Abstract
Regulatory T (Treg) cells can weaken antitumor immune responses, and inhibition of their function appears to be a promising therapeutic approach in cancer patients. Mice with targeted deletion of the gene encoding the Cl−/HCO3− anion exchanger AE2 (also termed SLC4A2), a membrane-bound carrier involved in intracellular pH regulation, showed a progressive decrease in the number of Treg cells. We therefore challenged AE2 as a potential target for tumor therapy, and generated linear peptides designed to bind the third extracellular loop of AE2, which is crucial for its exchange activity. Peptide p17AE2 exhibited optimal interaction ability and indeed promoted apoptosis in mouse and human Treg cells, while activating effector T-cell function. Interestingly, this linear peptide also induced apoptosis in different types of human leukemia, lymphoma and multiple myeloma cell lines and primary malignant samples, while it showed only moderate effects on normal B lymphocytes. Finally, a macrocyclic AE2 targeting peptide exhibiting increased stability in vivo was effective in mice xenografted with B-cell lymphoma. These data suggest that targeting the anion exchanger AE2 with specific peptides may represent an effective therapeutic approach in B-cell malignancies.
Introduction
Despite the use of new diagnostic and therapeutic strategies that have improved the prognosis of mature B-cell malignancies, most patients cannot be cured with currently available therapies.1,2 To improve the clinical outcome of these patients, novel agents against specific cellular targets are being developed and tested.3,4 In addition, different types of therapy have become standard treatments for certain hematologic malignancies, while others undergo clinical testing.5,6 Among these, a promising experimental approach aims to inhibit the CD4+CD25+Foxp3+ T regulatory (Treg) cells, and prevent their suppressor activity against antitumoral T helper and cytotoxic T cells.7,8 The use of therapies combining a direct antitumoral effect with an enhancement of the T cell-mediated immune responses would represent a major advance in the treatment of B-cell malignancies.9
The regulation of intracellular pH (pHi) is critical for important cellular processes and functions in many cell types, including lymphocytes.10–12 To achieve acid–base homeostasis, lymphoid cells are equipped with a coordinated network of ion channels and transporters in the plasma cell membrane that orchestrate the input and output of acid/base ions H+ and HCO3− to maintain the pHi within a narrow physiological range that is generally ~7.2.10–13 On the other hand, cancer cells with a high rate of metabolic activity have increased pHi while the extracellular space becomes acidified.1014–17 Extracellular acidification of the tumor microenvironment suppresses the effector function of antitumor cytotoxic T cells and promotes tumor evasion.18,19 Moreover, early in vitro studies have shown that inhibition of the acid extruder Na+/H+ exchanger 1 (NHE1) in leukemic cells decreases their pHi leading to apoptosis.20,21 Accordingly, physiological pH sensors involved in the modulation of acid-loading and acid-extruding mechanisms hold promise as targets in cancer therapeutics.22–24 Among the SLC4 family of HCO3− transporters, the Na+-independent Cl−/HCO3− anion exchanger 2 (AE2, also referred to as solute carrier family 4 member 2, SLC4A2) is considered a master acid loader in many cell types.25,26 Under physiological conditions, AE2 favors the extrusion of intracellular HCO3− in exchange for extracellular Cl−, resulting in an acid load.27–29 Our group has shown that mice carrying targeted deletion of AE2 (Ae2a,b−/− mice) have lymphocytes with alterations in pHi, which eventually leads to a reduction in the number of Treg cells, among other alterations.30–33 These data prompted us to investigate the role of AE2 as a potential target for tumor immunotherapy.
Here we report the generation and characterization of specific peptides targeting AE2 exchanger function. Our results show that AE2 binding peptides induced opposite effects on different T-cell subsets, promoting apoptosis in Treg cells while activating effector T-cell function. Targeting peptides also promoted apoptosis in tumor cells from different types of leukemia, lymphoma and multiple myeloma, while showing only moderate effects on non-tumoral B lymphocytes. These data suggest that targeting AE2 represents a novel therapeutic approach that may simultaneously promote apoptosis of tumor cells and enhance T cell-mediated immune responses.
Methods
Peptides
A series of 24 linear peptides of 15 amino acids that potentially bind a short stretch of highly conserved amino acid sequences (NMTWAGARPT in human and NMTWATTI in mouse AE2), were designed. These conserved target sequences are within the third extracellular loop of the protein, which has been shown to play a key role in Cl−/HCO3− exchange function.25,34 The design of binding peptides followed a methodology that assigns potential interactions between peptides based on the hydrophilicity/hydrophobicity and the net charge of the amino acid side chains of the involved peptides, as described.35 AE2 binding peptides were synthesized by the solid phase method of Merrifield using the Fmoc alternative, as described.36 The purity of the peptides was analyzed by HPLC. To generate cycled peptides, based on the 3D structure of the p17AE2 peptide predicted using the de novo prediction server PEP-FOLD,37,38 two macrocyclic peptides with a head-to-tail cyclization (termed p17AE2-HT) and with a secondary amide as linker (termed p17AE2-Amide) were synthesized by solid phase synthesis (Wuxi AppTech, Shanghai, China). Synthesis and measurement of peptide metabolic stability procedures are detailed in Online Supplementary Methods.
Surface plasmon resonance
Peptide binding to the third extracellular loop of AE2 was analyzed by surface plasmon resonance using the ProteOn XPR36 (Bio-Rad) optical biosensor, as described.39 Briefly, N-terminally biotinylated peptide biot-DSEAGSSSSSNMTWATTILVPDNSSASGQSGQEKPR, encompassing the AE2-specific third extracellular loop of AE2, was immobilized into a NLC-neutravidin chip (Biorad). Individual peptide solutions (from 0.15 to 2.5 μM) were injected by triplicate in running buffer (phosphate buffered saline, 0.005% (v/v) Tween 20, pH 7.4) at a flow of 30 μL/min. The interspot signal (obtained in the chip surface not immobilized with protein) was used as reference.
Cell lines and patient samples
The human lymphoma, leukemia and myeloma cell lines used in the study were cultured in RPMI or IMDM media supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin (P-S) as described.40,41 A series of ten fresh peripheral blood cells obtained from untreated patients with B-cell lymphoma with peripheral blood dissemination were included in the study. Primary B cells were isolated from human peripheral blood using a CD19+ isolation kit (Miltenyi Biotec). To determine cell viability and apoptosis, cells were cultured in IMDM medium with 20% fetal bovine serum and 1% P-S. The research protocol on human subjects was approved by the University of Navarra Institutional Review Board. Informed consent was obtained from patients and healthy donors, in accordance with the Helsinki Declaration.
Cell viability and apoptosis assays
Both primary cells and established cell lines were cultured in flat bottom M96 wells, at a density of 50,000 cells/well, and incubated in 100 mL of cell medium supplemented with the corresponding isoform of the peptide at a final concentration of 50 μg/mL for 24 hours. To determine cell viability, 10 μL of MTS (Promega) were added to each well and plates were read at a wavelength of 450/690 nm. Cell viability was normalized to control group for each experiment. Apoptosis was measured using the FITC Annexin V Apoptosis Detection Kit I (BD), as reported.41,42 Briefly, cell were stained with Annexin V-FITC and ToPro3 and analyzed in a FACS Calibur cytometer (BD).
In vitro assays for murine effector and regulatory T cells
Regulatory (CD4+CD25+) and effector (CD4+CD25−) T cells were isolated from BALB/c mouse spleens using Treg isolation kits (Miltenyi Biotec). For the screening of the peptides targeting AE2, CD3-stimulated effector T cells were cultured in the presence or absence of purified Treg cells and peptides, as previously described.43 Then, cell proliferation was calculated by adding [methyl-3H] thymidine, and incorporated radioactivity was measured using a scintillation counter.44 The same procedure was carried out to determine the effect of p17AE2 in effector and regulatory T cell populations. IL-2 and IL-10 secreted to the supernatant of cell cultures were measured by ELISA (Pharmingen).
Intracellular pH measurement
To asses pHi, cells were stained with the intracellular fluorescent pH indicator BCECF-AM (Biotium), as described.32,45 Briefly, cells were stained and washed in MACS buffer supplemented with the corresponding peptide at a final concentration of 50 μg/mL. Then, cells were excited at 488 nm and the ratio of emission wavelengths 530/661 nm was determined in a FACS Calibur cytometer. The nigericin clamp technique was used to estimate pHi values from calibration curves as described.31,46
Determination of anion exchange activity
To determine the Cl−/HCO3− exchange activity, cells were stained with BCECF-AM in Krebs-Ringer bicarbonate buffer (KRB, which contains in mM: 115 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1 Na+-pyruvate, 1.2 KH2PO4,1 MgSO4, 5 glucose, and carbogen − 95% CO2 + 5% O2) and incubated as described above. Then, cells were washed and resuspended in Cl−-free buffer (in mM: 115 Na+-isethionate, 4.7 K+-gluconate, 1.5 Ca2+-gluconate, 25 NaHCO3, 1 Na+-pyruvate, 1.2 KH2PO4, 1 MgSO4, 5 glucose, and carbogen) supplemented with 50 μg/mL of the corresponding peptide, and pHi was measured every 2 minutes by flow cytometry. Cl− was replaced by isothionate in order to maintain osmolality. Changes in anion exchange activity were represented as the variation of pHi along the time after extracellular Cl− removal. This removal forces the efflux of Cl− and the influx of HCO3−, and intracellular pH increases as a result of a reverse activity of the AE2 anion exchanger.29,32
Therapeutic in vivo assays in mouse xenografts
OCI-Ly7, L363 and UPN1 tumor cells (5×106 cells per animal) were injected subcutaneously in 6-week-old male immunodeficient Rag2−/−IL2γc−/− mice. Eight animals per group were used on each experiment. Once tumors reached volumes of 100 mm3, treatment was started by intratumorally injecting 50, 200 or 400 μg of the peptide (a truncated peptide was used for control mice at the same dosage). Tumor size was measured every 2 days, as described previously.47 When tumors reached volumes of 2000 mm3, animals were euthanized.
Statistical Analysis
Results are expressed as mean ± SEM. At least three independent experiments for cell viability, apoptosis and pHi assessment assays were performed (in duplicate each). The normal distribution of values was assessed by using the Shapiro-Wilks and Kolmogorof-Smirnov tests, and the statistical significance of differences was determined with the student’s-t test, taking two-tailed P<0.05 as the criterion for significance. For in vivo experiments, data were analyzed by two way ANOVA test and the Bonferroni post-tests to compare replicate means. Statistical analyses were performed using the Graph Pad Prism 5 program.
Results and Discussion
Generation and characterization of functional peptides targeting AE2
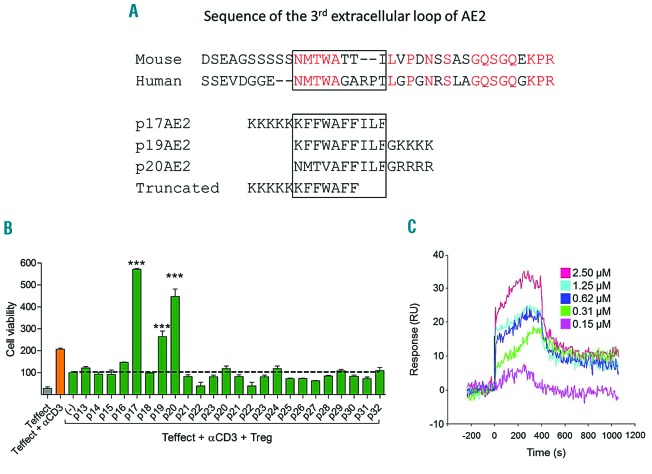
A series of 24 linear peptides were designed to bind a short, highly conserved region in human and mouse AE2 (Figure 1A). Once synthesized, peptides were tested for their ability to inhibit the suppressor activity of natural CD4+CD25+Treg cells in vitro. Thus, CD4+CD25− effector T cells activated with anti-CD3 monoclonal antibody in the presence of Treg cells were used to analyze the capacity of each peptide to restore T-cell proliferation inhibited by Treg cells. Among the 24 peptides, three (p17AE2, p19AE2 and p20AE2) were able to restore and even enhance the proliferation of effector T cells (Figure 1B). Surface plasmon resonance showed a dose-dependent binding of the p17AE2 peptide to a 36 amino acids long peptide encompassing the third extracellular loop of human AE2 that is crucial for its exchange function,25,34 thus revealing physical binding (Figure 1C). The p17AE2 peptide was therefore selected for further experiments.
Figure 1.
Generation of functional peptides interacting specifically with the third extracellular loop of AE2 protein. (A) Alignment of the third extracellular loop of murine and human AE2 and linear p17AE2, p19AE2, p20AE2 and truncated peptides. (B) p17AE2, p19AE2 and p20AE2 increased proliferation of effector T cells co-cultured with Treg cells in vitro. (C) Sensogram of surface plasmon resonance showing p17AE2 binding to AE2 in a dose dependent manner. ***, P<0.001.
Functional targeting peptide p17AE2 shows opposite functions in different T-cell subsets
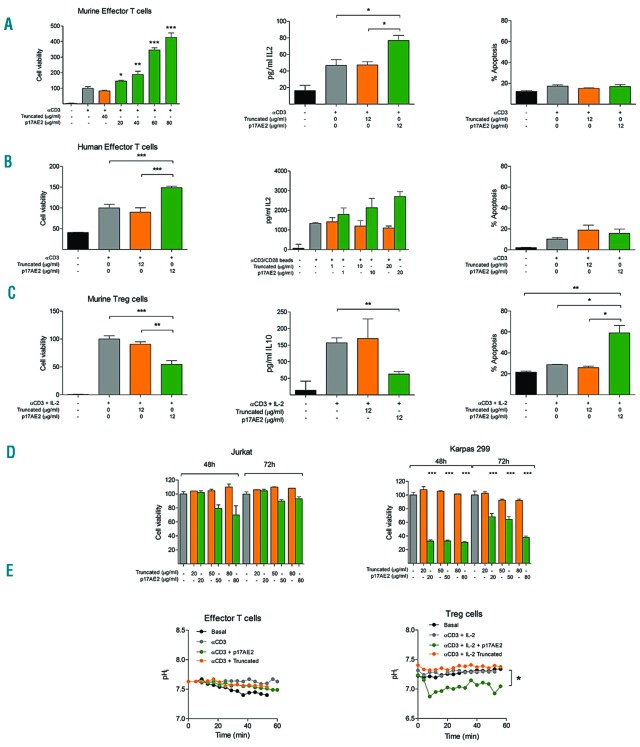
Next, we tested the effect of p17AE2 on conventional effector T-cell proliferation in the absence of Treg cells. In vitro experiments showed that the addition of p17AE2 to murine effector T cells slightly increased cell proliferation in response to anti-CD3 stimulation, and similarly promoted IL-2 secretion, while apoptosis was not affected (Figures 2A). This capacity to promote effector T-cell proliferation was also observed in human effector T cells from healthy donors stimulated with anti-CD3/CD28 beads (Figure 2B). Conversely, p17AE2 reduced cell proliferation of activated Treg cells in culture and induced cell apoptosis, while the production of IL-10 was not significantly altered, (Figures 2C). Likewise, p17AE2 decreased cell viability of the human-derived T-cell leukemia cell line Karpas299, which shows characteristics typical of natural Treg cells with a CD4+CD25+Foxp3+ phenotype.48 On the other hand, p17AE2 did not affect cell survival of Jurkat T-cell leukemia cells with a CD4+CD25− T-cell effector phenotype (Figure 2D).39 We then determined whether changes in cell survival were related to variations of the pHi. Upon incubation with p17AE2, the pHi in effector T lymphocytes remained similar to that in control cells or cells treated with the truncated peptide, while pHi values decreased in Treg cells over time (Figure 2E).
Figure 2.
p17AE2 peptide induces proliferation of effector T cells and apoptosis of regulatory T lymphocytes. Effect of p17AE2 on cell viability, IL-2 secretion and apoptosis in murine (A) and human (B) effector T lymphocytes. (C) Effect of p17AE2 on cell proliferation, IL-10 secretion and apoptosis in murine regulatory T lymphocytes. (D) Cell viability of human Jurkat (T effector) and Karpas299 (Treg) cell lines upon incubation with p17AE2 peptide. (E) pHi of murine effector and regulatory T cells after p17AE2 treatment. *P<0.05; **P<0.01; ***P<0.001.
AE2 targeting promotes apoptosis of B-cell leukemia, lymphoma and multiple myeloma cells
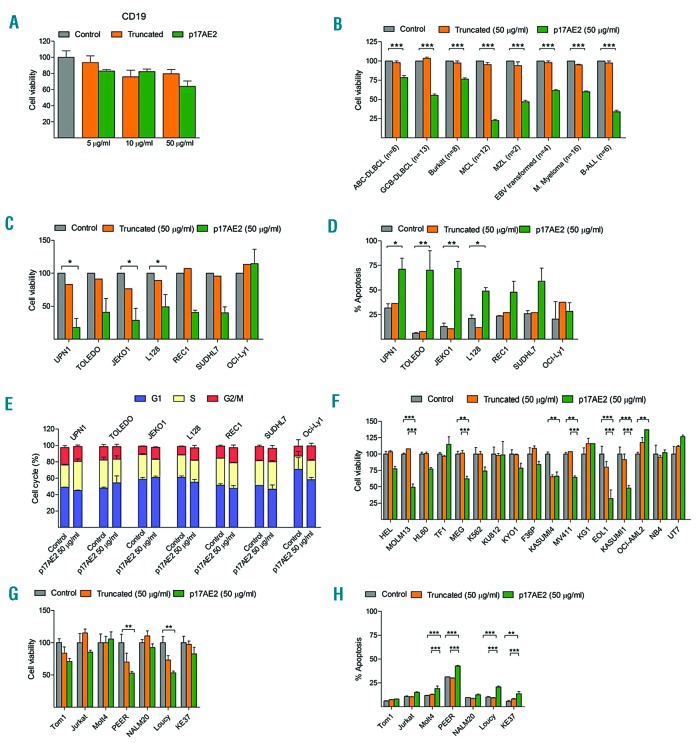
The effect of the p17AE2 peptide in vitro was also evaluated on peripheral blood B lymphocytes isolated from healthy donors as well as on cell lines derived from patients with a variety of B-cell malignancies. While p17AE2 showed a moderate effect on normal B lymphocytes, decreasing B-cell viability only at high dosage (50 μg/mL) (Figure 3A), this peptide markedly reduced cell growth in 44 of 69 (64%) malignant cell lines, as follows: 28 of 43 (65%) mature B-cell lymphomas (8 of 12 mantle cell lymphoma, 5 of 8 Burkitt lymphoma, 14 of 21 diffuse large B-cell lymphoma and 1 of 2 splenic marginal-zone B-cell lymphoma); 2 of 4 EBV transformed lymphoblastoid B-cell lines; 9 of 16 (56%) multiple myelomas; and 5 of 6 (83%) B-cell acute lymphoblastic leukemias (Figure 3B). The effects of p17AE2 on cell viability and apoptosis were dose-dependent in p17AE2 sensitive B-cell lymphoma cell lines, but not in resistant OCI-Ly1 B-cell lymphoma cells (Figure 3 C–D). Cell cycle, however, was not affected either in sensitive or in resistant cell lines (Figure 3E). Besides, p17AE2 also affected cell viability and apoptosis of several acute myeloid leukemia (AML) and T-cell acute lymphoblastic leukemia (T-ALL) (Figure 3 F–G). These results indicate that targeting AE2 with p17AE2 reduces cell survival in a variety of hematologic neoplasms, including B-cell tumors, while showing only moderate effects on non-tumoral B lymphocytes.
Figure 3.
Effect of p17AE2 on human normal B lymphocytes and tumor B-cell lines. (A) Cell viability of CD19+ cell isolated from human peripheral blood lymphocytes (PBLs) upon treatment with 5, 10 or 50 μg/mL of truncated or p17AE2 peptides. (B) Effect of p17AE2 on cell viability of human B-cell leukemia, lymphoma and multiple myeloma cell lines. Cell viability, apoptosis, and cell cycle abnormalities in tumor B-cell (C, D, E), AML (F) and T-ALL cell lines (G, H) after 48 hour incubation with p17AE2 peptide. *P<0.05; **P<0.01; ***P<0.001.
Functional targeting peptide p17AE2 induces apoptosis in tumor cells by modulating intracellular pH and AE2 function
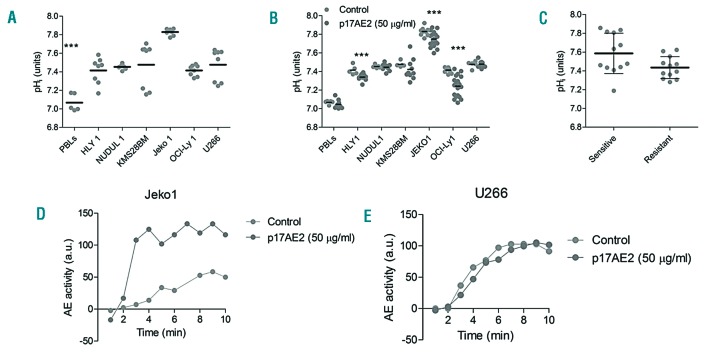
To study how p17AE2 reduces cell viability of tumor B cells after targeting AE2 protein, basal pHi values were measured in normal B cells and in tumor B-cell lines. Consistent with previous studies, human peripheral blood B lymphocytes from healthy donors showed basal pHi values ranging from 7.0 to 7.2, whereas cell lines derived from different B-cell malignancies exhibited higher pHi values ranging from 7.4 to 7.8 (Figure 4A). The alkaline pHi is permissive for tumor cell proliferation and evasion of apoptosis, while acidic pHi values promote cell apoptosis.10,14 Incubation of tumor B cells with p17AE2 led to dose-dependent pHi acidification in sensitive cells, which correlated with apoptosis rates (Figure 4B). In B lymphocytes and in tumor resistant cells, however, p17AE2 treatment did not affect pHi values. Nevertheless, there was not a correlation between basal pHi values in the different tumor B-cell lines and response to p17AE2 (Figure 4C). To evaluate whether the Cl−/HCO3− exchange activity of AE2 was modulated by p17AE2, cells were subjected to removal of extracellular Cl−, and the pHi changes with and without p17AE2 were measured at different time intervals; under these forced conditions, a reversed exchange activity of AE2 is reflected by an increase in pHi (see Methods for details). In the sensitive cell line Jeko1, p17AE2 induced an increase in pHi with respect to cells incubated in the absence of the peptide, while in the resistant cell line U266 the activity of AE2 remained unchanged (Figure 4 D–E). These results suggest that in sensitive cell lines, like Jeko1, p17AE2 is able to modulate AE2 activity, altering pHi and thus inducing cell apoptosis, while in resistant cell lines, such as U266, the peptide would not be able to alter AE2 activity and pHi would remain stable, not compromising cell viability. Hence, in sensitive cell lines, p17AE2 would activate the physiological Cl−/HCO3− exchange function of AE2, favoring HCO3− export in exchange with Cl−, thus reducing pHi and promoting apoptosis.
Figure 4.
Modulation of the exchanger function of AE2 driven by p17AE2 peptide. (A) Basal pHi in human peripheral blood B lymphocytes (PBLs) and tumor B-cell lines. (B) Changes in pHi values upon treatment with p17AE2. (C) Average basal pHi in sensitive and resistant tumor B-cell lines. (D-E) Effect of p17AE2 treatment on the AE2 activity in sensitive Jeko1 and resistant U266 cell lines. ***P<0.001.
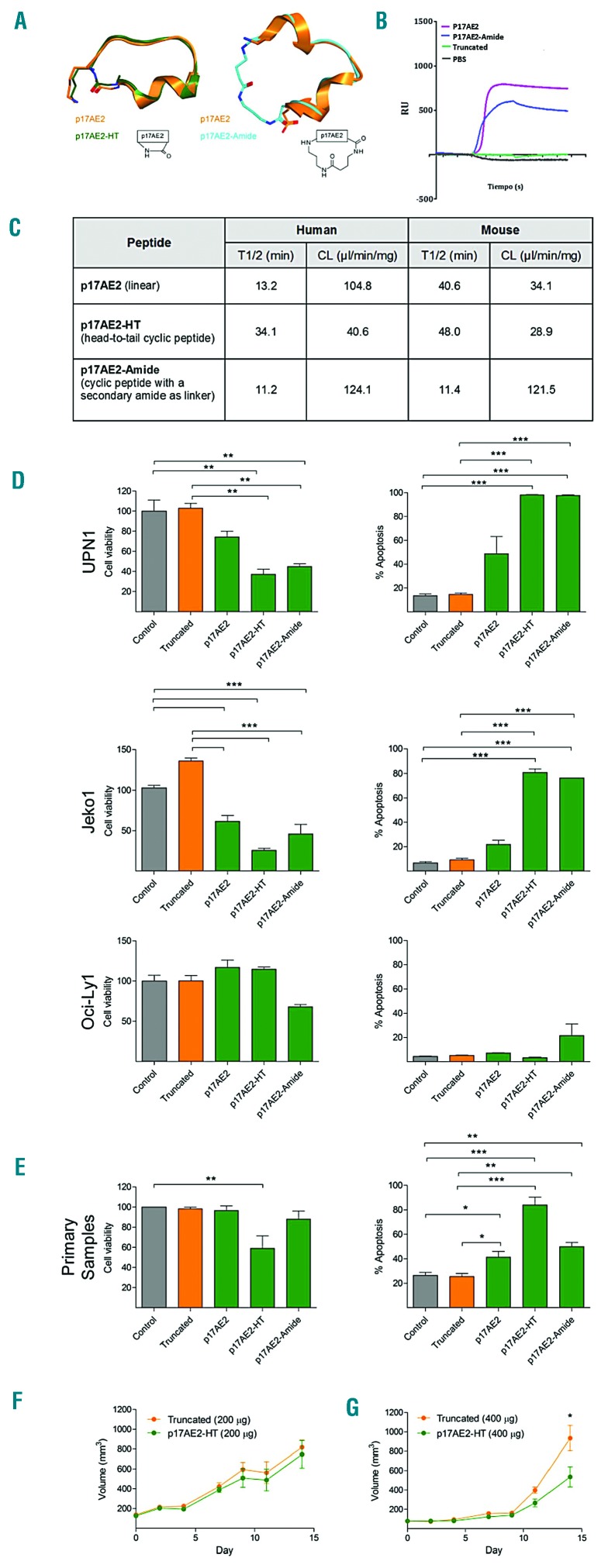
Therapeutic effect of AE2 targeting peptides in mouse xenograft models in vivo
Finally, we assessed the effects of targeting AE2 in vivo. For that purpose, 5×106 cells from three sensitive cell lines (Mantle cell lymphoma- UPN1, DLBCL-OCI-Ly7 and multiple myeloma-L363) were injected subcutaneously in Rag2−/−IL-2γc−/− immunodeficient mice. When tumors achieved volumes of 100 mm3, mice received daily intra-tumoral injection of p17AE2 or a truncated peptide (50 μg each). However, no changes in tumor size between control and treated mice were observed after 14 days of treatment, suggesting that p17AE2 effects could be limited by a poor bio-availability and susceptibility to degradation by proteases (data not shown). Peptide cyclization has been used as a strategy for stabilizing small peptides.49 Therefore, based on computational studies, we used this strategy to design and synthesize two macrocyclic peptides, one with a head-to-tail cyclization (termed p17AE2-HT) and another one with a secondary amide as linker (termed p17AE2-Amide) (Figure 5A). Surface plasmon resonance showed a dose-dependent binding of both p17AE2-HT and p17AE2-Amide peptides to the third extracellular loop of human AE2 (Figure 5B). However, only the p17AE2-HT peptide, but not the p17AE2-Amide, showed longer half-life and reduced clearance with respect to the linear p17AE2 peptide (Figure 5C). In addition, p17AE2-HT decreased cell proliferation and promoted apoptosis in different B-cell lymphoma cell lines and in primary B-cell lymphoma samples, even more potently than the p17AE2 linear peptide (Figures 5D–E). These results indicated that p17AE2-HT was more efficient than linear peptides and hence a good candidate for therapeutic testing in vivo. To assess the therapeutic role of the macrocyclic peptides in vivo, we inoculated subcutaneously 5×106 cells from the UPN1 B-cell lymphoma-derived cell line into Rag2−/−IL-2γc−/− mice, due to its sensitivity in the in vitro model. When tumors reached a size of 100 mm3, 200 μg or 400 μg per day of p17AE2-HT or truncated peptides were injected intratumorally during 14 days. Mice treated with 400 μg of p17AE-HT exhibited reduction in tumor volumes with respect to those treated with 200 μg or with truncated peptide (Figures 5F–G). These results indicate that p17AE2-HT treatment moderately reduced tumor growth in vivo.
Figure 5.
Therapeutic effect of the linear and the macrocyclic p17AE2 peptides on B-cell lymphomas. (A) Modelled 3D structures of p17AE2, p17AE2-HT and p17AE2-Amide. Left, superposition of conformation 1 of p17AE2 (< 5 Å, orange) and p17AE2-HT (green). Right, superposition of conformation 2 of p17AE2 (>10 Å, orange) and p17AE2-Amide (cyan). (B) Sensogram of surface plasmon resonance showing binding of p17AE2-Amide and p17AE2-HT to AE2 in a dose dependent manner. (C) Half-life and clearance of the different peptides in humans and mice. (D) Cell viability and apoptosis in UPN1, Jeko1, OCI-Ly1 cell lines after incubation with 50 μg/mL of truncated, p17AE2, p17AE2-HT and p17AE2-Amide peptides for 24 hours. (E) Similarly, the peptides reduced cell viability and promoted apoptosis of primary samples obtained from patients with B-cell lymphomas (n=10). (F, G) Representation of volumes of subcutaneous tumors in xenografted mice after intratumoral injection of 200 mg or 400 mg of truncated or p17AE2-HT peptides. *P<0.05; **P<0.01; ***P<0.001.
In summary, our results suggest that targeting AE2 might exert a dual therapeutic effect in B-cell malignancies. In our in vitro studies, the linear p17AE2 was able to induce apoptosis of tumor B cells, while potentially boosting anti-tumor immune responses by reducing the number of Treg cells. In in vivo models, however, the linear peptide had no effect, probably because of its low stability, while the head-to-tail cycled p17AE2-HT showed increased stability and managed to reduce tumor growth in vivo when given at a high dosage. Thus, AE2 seems to be a promising target in different B-cell malignancies, and modifications of AE2 targeting peptides may increase their potential therapeutic value in vivo.
Supplementary Material
Acknowledgments
We thank Elena Ciordia and Eneko Elizalde (CIMA) for excellent animal care.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/6/1065
Funding
The work was supported by grants from Fundación Ramón Areces, BAYER Pharma HealthCare (Grant4Targets 2015), Fundación Arnal Planelles, and Roche Spain (Hemato-Oncology award 2014) (to JAM-C, JJL and JFM); Worldwide Cancer Research (WCR15-1322), ISCIII/FIS PI16/00581, and ISCIII/FIS-CIBERONC (to JAM-C); Ministerio de Educación y Ciencia SAF2013-42772-R and SAF2016-78568-R and Fundación Bancaria La Caixa-Hepacare Project, Ministerio de Educación y Ciencia SAF2013-42772-R and SAF2016-78568-R (to JJL), and Ministerio de Economia y Competitividad by the Torres-Quevedo subprogram PTQ-14-07320 (to IdM). JC is a Juan de la Cierva post-doctoral fellow.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127(20):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer AL, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30(1):565–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roschewski M, Staudt LM, Wilson WH. Diffuse large B-cell lymphoma: treatment approaches in the molecular era. Nat Rev Clin Oncol. 2013;11(1):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowakowski GS, Blum KA, Kahl BS, et al. Beyond RCHOP: A blueprint for diffuse large B cell lymphoma research. J Natl Cancer Inst. 2016;108(12):djw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavanello F, Zucca E, Ghielmini M. Rituximab: 13 open questions after 20 years of clinical use. Cancer Treat Rev. 2017;53:38–46. [DOI] [PubMed] [Google Scholar]
- 6.Goodman A, Patel SP, Kurzrock R. PD-1– PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2016;14(4):203–220. [DOI] [PubMed] [Google Scholar]
- 7.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. [DOI] [PubMed] [Google Scholar]
- 8.Lozano T, Casares N, Lasarte JJ. Searching for the Achilles Heel of FOXP3. Front Oncol. 2013;3:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017; 17(5):286–301. [DOI] [PubMed] [Google Scholar]
- 10.Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol. 2012;12(7):532–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231(1):59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King LB, Freedman BD. B-lymphocyte calcium inFlux. Immunol Rev. 2009; 231(1):265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vito P. The sodium/hydrogen exchanger: A possible mediator of immunity. Cell Immunol. 2006;240(2):69–85. [DOI] [PubMed] [Google Scholar]
- 14.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671–677. [DOI] [PubMed] [Google Scholar]
- 15.Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival and growth. J Cell Physiol. 2011;226(2):299–308. [DOI] [PubMed] [Google Scholar]
- 16.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5(10):786–795. [DOI] [PubMed] [Google Scholar]
- 17.Lang F, Huber SM, Szabo I, Gulbins E. Plasma membrane ion channels in suicidal cell death. Arch Biochem Biophys. 2007;462(2):189–194. [DOI] [PubMed] [Google Scholar]
- 18.Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J cancer. 2012;131(3):633–640. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa Y, Negishi Y, Shimizu M, Takahashi M, Ichikawa M, Takahashi H. Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol Lett. 2015;167(2):72–86. [DOI] [PubMed] [Google Scholar]
- 20.Rich IN, Worthington-White D, Garden OA, Musk P. Apoptosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na(+)/H(+) exchanger. Blood. 2000;95(4):1427–1434. [PubMed] [Google Scholar]
- 21.Marches R, Vitetta ES, Uhr JW. A role for intracellular pH in membrane IgM-mediated cell death of human B lymphomas. Proc Natl Acad Sci USA. 2001;98(6):3434–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue Loo S, Ker Xing Chang M, Sui Huay Chua C, Prem Kumar A, Pervaiz S, Veronique Clement M. NHE-1: a promising target for novel anti-cancer therapeutics. Curr Pharm Des. 2012;18(10):1372–1382. [DOI] [PubMed] [Google Scholar]
- 23.Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13(9):611–623. [DOI] [PubMed] [Google Scholar]
- 24.Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14(8):543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212(11):1672–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.César-Razquin A, Snijder B, Frappier-Brinton T, et al. A call for systematic research on solute carriers. Cell. 2015; 162(3):478–487. [DOI] [PubMed] [Google Scholar]
- 27.Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol. 2006; 91(1):153–161. [DOI] [PubMed] [Google Scholar]
- 28.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3-) transporters. Mol Aspects Med. 2013;34(2–3):159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uriarte I, Banales JM, aez E, Arenas F, et al. Bicarbonate secretion of mouse cholangiocytes involves Na-HCO3 cotransport in addition to Na-independent Cl/HCO3 exchange. Hepatology. 2010;51(3):891–902. [DOI] [PubMed] [Google Scholar]
- 30.Medina JF, Recalde S, Prieto J, et al. Anion exchanger 2 is essential for spermiogenesis in mice. Proc Natl Acad Sci USA. 2003;100(26):15847–15852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salas JT, Banales JM, Sarvide S, et al. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134(5):1482–1493. [DOI] [PubMed] [Google Scholar]
- 32.Concepcion AR, Salas JT, Sarvide S, et al. Anion exchanger 2 is critical for CD8+ T cells to maintain pHi homeostasis and modulate immune responses. Eur J Immunol. 2014;44(5):1341–1351. [DOI] [PubMed] [Google Scholar]
- 33.Concepcion AR, Salas JT, Sáez E, et al. CD8+ T cells undergo activation and programmed death-1 repression in the liver of aged Ae2a,b −/− mice favoring autoimmune cholangitis. Oncotarget. 2015; 6(30):28588v28606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004;447(5):495–509. [DOI] [PubMed] [Google Scholar]
- 35.Ezquerro IJ, Lasarte JJ, Dotor J, et al. A synthetic peptide from transforming growth factor type III receptor inhibits liver fibro-genesis in rats with carbon tetrachloride liver injury. Cytokine. 2003;22(1–2):12–20. [DOI] [PubMed] [Google Scholar]
- 36.Borrás-Cuesta F, Golvano J, Sarobe P, et al. Insights on the amino acid side-chain interactions of a synthetic T-cell determinant. Biologicals. 1991;19(3):187–190. [DOI] [PubMed] [Google Scholar]
- 37.Maupetit J, Derreumaux P, Tufféry P. A fast method for large-scale de novo peptide and miniprotein structure prediction. J Comput Chem. 2010;31(4):72–738. [DOI] [PubMed] [Google Scholar]
- 38.Thévenet P, Shen Y, Maupetit J, Guyon F, Derreumaux P, Tufféry P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012;40(W1):W288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lozano T, Villanueva L, Durántez M, et al. Inhibition of FOXP3/NFAT interaction enhances T Cell function after TCR stimulation. J Immunol. 2015;195(7):3180–3189. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez-Ortigosa CM, Celay J, Olivas I, et al. A GAPDH-mediated trans-nitrosylation pathway is required for feedback inhibition of bile salt synthesis in rat liver. Gastroenterology. 2014;147(5):1084–1093. [DOI] [PubMed] [Google Scholar]
- 41.Beltran E, Fresquet V, Martinez-Useros J, et al. A cyclin-D1 interaction with BAX underlies its oncogenic role and potential as a therapeutic target in mantle cell lymphoma. Proc Natl Acad Sci USA. 2011; 108(30):12461–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sagardoy A, Martinez-Ferrandis JI, Roa S, et al. Downregulation of FOXP1 is required during germinal center B-cell function. Blood. 2013;121(21):4311–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lozano T, Soldevilla MM, Casares N, et al. Targeting inhibition of Foxp3 by a CD28 2′-Fluro oligonucleotide aptamer conjugated to P60-peptide enhances active cancer immunotherapy. Biomaterials. 2016;91:73–80. [DOI] [PubMed] [Google Scholar]
- 44.Gil-Guerrero L, Dotor J, Huibregtse IL, et al. In vitro and in vivo down-regulation of regulatory T cell activity with a peptide inhibitor of TGF-beta1. J Immunol. 2008;181(1):126–135. [DOI] [PubMed] [Google Scholar]
- 45.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18(11):2210–2218. [DOI] [PubMed] [Google Scholar]
- 46.Musgrove E, Rugg C, Hedley D. Flow cytometric measurement of cytoplasmic pH: A critical evaluation of available fluorochromes. Cytometry. 1986;7(4):347–355. [DOI] [PubMed] [Google Scholar]
- 47.Robles EF, Mena-Varas M, Barrio L, et al. Homeobox NKX2-3 promotes marginal-zone lymphomagenesis by activating B-cell receptor signalling and shaping lymphocyte dynamics. Nat Commun. 2016;7:11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolke C, Tadje J, Bukowska A, et al. Assigning the phenotype of a natural regulatory T-cell to the human T-cell line, KARPAS-299. Int J Mol Med. 2006;17(2):275–278. [PubMed] [Google Scholar]
- 49.Bhat A, Roberts LR, Dwyer JJ. Lead discovery and optimization strategies for peptide macrocycles. Eur J Med Chem. 2015; 94:471–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.