cell (RBC) adhesion and Lu/BCAM expression and activation in 4 polycythemia vera (PV) patients during hydroxycarbamide (HC) treatment. All results were obtained with RBCs from 4 PV patients before (UT) and during (HC) HC treatment. (A) Typical images of RBCs adhering to laminin 521 at 3 dyn/cm2. (B) Mean number of RBCs/mm2. (C) Flow cytometry analysis of Lu/BCAM expression: (top) histograms (before: dotted; during: solid); (bottom) percentage of RBCs expressing Lu/BCAM and mean fluorescence intensity (MFI). (D) Western blot analysis of Lu/BCAM expression; the upper band corresponds to the long isoform Lu and the lower one to the short isoform Lu(v13). (E) Lu/BCAM phosphorylation rate. The top (P) and bottom (T) panels show the phosphorylation and the total amount of the immunopurified proteins, respectively. The phosphorylated fraction is determined by the P/T ratio.
Results
Altered membrane protein expression in RBCs of HC-treated patients
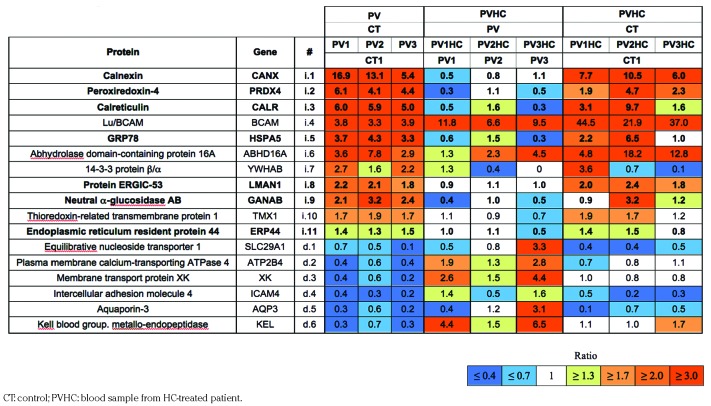
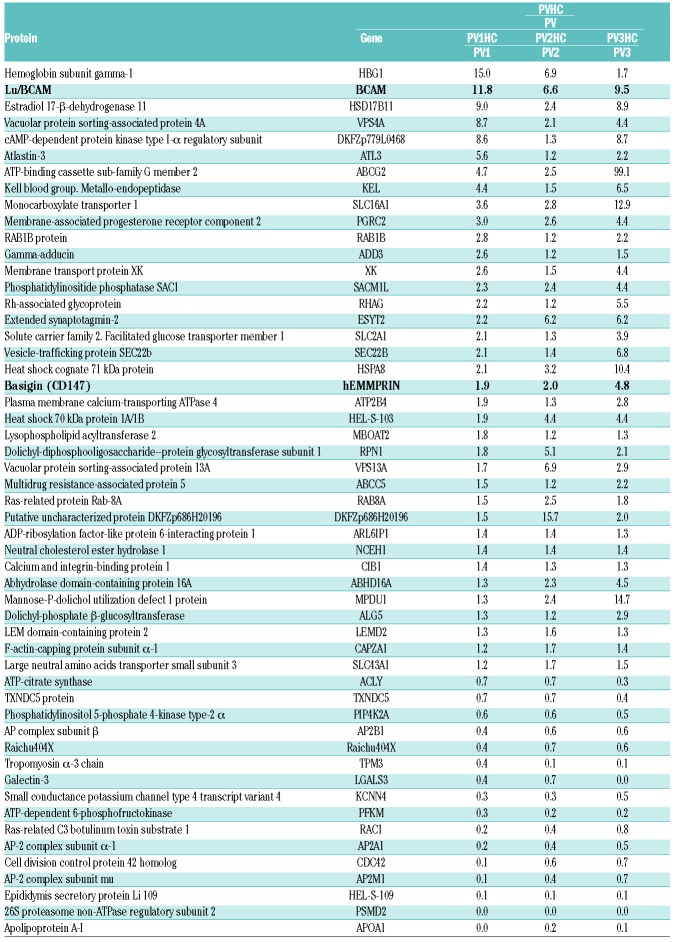
In our recent work, we showed abnormal expression of several proteins at the membrane of PV RBCs, with a high number of proteins from the endoplasmic reticulum, including calreticulin (Calr).19 To verify if HC treatment restores normal expression of these proteins, we performed a proteomic analysis with RBC ghosts from 3 patients before and during their treatment with HC (pre-post patients). Three hundred and seventy-five proteins were confidently identified, with a number of peptides that allowed 358 distinct protein quantification (Online Supplementary File 2). Among the deregulated proteins initially reported,19 HC decreased the expression of several over-expressed proteins and increased the expression of those down-regulated (Table 1). Nevertheless, the treatment did not seem to restore a normal expression pattern of these proteins for all 3 patients when compared to control (Table 1 and Online Supplementary Table S1). In addition, the comparative analysis showed that HC deregulated the expression of 53 proteins that had normal expression before the treatment started, with overexpression of 37 proteins (ratio ≥1.3) and downregulation of 16 proteins (ratio ≤0.7) (Table 2).
Table 1.
ITRAQ ratios of proteins with increased (i) or decreased (d) expression at the membrane of polycythemia vera (PV) red blood cells, and effect of hydroxycarbamide (HC) treatment. Proteins from endoplasmic reticulum are in bold.
Table 2.
ITRAQ ratios of 53 proteins modulated by hydroxycarbamide (HC) in polycythemia vera (PV) red blood cell ghosts.
Expression of Calr was diminished during HC treatment but did not reach control levels (Table 1). We analyzed Calr by western blot in RBC ghosts from a total of 4 pre-post patients, including the 3 patients of the proteomics study, and found that its expression was decreased during the treatment without reaching significance (Figure 1A), confirming the proteomics results. This was probably due to the variability and relatively short duration of the treatment in this group (mean duration 1.4 years, range 0.5–3 years). Therefore, we tested for the presence and expression level of Calr by western blot in a group of 11 patients treated with HC for a longer period of time (mean duration 5.9 years, range 0.5–20 years), a group of 19 untreated (UT) patients (i.e. with no cytotoxic antiproliferative treatment), and a group of 11 healthy donors (Figure 1B). We found no significant difference between the HC and healthy donor groups indicating that long-term HC treatment restores the expression of Calr. Similarly, we investigated Calr in a group of 7 patients treated with IFN and found no difference with the healthy donors group, strongly suggesting that IFN restores normal expression of Calr in PV RBCs (Figure 1B).
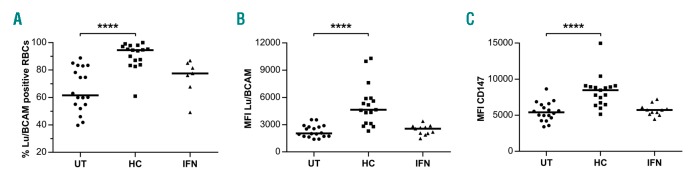
Overexpression of Lu/BCAM and CD147 in HC-treated patients
In the group of membrane proteins abnormally expressed in PV RBCs,19 Lu/BCAM was the only protein whose overexpression was further exacerbated by HC in all 3 patients, with a fold increase comprised between 6.6 and 11.8 (Table 1). Lu/BCAM is a low abundance surface protein that is expressed on a subpopulation of circulating RBCs. To determine whether HC increases the percentage of Lu/BCAM-positive RBCs or Lu/BCAM expression level per RBC, or both, we performed flow cytometry assays with 18 UT and 17 HC blood samples, using a specific mouse monoclonal anti-Lu/BCAM antibody. The percentage of Lu/BCAM-expressing cells and the number of Lu/BCAM molecules per RBC, estimated by the mean fluorescence intensity (MFI) of Lu/BCAM-positive RBCs, were significantly higher in the HC group (median 94.35%, MFI=4570) than in the UT group (median 73%, MFI=1819) (P<0.001, Mann-Whitney test) (Figure 2A and B, and Online Supplementary Figure S1A). As Lu/BCAM expression is known to be higher in young (reticulocytes) than mature RBCs,25 we determined the percentage of reticulocytes in all blood samples. There was no significant difference between both patient groups (mean UT:0.6 ±0.05; HC: 0.9±0.17, P=0.097) (Online Supplementary Figure S1B) indicating that the increase of Lu/BCAM was not due to an HC-induced imbalance between reticulocytes and mature RBCs.
Figure 2.
Lu/BCAM and CD147 erythroid expression are increased under hydroxycarbamide (HC) treatment. Flow cytometry analyses of (A and B) Lu/BCAM and (C) CD147 expression on red blood cells (RBC) from 17 untreated (UT), 16 HC-treated (HC), and 11 interferon-α-treated (IFN) polycythemia vera patients. MFI: mean fluorescence intensity. Horizontal lines represent medians.
Flow cytometry analysis was conducted with 11 blood samples from IFN-treated patients and no significant difference was found with the UT group (Lu/BCAM-positive RBCs: 81.4% vs. 73%, P=0.3722; MFI: 2479 vs. 1819, P=0.5588, respectively) (Figure 2A and B), indicating that IFN did not influence Lu/BCAM protein expression.
In order to explore the potential effect of HC and IFN on the expression of other erythroid adhesion proteins, we performed flow cytometry analysis of four additional adhesion markers: CD44, CD47, CD147 and CD242. All 4 markers are expressed on almost 100% of circulating RBCs and there was no effect of HC or IFN on this percentage (data not shown). Likewise, there was no effect of either HC or IFN treatment on the expression level per RBC of CD44, CD47 and CD242, estimated by the MFI, when compared to UT patients. However, we observed increased expression of CD147 in the HC group (median MFI=8472) when compared to the UT group (median MFI=5406) (P<0.0001, Mann-Whitney test) (Figure 2C) suggesting a common upregulation of Lu/BCAM and CD147 by HC. This increase was confirmed by proteomics in the 3 pre-post patients who showed increased CD147 expression during HC treatment (Table 2).
Increased RBC adhesion to laminin in HC-treated patients
As Lu/BCAM mediates abnormal adhesion of PV RBCs to laminin,17 we examined the effect of HC treatment on PV RBC adhesion by performing adhesion assays under flow conditions. RBCs from HC-treated patients adhered much more than those from UT patients, with respective medians of 969 and 231 RBCs/mm2 at 3 dyn/cm2 (P=0.0047, Mann-Whitney test) whereas no significant difference was found between the IFN (271 RBCs/mm2) and UT groups (P=0.8508, Mann-Whitney test) (Figure 3A). Adhesion of RBCs from 6 control donors was minor (median=37 RBCs/mm2), and significantly lower than in the UT, HC and IFN groups (P<0.01, Mann-Whitney test).
The impact of HC and IFN on cell adhesion was also analyzed by comparing the UT, HC and IFN groups in terms of RBC adhesion value distributions. We found that these values were broadly dispersed in the UT and HC groups but were less scattered in the IFN group (Figure 3A), with a significant difference between the variance of the IFN group as compared to the UT (P<0.05) or the HC group (P<0.05) (Fisher exact test). This was probably linked to the JAK2V617F allele burden of each group. As a matter of fact, we have previously shown that PV RBC adhesion to laminin in UT patients was correlated with the JAK2V617F allele burden, defined as the percentage of circulating JAK2 alleles with the V617F mutation (%V617F).26 We determined the %V617F of all patients and, as expected and as reported in previous studies,13 the median was significantly lower in the IFN than in the UT and HC groups (Figure 3B). Moreover, we found no significant difference between the UT and the HC groups, despite a lower median in the latter (Figure 3B), indicating that increased RBC adhesion in the HC group was independent of the %V617F and was most probably due to the expression level of Lu/BCAM. This was demonstrated by plotting RBC adhesion values against the percentage of JAK2V617F (Figure 3C) and the MFI of Lu/BCAM (Figure 3D).
Longitudinal study of HC treatment in 4 PV patients
As only HC had activating effects on RBC adhesion, and because of wide interindividual variability in PV, we further investigated the effects of HC treatment in the 4 pre-post HC patients of our cohort by performing adhesion and flow cytometry assays. This longitudinal analysis showed a great increase of adhesion after HC treatment for all 4 patients (Figure 4A and B), confirming the results obtained with the UT and HC groups. As seen in the UT and HC groups, we found that the increased adhesion was associated with increased Lu/BCAM expression, both in terms of percentage of Lu/BCAM-positive RBCs and associated mean fluorescence intensity (Figure 4C), confirming that HC increases both the percentage of Lu/BCAM-positive RBCs and the expression of this protein within each positive RBC. This increase was reflected by higher amounts of Lu/BCAM during HC treatment detected by western blot (Figure 4D).
We have shown that Lu/BCAM long isoform was constitutively phosphorylated in PV RBCs18 and that inhibi tion of this phosphorylation was associated with less PV RBC adhesion to laminin.17 In order to determine whether the increased adhesion induced by HC was associated with Lu/BCAM activation, we tested the phosphorylation state of Lu/BCAM long isoform. Radiophospholabeling experiments confirmed that Lu/BCAM was phosphorylated in PV RBCs and showed increased phosphorylation during HC treatment (Figure 4E, top panel). This increased phosphorylation was not only due to increased Lu/BCAM expression, as the phosphorylation ratio (P/T), in which P is the measured phosphorylation and T the expression of total Lu/BCAM, was higher during than before HC treatment (Figure 4E, bottom panel), indicating that HC both increases the expression of Lu/BCAM and the proportion of phosphorylated molecules.
Discussion
Our recent work showed abnormal expression of several proteins in RBCs of PV patients,19 revealing qualitative alterations in these cells in addition to their increased number in the circulation. The aim of the current study was to address the effects of HC treatment on the expression of erythroid membrane proteins of PV patients using a global proteomic approach.
Our proteomic results obtained with ghosts from 3 pre-post patients showed that HC treatment tends to restore the expression of deregulated membrane proteins. Nevertheless, normal expression of these membrane proteins was not reached, suggesting that the duration of the treatment for these patients was not sufficient, which is supported by the results of Calr expression in the group of 11 patients treated with HC for a longer duration.
Since we have shown that Calr overexpression was associated with the presence of JAK2V617F,19 the decreased Calr expression in the HC group could have been the consequence of fewer JAK2V617F-positive clones in the bone marrow of these patients. This was reflected by their lower %V617F when compared to the UT group (median=42.5 and 60% for HC and UT, respectively), although the difference was not significant, which is in accordance with a study showing that HC does not appreciably reduce the JAK2V617F allele burden.27 The IFN group also showed lower expression levels of Calr than the UT group, along with a significant decrease of the %V617F (median=15%). This decrease was expected as IFN treatment is known to reduce the JAK2V617F allele burden, with some cases of complete remission.13 Calr is a calcium-binding chaperone that promotes efficient folding of glycoproteins but whose role in circulating RBCs has not been elucidated. Because calcium regulates several erythrocyte functions,28 we believe that the decrease in Calr expression triggered by HC and IFN might be beneficial to the calcium homeostasis and subsequent calcium-regulated functions of PV RBCs.
However, despite the tendency to normalize Calr expression, the proteomic analysis showed that HC treatment clearly affects the expression of several membrane proteins. This is not the first example of such deregulation, as HC is known to induce the neosynthesis of fetal hemoglobin in the erythroid lineage and is used to this purpose in sickle cell disease (SCD) patients.29,30 Among the proteins over-expressed by HC in PV patients, Lu/BCAM and CD147 were also reported to be up-regulated in RBCs of sickle cell disease patients treated with HC,31 indicating that the observed effect of HC is not specific to PV. This suggests the triggering of common pathways by HC independently of the underlying illness, most likely through the activation of gene transcription. This is supported by the findings of Odievre et al.31 showing increased expression of Lu/BCAM in erythroid progenitors from SCD patients and healthy donors differentiated in vitro in the presence of HC.31 Moreover, we have previously shown activation of Lu/BCAM gene transcription by HC in endothelial cells grown in vitro.32
Similar to our previous reports in sSCD, we found that HC significantly increases erythroid Lu/BCAM expression by enhancing both the percentage of Lu/BCAM-positive RBCs and the Lu/BCAM copy number per RBC. The increase in the percentage of Lu/BCAM-positive RBCs indicates that the observed higher expression is not due to the documented increase of the RBC volume consecutive to the HC treatment. Opposite to RBCs from HC-treated SCD patients, those from treated PV patients had increased adhesion to laminin. In SCD, increased Lu/BCAM phosphorylation is achieved in a PKA/cAMP-dependent manner, with patients showing high cAMP levels that are decreased during HC treatment.23 In PV, Lu/BCAM phosphorylation is driven by a JAK2V617F-dependent pathway involving Akt, and not PKA, which could explain the difference of HC effects between SCD and PV. HC is a nitric oxide (NO) donor in vivo33–35 and its effect in PV RBCs might be due to Akt activation through an NO-dependent pathway. Indeed, RBCs have been shown to express an active NO synthase (NOS)36 and are thus capable of generating NO and activating downstream effectors like Akt. Such activation of Akt by NO donors has been reported in endothelial cells37 and chick retinal neurons;38 it occurs through the activation of soluble guanylyl cyclase (sGC) and protein kinase G. Interestingly, HC has also been shown to activate sGC in erythroid cells,39 such activation might trigger Akt activity in PV RBCs and lead to Lu/BCAM activation and increased RBC adhesion.
Activation of leukocytes, platelets and endothelial cells is known to promote a prothrombotic state. Markers for such activation have been reported in PV patients, including activation of polymorphonuclear leukocytes and endothelial cells,40 and increased levels of leukocyte-platelet aggregates.41 A previous report showed that treatment of PV patients with HC did not influence these marker levels.42 Our study reveals a new pro-adhesive effect of HC in PV patients that acts by increasing the expression of two adhesion molecules, Lu/BCAM and CD147, and by activating RBC adhesion to laminin. CD147, also named basigin or neurothelin, is a member of the immunoglobulin superfamily that is expressed in various tissues, including brain, leukocytes, endothelial cells, and most tumor cell lines. CD147 is expressed during erythroid differentiation43 and is carrier molecule for the blood group antigen Oka.44 Its erythroid adhesive function is important during the circulation of RBCs in the spleen.45 It was shown to bind endothelial cells and fibroblasts,46 and to be a receptor essential for erythrocyte invasion by Plasmodium falciparum.47 It is noteworthy that Lu/BCAM and CD147 are adhesion markers linked to progression of solid tumors. Lu/BCAM is over-expressed in carcinomas in vivo and up-regulated following malignant transformation in some cell types.48–51 Likewise, CD147 plays a central role in the progression of many cancers by inducing the secretion of matrix metalloproteinases and various cytokines (reviewed by Xiong, Edwards and Zhou52). Several studies have indicated that CD147 is a multifunctional glycoprotein that inhibits tumor cell anoikis,53 enhances tumor angiogenesis,54 and promotes invasion, metastasis,55 and glycolytic energy metabolism.56 Considering the wide tissue distribution of both proteins, and the fact that HC distributes throughout the body reaching approximately all tissues, one would expect that Lu/BCAM and CD147 would be also over-expressed in cell types other than RBCs, such as endothelial and epithelial cells. This is supported by our study showing that HC increases the endogenous expression of Lu/BCAM in endothelial cells ex vivo.32 Consequently, overexpression of Lu/BCAM and CD147 in HC-treated patients might possibly have a negative impact both in the vascular territory by promoting abnormal cellular interactions, and in the higher incidence of skin cancer reported in HC-treated PV patients.57
Although HC and IFN inhibit the proliferation of progenitor cells, nothing was known about their impact on PV RBCs once they exit the bone marrow. Our study is the first to address the effects of these molecules on PV RBCs, and to show that HC and IFN have a different impact on RBC protein expression and adhesive function. Leukocytosis and high hematocrit are two parameters involved in thrombotic events in MPN patients, and are both decreased during HC treatment.10 A PV Study Group non-randomized trial showed that HC was associated with a lower incidence of early thrombosis compared to a historical cohort treated with phlebotomy alone (6.6% vs. 14% at 2 years).58 Nevertheless, thrombosis is not totally abrogated in HC-treated patients. Patients treated with IFN also encounter less thrombotic events,59 but whether this happens more or less than those treated with HC is still unknown because there had been no clinical trials comparing the effects of both drugs in the same cohort of MPN patients. Such trials are currently ongoing and might show differences between HC and IFN regarding circulatory complications.
Altogether, our study shows that HC and IFN each have a different impact on RBCs and reveals unexpected adverse effects of HC on RBC physiology in PV. Our findings show that HC deregulates the expression of several proteins at the red cell membrane providing new insights into the effects of this molecule on gene regulation and protein recycling or maturation during erythroid differentiation. Furthermore, our study shows that HC increases the expression of two ubiquitously expressed proteins that are linked to progression of solid tumors. Investigating such overexpression in tissues other than blood cells will be of interest in MPNs.
Supplementary Material
Acknowledgments
We thank Ms. Dominique Gien, Sirandou Tounkara and Eliane Véra at the Centre National de Référence pour les Groupes Sanguins for the management of blood samples, Emilie-Fleur Gautier and Morgane Le Gall for assistance in proteomics data analyses, and the France Intergroupe Syndromes Myéloprolifératifs (FIM) for helpful discussions.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/6/972
Funding
The research leading to these results has received funding from the Institut National de la Santé et de la Recherche Médicale (Inserm), the Institut National de la Transfusion Sanguine, the European Union’s Horizon 2020 research and innovation program through the RELEVANCE project under the Marie Skłodowska-Curie grant agreement n. 675115, and the Laboratory of Excellence GR-Ex, reference ANR-11-LABX-0051. GR-Ex is funded by the program “Investissements d’Avenir” of the French National Research Agency, reference ANR-11-IDEX-0005-02. MB and MDG were funded by the Ministère de l’Enseignement Supérieur et de la Recherche (Ecole Doctorale BioSPC). They received a financial support from: Club du Globule Rouge et du Fer and Société Française d’Hématologie.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005; 434(7037):1144–1148. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005; 7(4):387–397. [DOI] [PubMed] [Google Scholar]
- 5.Schafer AI. Bleeding and thrombosis in the myeloproliferative disorders. Blood. 1984; 64(1):1–12. [PubMed] [Google Scholar]
- 6.Tefferi A, Barbui T. Essential Thrombocythemia and Polycythemia Vera: Focus on Clinical Practice. Mayo Clin Proc. 2015;90(9):1283–1293. [DOI] [PubMed] [Google Scholar]
- 7.Nazha A, Khoury JD, Verstovsek S, Daver N. Second line therapies in polycythemia vera: What is the optimal strategy after hydroxyurea failure? Crit Rev Oncol Hematol. 2016;105:112–117. [DOI] [PubMed] [Google Scholar]
- 8.Gwilt PR, Tracewell WG. Pharmacokinetics and pharmacodynamics of hydroxyurea. Clin Pharmacokinet. 1998; 34(5):347–358. [DOI] [PubMed] [Google Scholar]
- 9.Yarbro JW. Mechanism of action of hydroxyurea. Semin Oncol. 1992;19(3 Suppl 9):1–10. [PubMed] [Google Scholar]
- 10.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. [DOI] [PubMed] [Google Scholar]
- 11.Kiladjian JJ, Chevret S, Dosquet C, Chomienne C, Rain JD. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol. 2011; 29(29):3907–3913. [DOI] [PubMed] [Google Scholar]
- 12.Kiladjian JJ, Masse A, Cassinat B, et al. Clonal analysis of erythroid progenitors suggests that pegylated interferon alpha-2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia. 2010;24(8):1519–1523. [DOI] [PubMed] [Google Scholar]
- 13.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–3072. [DOI] [PubMed] [Google Scholar]
- 14.Kwaan HC, Wang J. Hyperviscosity in polycythemia vera and other red cell abnormalities. Semin Thromb Hemost. 2003;29(5):451–458. [DOI] [PubMed] [Google Scholar]
- 15.Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2(8102):1219–1222. [DOI] [PubMed] [Google Scholar]
- 16.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100(13):4272–4290. [DOI] [PubMed] [Google Scholar]
- 17.De Grandis M, Cambot M, Wautier MP, et al. JAK2V617F activates Lu/BCAM-mediated red cell adhesion in polycythemia vera through an EpoR-independent Rap1/Akt pathway. Blood. 2013;121(4):658–665. [DOI] [PubMed] [Google Scholar]
- 18.Wautier MP, El Nemer W, Gane P, et al. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin alpha5 chain and Lu/BCAM. Blood. 2007; 110(3):894–901. [DOI] [PubMed] [Google Scholar]
- 19.Brusson M, Cochet S, Leduc M, et al. Enhanced calreticulin expression in red cells of polycythemia vera patients harboring the JAK2V617F mutation. Haematologica. 2017;102(7):e241–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautier EF, Ducamp S, Leduc M, et al. Comprehensive Proteomic Analysis of Human Erythropoiesis. Cell Rep. 2016; 16(5):1470–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouroupi E, Kiladjian JJ, Dosquet C, et al. Does increasing the JAK2V617F assay sensitivity allow to identify more patients with MPN? Blood Cancer J. 2012;2(5):e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartolucci P, Chaar V, Picot J, et al. Decreased sickle red blood cell adhesion to laminin by hydroxyurea is associated with inhibition of Lu/BCAM protein phosphorylation. Blood. 2010;116(12):2152–2159. [DOI] [PubMed] [Google Scholar]
- 24.Gauthier E, Rahuel C, Wautier MP, et al. Protein kinase A-dependent phosphorylation of Lutheran/basal cell adhesion molecule glycoprotein regulates cell adhesion to laminin alpha5. J Biol Chem. 2005;280(34):30055–30062. [DOI] [PubMed] [Google Scholar]
- 25.El Nemer W, Gane P, Colin Y, et al. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J Biol Chem. 1998; 273(27):16686–16693. [DOI] [PubMed] [Google Scholar]
- 26.De Grandis M, Cassinat B, Kiladjian JJ, Chomienne C, El Nemer W. Lu/BCAM-mediated cell adhesion as biological marker of JAK2V617F activity in erythrocytes of polycythemia vera patients. Am J Hematol. 2015;90(7):E137–138. [DOI] [PubMed] [Google Scholar]
- 27.Antonioli E, Carobbio A, Pieri L, et al. Hydroxyurea does not appreciably reduce JAK2 V617F allele burden in patients with polycythemia vera or essential thrombocythemia. Haematologica. 2010;95(8):1435–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L. Calcium in red blood cells-a perilous balance. Int J Mol Sci. 2013; 14(5):9848–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charache S. Fetal hemoglobin, sickling, and sickle cell disease. Adv Pediatr. 1990;37:1–31. [PubMed] [Google Scholar]
- 30.Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984;74(2):652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odievre MH, Bony V, Benkerrou M, et al. Modulation of erythroid adhesion receptor expression by hydroxyurea in children with sickle cell disease. Haematologica. 2008;93(4):502–510. [DOI] [PubMed] [Google Scholar]
- 32.Chaar V, Laurance S, Lapoumeroulie C, et al. Hydroxycarbamide decreases sickle reticulocyte adhesion to resting endothelium by inhibiting endothelial lutheran/basal cell adhesion molecule (Lu/BCAM) through phosphodiesterase 4A activation. J Biol Chem. 2014;289(16):11512–11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glover RE, Ivy ED, Orringer EP, Maeda H, Mason RP. Detection of nitrosyl hemoglobin in venous blood in the treatment of sickle cell anemia with hydroxyurea. Mol Pharmacol. 1999;55(6):1006–1010. [DOI] [PubMed] [Google Scholar]
- 34.Jiang J, Jordan SJ, Barr DP, Gunther MR, Maeda H, Mason RP. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol. 1997;52(6):1081–1086. [DOI] [PubMed] [Google Scholar]
- 35.Nahavandi M, Wyche MQ, Perlin E, Tavakkoli F, Castro O. Nitric Oxide Metabolites in Sickle Cell Anemia Patients after Oral Administration of Hydroxyurea; Hemoglobinopathy. Hematology. 2000; 5(4):335–339. [DOI] [PubMed] [Google Scholar]
- 36.Kleinbongard P, Schulz R, Rassaf T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107(7):2943–2951. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23(16):5726–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mejia-Garcia TA, Portugal CC, Encarnacao TG, Prado MA, Paes-de-Carvalho R. Nitric oxide regulates AKT phosphorylation and nuclear translocation in cultured retinal cells. Cell Signal. 2013;25(12):2424–2439. [DOI] [PubMed] [Google Scholar]
- 39.Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood. 2008;111(3):1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falanga A, Marchetti M, Evangelista V, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96(13):4261–4266. [PubMed] [Google Scholar]
- 41.Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol. 2005;33(5):523–530. [DOI] [PubMed] [Google Scholar]
- 42.Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet-leukocyte interactions in thrombosis. Thromb Res. 2012;129(3):263–266. [DOI] [PubMed] [Google Scholar]
- 43.Papayannopoulou T, Brice M. Integrin expression profiles during erythroid differentiation. Blood. 1992;79(7):1686–1694. [PubMed] [Google Scholar]
- 44.Spring FA, Holmes CH, Simpson KL, et al. The Oka blood group antigen is a marker for the M6 leukocyte activation antigen, the human homolog of OX-47 antigen, basigin and neurothelin, an immunoglobulin superfamily molecule that is widely expressed in human cells and tissues. Eur J Immunol. 1997;27(4):891–897. [DOI] [PubMed] [Google Scholar]
- 45.Coste I, Gauchat JF, Wilson A, et al. Unavailability of CD147 leads to selective erythrocyte trapping in the spleen. Blood. 2001;97(12):3984–3988. [DOI] [PubMed] [Google Scholar]
- 46.Mutin M, Dignat-George F, Sampol J. Immunologic phenotype of cultured endothelial cells: quantitative analysis of cell surface molecules. Tissue Antigens. 1997;50(5):449–458. [DOI] [PubMed] [Google Scholar]
- 47.Crosnier C, Bustamante LY, Bartholdson SJ, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480(7378):534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernemann TM, Podda M, Wolter M, Boehncke WH. Expression of the basal cell adhesion molecule (B-CAM) in normal and diseased human skin. J Cutan Pathol. 2000;27(3):108–111. [DOI] [PubMed] [Google Scholar]
- 49.Garinchesa P, Sanzmoncasi M, Campbell I, Rettig W. Non-polarized expression of Basal-cell adhesion molecule B-cam in epithelial ovarian cancers. Int J Oncol. 1994;5(6):1261–1266. [DOI] [PubMed] [Google Scholar]
- 50.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci USA. 1988;85(9):3110–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schon M, Klein CE, Hogenkamp V, Kaufmann R, Wienrich BG, Schon MP. Basal-cell adhesion molecule (B-CAM) is induced in epithelial skin tumors and inflammatory epidermis, and is expressed at cell-cell and cell-substrate contact sites. J Invest Dermatol. 2000;115(6):1047–1053. [DOI] [PubMed] [Google Scholar]
- 52.Xiong L, Edwards CK, 3rd, Zhou L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int J Mol Sci. 2014;15(10):17411–17441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang JM, O’Neill P, Jin W, et al. Extracellular matrix metalloproteinase inducer (CD147) confers resistance of breast cancer cells to Anoikis through inhibition of Bim. J Biol Chem. 2006;281(14):9719–9727. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Zhang H, Gou X, Horikawa Y, Xing J, Chen Z. Upregulation of HAb18G/CD147 in activated human umbilical vein endothelial cells enhances the angiogenesis. Cancer Lett. 2009;278(1):113–121. [DOI] [PubMed] [Google Scholar]
- 55.Dai JY, Dou KF, Wang CH, et al. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer. 2009;9:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Floch R, Chiche J, Marchiq I, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci USA. 2011;108(40):16663–16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Najean Y, Rain JD. Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood. 1997;90(9):3370–3377. [PubMed] [Google Scholar]
- 58.Fruchtman SM, Mack K, Kaplan ME, Peterson P, Berk PD, Wasserman LR. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34(1):17–23. [PubMed] [Google Scholar]
- 59.Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006; 107(3):451–458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.