Leukocyte adhesion deficiency type III (LAD-III) is a recessive autosomal condition characterized by bleeding events and life-threatening infections. This condition is due to variations in the FERMT3 gene (encoding the kindlin-3 protein) that impair integrin function. Less than 40 LAD-III cases have been reported.1 The primary treatment for this condition is early hematopoietic stem cell transplantation (HSCT), which is, however, associated with severe complications and high rates of treatment-related mortality.
In 2011, our team described a patient of gypsy ethnicity carrying a novel biallelic FERMT3 variant.2 Herein, we discuss the long-term data regarding innovative therapeutic management of the disorder, which involves hemostatic and antimicrobial treatment, without HSCT. We performed a systematic literature review, which provides exhaustive data concerning complications, therapeutic strategies and prognosis of LAD-III. Furthermore, we investigated in vitro the effect of recombinant factor VIIa (rFVIIa) on kindlin-3-deficient platelet aggregation.
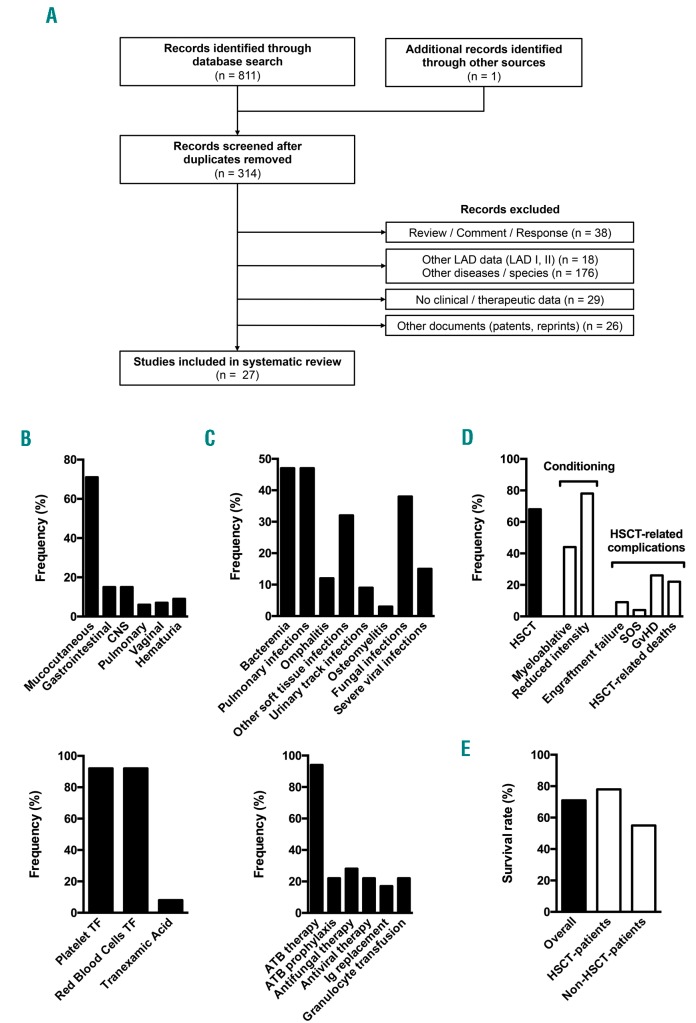
The patient was included in the study after written informed consent was obtained from his parents, in accordance with the Declaration of Helsinki. The systematic review was included in the PROSPERO international prospective register (registration number CRD42017064092). The electronic databases MEDLINE, Cochrane Library and Web of Science were included in the literature search according to the strategy described on the PROSPERO website. The flow diagram of the systematic review is presented in Figure 1A. Thirty-four LAD-III patients were included in the review. Data from the systematic review are compiled in Figure 1B–E and Online Supplementary Table S1.
Figure 1.
Systematic review of leukocyte adhesion deficiency type III complications, treatments and outcome. (A) Flow diagram of the systematic review. The number or records included or excluded is indicated at each step. Twenty-seven records reporting data from 34 patients were ultimately included in the analysis. The previous report from our group2 was excluded. (B) Prevalence of bleeding events with regards to topography (top; n=27) and associated treatments (bottom; n=24) among LAD-III patients reported in the literature. Frequency of vaginal bleeding was calculated for female patients. (C) Prevalence of infections with regards to topography/type (top; n=27) and associated treatments (bottom; n=18) among LAD-III patients reported in the literature. (D) Description of LAD-III patients treated with hematopoietic stem cell transplantation (HSCT). The percentage of patients treated with HSCT was calculated for all reported LAD-III patients (n=34). The frequency of conditioning regimens and HSCT-related complications was calculated for transplanted patients (n=23). Some patients underwent two HSCTs with different types of conditioning regimen, thus the sum of the conditioning regimen frequencies exceeds 100%. Conditioning regimen intensity was defined as previously reported.13 (E) Survival rate of reported LAD-III patients. LAD: leukocyte adhesion deficiency CNS: central nervous system; TF: transfusion. ATB: antibiotics; Ig: immunoglobulin; GvHD: graft-versus-host disease; SOS: sinusoidal obstruction syndrome.
The patient presented with bleeding symptoms starting at birth, as is common in most LAD-III patients. He constantly suffered from mild to moderate mucocutaneous bleeding (petechiae, buccal hemorrhaging and epistaxis). A total of 71% of reported LAD-III cases presented with mucocutaneous bleeding, which constitutes a hallmark of the disease (Figure 1B, top). The patient’s medical history involved severe bleeding episodes (traumatic penile hematoma, hematuria and traumatic bleeding of the tongue). The patient’s International Society on Thrombosis and Hemostasis – Bleeding Assessment Tool (ISTH-BAT) bleeding score was 13. The LAD-III bleeding phenotype may be more severe than that of Glanzmann thrombasthenia.3 Indeed, intracranial hemorrhage, gastrointestinal bleeding and pulmonary bleeding occurred in 15%, 15% and 6% of reported patients, respectively (Figure 1B, top). The management of these complications remains very challenging, as illustrated by the reports of three deaths due to bleeding complications.
Erythrocyte and platelet transfusions were performed in >90% of LAD-III cases, constituting a pivotal treatment for bleeding events (Figure 1B, bottom). Some patients received >20 erythrocyte transfusions and >50 platelet transfusions per year.4 Our patient received only one platelet transfusion, which may be due to the use of rFVIIa as a first-line treatment for severe bleeding as well as a preventive treatment in high-risk situations (off-label use). Ten doses of 90–120 μg/kg rFVIIa were successfully administered to treat penile hematoma (one dose), tongue bleeding (five doses) and three preventive circumstances (one to two doses; dental surgeries). The first dose of rFVIIa was administered at 17 months of age. This treatment has not been previously reported in LAD-III cases. Tranexamic acid was used in 8% of reported cases. Our patient received this antifibrinolytic drug as a long-term prophylaxis, which has never been reported in LAD-III cases. This treatment has been used from the age of three at a dose of 25 mg/kg/day divided into three oral doses.
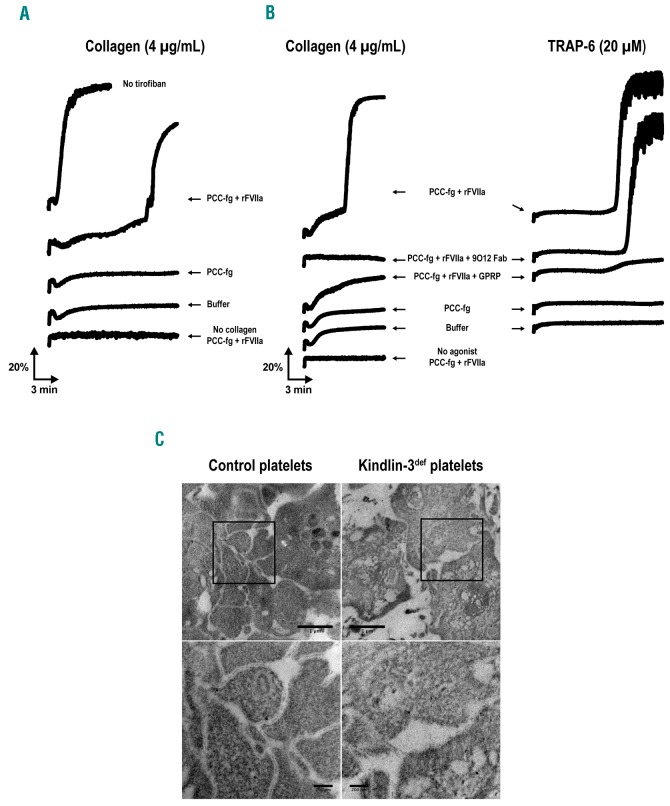
The clinical efficacy of rFVIIa to control bleeding in Glanzmann thrombasthenia patients has been clearly demonstrated,3 although the mechanism of action of rFVIIa in this disease is not fully understood. Direct activation of Factor X on the platelet surface by rFVIIa may improve local thrombin generation.5 Furthermore, rFVIIa treatment restores aggregation of Glanzmann thrombasthenia platelets in vitro.6 We observed rFVIIa-mediated platelet aggregation for αIIbβ3-inhibited platelets and kindlin-3-deficient platelets (Figures 2A,B), thereby possibly explaining the clinical efficacy of rFVIIa in LAD-III patients. Platelet aggregates were analyzed using transmission electron microscopy (Figure 2C). The interplatelet distance within rFVIIa-mediated aggregates of kindlin-3-deficient platelets was increased and less regular compared with that of control platelet aggregates (mean ± standard deviation 359 ± 373 nm, n=31 versus 59 ± 18 nm, n=59, respectively; n referring to the number of interplatelet distances measured). However, interplatelet close interactions (<100 nm) were also observed.
Figure 2.
rFVIIa-mediated aggregation of αIIbβ3-inhibited and kindlin-3-deficient platelets. (A–B) Light transmission aggregometry. Washed platelets were prepared from citrated blood samples obtained by venipuncture and resuspended in Hepes-Tyrode buffer at pH 7.4 (final platelet concentration = 200 × 109/l). The platelet suspension was recalcified with 3 mM CaCl2. Aggregation of (A) control platelets pretreated for 10 minutes with the αIIbβ3 inhibitor tirofiban (50 μg/ml; Sigma-Aldrich) and (B) kindlin-3-deficient platelets was monitored using a PAP-8E aggregometer (Bio/Data Corporation) at 37°C, with a stir speed of 900 rpm. The platelets were activated with 4 μg/mL collagen (Hyphen) or 20 mM TRAP-6 (SFLLRN; PolyPeptide Group). The effect of (1) a fibrin-generating system consisting of fibrinogen (fg; 50 μg/ml), factor II (0.13 IU/ml), factor VII (0.07 IU/ml), factor IX (0.16 IU/ml), factor X (0.18 IU/ml), and recombinant factor VIIa (rFVIIa; 1.2 μg/ml; Novoseven®, NovoNordisk), (2) the fibrin polymerization inhibitor Gly-Pro-Arg-Pro (GPRP; 5 mM; AnaSpec/Eurogentec), and (3) the anti-GPVI blocking Fab 9O128 (30 μg/ml) was evaluated. Factors II, VII, IX, and X – i.e., prothrombin complex concentrate (PCC) – were prepared using a Confidex® solution (CSL Behring GmbH). The data are representative of two independent experiments for the patient’s platelets and four independent experiments for the tirofiban-treated control platelets. (C) Representative transmission electron microscopy images of platelet aggregate ultrathin sections. The aggregates of control platelets (left) and kindlin-3-deficient platelets in the presence of the fibrin-generating system described above (right) were fixed and prepared as previously described,14 after a 10 min stimulation with 4 μg/ml collagen. Images of the same field acquired at higher magnification are shown at the bottom. Scale bar, 1 μm (top) and 200 nm (bottom). TRAP-6: thrombin receptor activating peptide 6; rFVIIa: recombinant factor VIIa; GPRP: Gly-Pro-Arg-Pro.
Platelet glycoprotein VI (GPVI) has recently been shown to bind to immobilized fibrin.7 We hypothesized that GPVI-fibrin interactions may mediate the aggregation of kindlin-3-deficient platelets in our model. In the presence of a fibrin generating system (prothrombin complex concentrate, fibrinogen, rFVIIa), washed kindlin-3-deficient platelets fully aggregated in response to collagen or thrombin receptor activating peptide-6 (TRAP-6) (Figure 2B). This aggregation was impeded if fibrin polymerization was inhibited by the Gly-Pro-Arg-Pro (GPRP) peptide (Figure 2B). Blocking GPVI using the anti-GPVI Fab fragment 9O128 inhibited collagen-induced aggregation of the kindlin-3-deficient platelets, which correlates with the fact that GPVI represents the receptor for platelet activation by collagen. However, the anti-GPVI Fab fragment 9O12, which was shown to inhibit GPVI-fibrin interactions,7 did not prevent rFVIIa-mediated aggregation upon TRAP-6 stimulation. This suggests that GPVI-fibrin interactions were not involved in this response (Figure 2B). Dependence on rFVIIa-mediated fibrin generation may explain the increased aggregation lag-time compared with that observed upon activation of normal platelets (Figure 2A,B). The use of collagen in this model probably results in collagen/thrombin double stimulation, which is known to strongly promote the formation of procoagulant platelets.9 This may explain the difference in aggregation lag-time following collagen or TRAP-6 stimulation (Figure 2B).
Regarding infections, the patient was treated with trimethoprim/sulfamethoxazole at five months of age for probable Pneumocystis jirovecii pneumonia, as reported for other LAD-III patients. Thereafter, preventive antibiotics (trimethoprim/sulfamethoxazole and itraconazole) were initiated. The patient did not suffer from additional severe infections, even after itraconazole was discontinued at 3.5 years of age. Severe bacterial infections have been reported in LAD-III patients, including bacteremias (47%), pulmonary infections (47%), omphalitis (12%) and other soft tissue infections (32%) (Figure 1C, top). Furthermore, fungal infections, such as Aspergillus pneumonia and Fusarium sepsis have been reported. Few authors have reported the use of bacterial or fungal prophylaxis in LAD-III patients (22%) (Figure 1C, bottom). Although these data emphasize individual variability, systematic antifungal and antibacterial prophylaxis should be considered for LAD-III patients.
With sustained hypogammaglobulinemia, our patient required long-term γ-globulin replacement at 10 months of age. This treatment has rarely been reported in the literature (17%; Figure 1C, bottom). γ-globulin levels were only evaluated in six cases. Two of these patients suffered from hypogammaglobulinemia.10,11 These patients presented no severe infection while undergoing γ-globulin replacement, and one patient developed severe sepsis a few weeks after discontinuing replacement therapy.11 In some patients, a defect in the adaptive immune response is thus an aspect of LAD-III, which suggests that kindlin-3 plays a role in B-cell biology.11 γ-globulin levels must be systematically evaluated, as long-term γ-globulin replacement may effectively prevent severe infections in LAD-III patients with hypogammaglobulinemia.
HSCT has been reported as the only curative therapy for LAD-III.1 Indeed, several groups have reported long-term, disease-free LAD-III survivors after HSCT. This therapy option has been applied in 68% of cases (Figure 1D). Furthermore, the reported survival of non-transplanted patients is relatively low (55%) (Figure 1E). Lethality is probably underestimated due to cases who died prior to diagnosis. Indeed, several siblings of LAD-III patients have died during the first months of life due to bleeding or infection. However, the mortality rate was 22% among transplanted patients (Figures 1D,E), and all deaths were directly due to HSCT complications. Severe complications were reported in 48% of cases, e.g., graft failure, sinusoidal obstruction syndrome or graft-versus-host disease (Figure 1D). Notably, 32% of patients had osteopetrosis, which likely compromises engraftment. Therefore, the balancing of the risk of rejection and toxicity is challenging in this disease. This may explain that the appropriate type of conditioning regimen remains controversial (Figure 1D). Furthermore, LAD-III survivors treated without HSCT have been reported.12 Regarding our patient, optimized treatment to manage bleeding and infection without HSCT appears to be an efficient therapeutic approach, with no significant adverse events. Indeed, this strategy led to a favorable outcome after a 10-year follow-up. Our patient exhibits normal growth and development and has been integrated into a standard school system. This approach may also apply to other LAD-III patients.
The study herein provides new insights regarding the complications, management and prognosis of LAD-III. Notably, we report the first effective use of rFVIIa to treat and prevent bleeding in a LAD-III patient, which was supported by in vitro platelet ultrastructural and aggregation data. Our results suggest that rFVIIa activity is platelet activation-dependent, fibrin-dependent and independent of GPVI-fibrin interaction. International registry and cohort studies should be implemented to identify prognostic factors that could guide therapeutic strategies for LAD-III.
Supplementary Material
Acknowledgments
The authors thank Laurence Panicot-Dubois and Christophe Dubois (Aix-Marseille Univ, INSERM, C2VN, Marseille, France) for the kind gift of Gly-Pro-Arg-Pro, David Gabriele for technical assistance, Sandra Moore for the revision of the paper, and the patient and his family for their participation in this study.
Footnotes
Funding: the study was funded by the “Fondation pour la Recherche Médicale” (grant to PSA: FDM20150633607).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Etzioni A. Leukocyte adhesion deficiency III - when integrins activation fails. J Clin Immunol. 2014;34(8):900–903. [DOI] [PubMed] [Google Scholar]
- 2.Robert P, Canault M, Farnarier C, et al. A novel leukocyte adhesion deficiency III variant: kindlin-3 deficiency results in integrin- and nonintegrin-related defects in different steps of leukocyte adhesion. J Immunol. 2011;186(9):5273–5283. [DOI] [PubMed] [Google Scholar]
- 3.Poon M-C, Di Minno G, d’Oiron R, Zotz R. New insights into the treatment of Glanzmann Thrombasthenia. Transfus Med Rev. 2016; 30(2):92–99. [DOI] [PubMed] [Google Scholar]
- 4.Kuijpers TW, van Bruggen R, Kamerbeek N, et al. Natural history and early diagnosis of LAD-1/variant syndrome. Blood. 2007; 109(8):3529–3537. [DOI] [PubMed] [Google Scholar]
- 5.Di Minno G. Eptacog alfa activated: a recombinant product to treat rare congenital bleeding disorders. Blood Rev. 2015;29 Suppl 1:S26–33. [DOI] [PubMed] [Google Scholar]
- 6.Lisman T, Adelmeijer J, Heijnen HFG, de Groot PG. Recombinant factor VIIa restores aggregation of alphaIIbbeta3-deficient platelets via tissue factor-independent fibrin generation. Blood. 2004; 103(5):1720–1727. [DOI] [PubMed] [Google Scholar]
- 7.Mammadova-Bach E, Ollivier V, Loyau S, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126(5):683–691. [DOI] [PubMed] [Google Scholar]
- 8.Lecut C, Feeney LA, Kingsbury G, et al. Human platelet glycoprotein VI function is antagonized by monoclonal antibody-derived Fab fragments. J Thromb Haemost. 2003;1(12):2653–2662. [DOI] [PubMed] [Google Scholar]
- 9.Agbani EO, Poole AW. Procoagulant platelets: generation, function, and therapeutic targeting in thrombosis. Blood. 2017;130(20):2171–2179. [DOI] [PubMed] [Google Scholar]
- 10.Kuijpers TW, Van Lier RA, Hamann D, et al. Leukocyte adhesion deficiency type 1 (LAD-1)/variant. A novel immunodeficiency syndrome characterized by dysfunctional beta2 integrins. J Clin Invest. 1997;100(7):1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suratannon N, Yeetong P, Srichomthong C, et al. Adaptive immune defects in a patient with leukocyte adhesion deficiency type III with a novel mutation in FERMT3. Pediatr Allergy Immunol. 2016; 27(2):214–217. [DOI] [PubMed] [Google Scholar]
- 12.Kuijpers TW, van de Vijver E, Weterman MAJ, et al. LAD-1/variant syndrome is caused by mutations in FERMT3. Blood. 2009; 113(19):4740–4746. [DOI] [PubMed] [Google Scholar]
- 13.Chiesa R, Veys P. Reduced-intensity conditioning for allogeneic stem cell transplant in primary immune deficiencies. Expert Rev Clin Immunol. 2012;8(3):255–266; quiz 267. [DOI] [PubMed] [Google Scholar]
- 14.Saultier P, Vidal L, Canault M, et al. Macrothrombocytopenia and dense granule deficiency associated with FLI1 variants: ultrastructural and pathogenic features. Haematologica. 2017;102(6):1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.