Following viral infections, considerable numbers of virus-specific memory T cells accumulate in the bone marrow (BM).1 This organ is rich in interleukin-15, which is required for the long-term maintenance of memory T cells.2 Whether this is driven by local homeostatic proliferation or by survival is actively debated.3 The ability of BM to harbor many (memory) T cells and support T-cell activation4 is intriguing, as activated T cells have a strong impact on the hematopoietic process; they can skew hematopoietic differentiation, affect self-renewal of hematopoietic stem cells (HSCs), and thereby cause anemia and even BM failure.5–7 T cells also influence allogeneic BM transplantation, as they drive graft-versus-host disease (GvHD).8 Interestingly, T-cell depletion from allogeneic BM grafts effectively lowers the risk of GvHD, but increases the risk of graft failure. This has led to the notion that T cells support HSC engraftment, which has been attributed to memory CD8+ T cells, whereas naïve T cells are responsible for GvHD development.8 Based on the impact that T cells have on the hematopoietic process, we questioned whether there is a functional crosstalk between T cells and HSCs. We found that mice lacking (memory) T cells had less HSCs, and that memory CD8+ T cells enhanced the maintenance of HSCs in vitro and in vivo. Both polyclonal and virus-specific BM memory CD8+ T cells exerted this effect, which was attributed to their production of soluble factor(s).
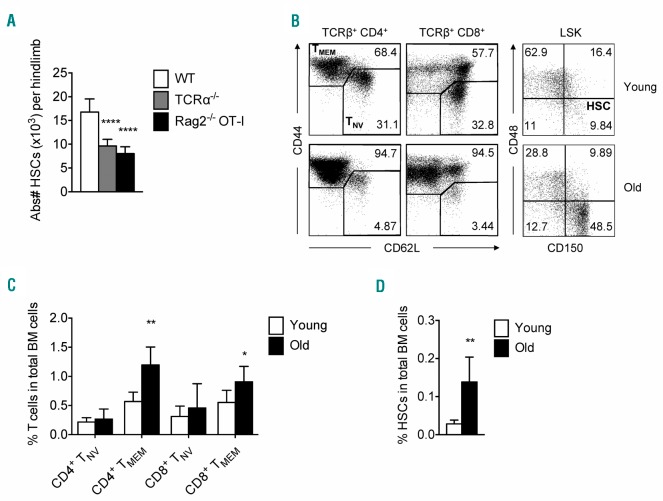
First, we assessed whether T cells contribute to HSC maintenance by analyzing the number of HSCs in T-cell deficient mice, and observed that TCRα−/− mice had significantly fewer HSCs compared to wild-type (WT) mice (Figure 1A). Interestingly, this was also observed in RAG2−/−-OT-I mice (Figure 1A) which contain naïve (TNV; CD44−CD62L+) T cells, but lack memory T cells,9 suggesting that memory T cells are responsible for the observed effect. In both strains, the level of HSC quiescence was comparable (Online Supplementary Figure S1A). We did not observe more HSCs in the spleen (data not shown), arguing against increased HSC mobilization from the BM. Reciprocally, old mice (>50 weeks), which have more memory T cells (TMEM; CD44+) in BM than young mice (<20 weeks) (Figure 1B and C), also have more HSCs (Figure 1B and D). This supports the hypothesis that memory T cells in BM can affect the function and/or maintenance of HSCs.
Figure 1.
Increase in memory T-cell numbers corresponds with higher frequency of hematopoietic stem cells (HSCs). (A) Absolute numbers of HSCs (Lin−/loSca-1+cKit+ CD150+CD48−) in wild-type (WT), TCRα−/−and Rag2−/−-OT-I mice (n=6 mice). (B) (Left) Representative FACS plots showing expression of CD44 and CD62L by bone marrow (BM) TCRβ CD4+ and TCRβ CD8+ T cells in young (top) versus old (bottom) mice. (Right) Representative FACS plots showing expression of CD150 and CD48 by Lin-/loSca-1+cKit+ (LSKs) of young and old mice; frequency of HSCs (CD150+CD48− cells) is indicated. (C) Frequency of naïve (CD44−) and total memory (CD44+; effector memory and central memory) CD4+ and CD8+ T cells in total BM cells. (D) Frequency of HSCs in total BM cells in young and old mice (n=6 mice). Graphs show mean±Standard Deviation of each tested group, pooled from 2 independent experiments.
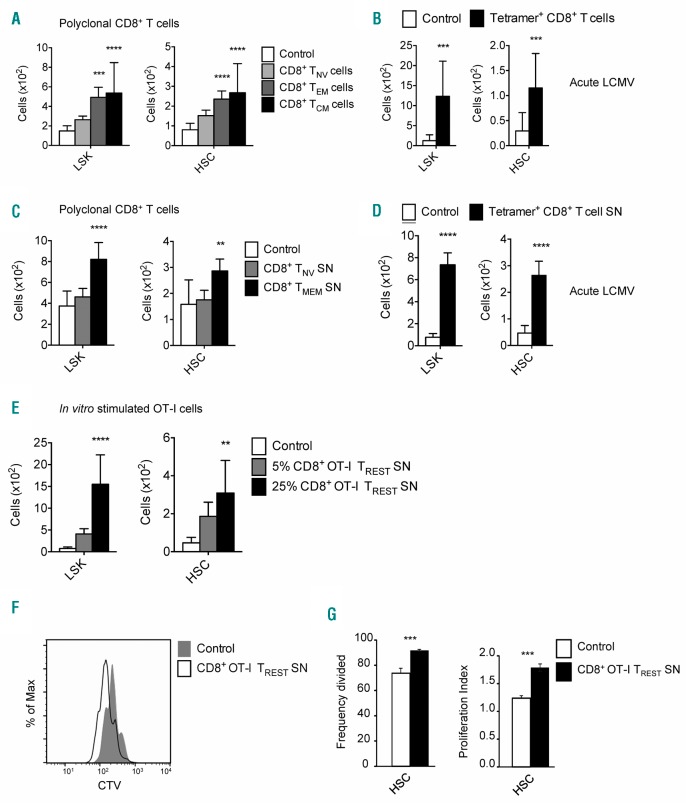
Next, we investigated whether T cells can directly affect the maintenance and function of HSCs, by co-culturing HSCs with BM-derived CD4+ or CD8+ T cells, and examined these cultures after seven days, as described previously.10 We observed that BM CD8+ T cells, but not CD4+ T cells, significantly increased the number of HSCs (Lin−/locKit+Sca−1+CD150+CD48−) and progenitors (LKS; Lin−/locKit+Sca-1+), without impairing their differentiation (Online Supplementary Figure S1B and C). Subset analysis revealed that both effector memory (TEM; CD44+CD62L−) and central memory (TCM; CD44+CD62L+) CD8+ T cells, but not TNV cells, were responsible for the observed effect (Figure 2A and Online Supplementary Figure S1D). Splenic memory CD8+ T cells also increased HSC numbers (data not shown), indicating that this feature is not restricted to BM memory CD8+ T cells. A similar supportive effect was observed with BM-derived memory CD8+ T cells specific for the acute strain of lymphocytic choriomeningitis virus (LCMV Armstrong) (Figure 2B). Interestingly, exhausted CD8+ T cells from mice chronically infected with LCMV Clone 13 were also able to support HSCs (Online Supplementary Figure S1E). Furthermore, we investigated whether the supporting effect of BM memory CD8+ T cells on HSCs is mediated by a soluble mediator. Therefore, we cultured naïve (CD44−) or memory BM CD8+ T cells (CD44+; TMEM cells) for four days in medium with IL-7 and IL-15 and collected the supernatants. HSCs cultured in medium supplemented with 25% supernatant from BM CD8+ TMEM cells, but not BM CD8+ TNV cells, gave rise to more LSKs and HSCs (Figure 2C). Similarly, the supporting effect of LCMV-specific CD8+ T cells on LSK and HSC numbers was also achieved with culture supernatant of cells generated during acute (Figure 2D) and chronic (Online Supplementary Figure S1F) LCMV infection. In addition, we investigated if we could generate HSC-supporting CD8+ T cells in vitro. We activated CD8+ OT-I T cells overnight with antigen-presenting cells and cognate peptide, followed by a resting period of six days without antigen.11 We harvested supernatant from the last four days (thereby excluding the presence of IFN-γ and TNF-α),11 and found that supernatant of these resting antigen-experienced CD8+ OT-I T cells (OT-I TREST SN) also increased LSK and HSC numbers in vitro in a dose-dependent manner (Figure 2E). Moreover, by labeling HSCs with a proliferation dye we observed that supernatant from CD8+ TMEM cells (data not shown) as well as OT-I TREST cells increased the proliferation of HSCs (Figure 2F and G, and Online Supplementary Figure S1G). Analysis of cell survival showed more than 80% viability for both LSKs and HSCs with control medium, which was further increased to more than 90% with CD8+ OT-I TREST SN (Online Supplementary Figure S1H). Thus, we demonstrate that antigen-experienced CD8+ T cells release soluble factor(s) that positively affect HSC maintenance in vitro by boosting their proliferation and survival.
Figure 2.
Co-culture of HSCs with memory T cells or their supernatant propagates the expansion of hematopoietic stem cells (HSCs). (A) Absolute cell numbers of Lin−/loSca-1+cKit+ (LSKs) and HSCs generated in the assay when HSCs are co-cultured with the different subsets of the bone marrow (BM) CD8+ T-cell population. (B) Absolute cell numbers of LSKs and HSCs generated in the assay when HSCs are co-cultured with acute BM lymphocytic choriomeningitis virus (LCMV)-specific memory CD8+ T cells isolated based on recognition of GP33–41, GP276–286 and NP396–404 epitopes on day 34 post infection. (C) Absolute cell numbers of LSKs and HSCs generated in the assay when HSCs are cultured with supernatant (SN) derived from BM naïve (CD44−) and memory (CD44+) CD8+ T cells. (D) Absolute cell numbers of LSKs and HSCs generated in the assay when HSCs are cultured with SN derived from acute BM LCMV-specific memory CD8+ T cells isolated based on recognition of GP33–41, GP276–286 and NP396–404 epitopes on day 37 post infection. (E) Absolute cell numbers of LSKs and HSCs generated in the assay when HSCs are cultured with 25% or 5% SN derived from in vitro generated resting antigen-experienced CD8+ OT-I T cells. (F) Flow cytometry analysis of the dilution of Cell Trace Violet (CTV) dye by HSCs when cultured with control medium or in vitro generated resting antigen-experienced CD8+ OT-I T cells. (G) Frequency divided and proliferation index of HSCs that are cultured with SN derived from in vitro generated resting antigen-experienced CD8+ OT-I T cells. Graphs show mean±Standard Deviation of each tested condition (n=3–16 wells), representative of 2–4 independent experiments.
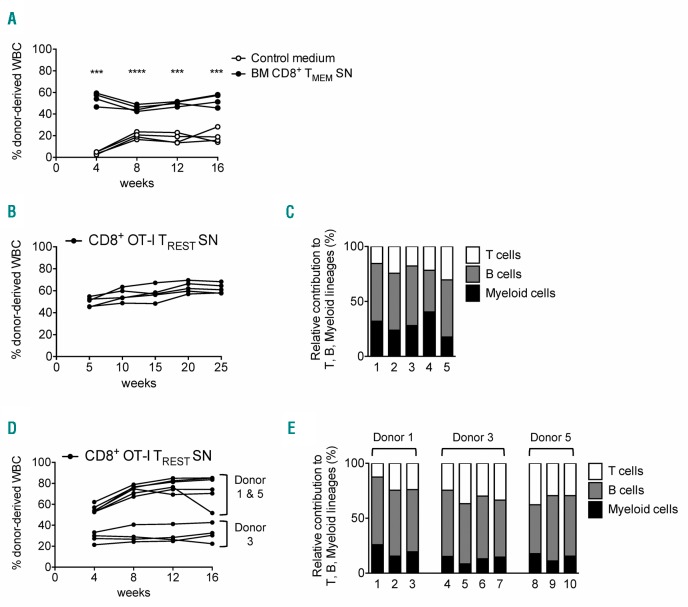
Lastly, in order to examine whether memory CD8+ T cells also preserved the in vivo functionality of HSCs, we performed a competitive transplantation assay. HSCs were cultured for three days with control medium (Ly5.1 HSCs) or BM CD8+ TMEM supernatant (Ly5.1/5.2 HSCs), and subsequently co-transplanted into lethally irradiated recipients (Ly5.2 mice). We observed that, at four weeks post transplantation, HSCs treated with CD8+ TMEM supernatant contributed more to leukocyte production than HSCs cultured with control medium (Figure 3A). This increased output was maintained over time and observed in both myeloid and lymphoid lineages (Online Supplementary Figure S2A–C). This long-term, multi-lineage repopulation capacity was also observed when HSCs were cultured with CD8+ OT-I TREST supernatant for seven days (Figure 3B and C). Secondary transplantations confirmed that donor cells reconstituted multiple lineages, formally demonstrating that the T-cell supernatant also preserved the self-renewal potential of HSCs (Figure 3D and E).
Figure 3.
Hematopoietic stem cells (HSCs) primed in vitro with bone marrow (BM) memory CD8+ T-cell supernatant (SN) have long-term, multi-lineage repopulation capacity. (A) Contribution of HSCs primed with control medium or SN derived from BM memory CD8+ T cells to the total circulating white blood cells (WBC) (n=4 mice). (B) Contributions of HSCs primed with CD8+ OT-I TREST SN to the total circulating WBC after primary transplant. (C) Relative contribution to lineage output of HSCs primed with CD8+ OT-I TREST SN 25 weeks after primary transplant (n=5 mice). (D) Contributions of HSCs originally primed (primary donors #1, #3, #5) with CD8+ OT-I TREST SN to the total circulating WBC after secondary transplant. (E) Relative contribution to lineage output of HSCs originally primed with CD8+ OT-I TREST SN 16 weeks after secondary transplant (n=10 mice). Graphs show results of 3 independent experiments.
Taken together, our data indicate that resting memory CD8+ T cells have a beneficial effect on the maintenance and survival of HSCs. The ability of virus-specific memory CD8+ T cells to boost HSC maintenance was surprising, as viral infections typically have a massive impact on the hematopoietic process, which is detrimental for HSCs.12 Inflammatory cytokines, such as type I IFNs, IFN-γ and TNF-α, induce HSC differentiation in order to promote the generation of immune cells that protect against invading pathogen. However, this comes at the cost of their self-renewal, thereby leading to a substantial loss in HSC numbers following viral infection.5–7,13 The observation that also virus-specific CD8+ T cells from chronically infected mice boost HSC maintenance is striking, as these T cells are functionally exhausted in terms of effector cytokine production. Yet, this could be the reason why chronically infected mice still have functional HSCs; the HSC-supporting capacity of exhausted T cells may counteract the deleterious impact of the ongoing wave of pro-inflammatory cytokines during these infections. Based on our novel findings, we postulate that the potential of memory CD8+ T cells to accumulate in the BM after viral infection supports the restoration of the HSC pool. Apart from infections, our data may also bear relevance for aging. It has been well-established that HSC numbers increase upon aging.14 Our data suggest that the accumulation of memory CD8+ T cells during aging may also contribute to this effect by supporting HSC maintenance and survival.
Our findings are of particular interest for HSC transplantation. For many years, scientists have tried to identify factors that expand human HSCs ex vivo, as the success of HSC transplantation is reduced when HSC numbers are limited.8 HSCs rapidly lose their long-term in vivo repopulating potential when they are expanded in vitro, and the synergistic action of multiple HSC-expansion agonists is required for maintenance of their long-term engraftment properties.15 It is, therefore, interesting that memory CD8+ T cells, including resting antigen-experienced CD8+ OT-I T cells, produce a yet to be identified soluble factor(s) that increases HSC numbers in vitro, while maintaining their ability for self-renewal and long-term hematopoietic reconstitution in vivo. Moreover, the functional characteristics of memory CD8+ T cells that we uncovered may also provide an explanation for the enigmatic observation that memory CD8+ T cells support HSC engraftment following allogeneic transplantation.8 It has been suggested that donor CD8+ T cells can eradicate remaining recipient HSCs or T cells, but also enhance the migration of donor HSCs.8 We now provide an additional explanation, namely that memory CD8+ T cells can directly enhance the self-renewal and maintenance of donor HSCs.
In summary, CD8+ T cells act as a double-edged sword in the BM: when activated, CD8+ T cells enhance HSC differentiation and inhibit their self-renewal capacity, while in a non-activated state, memory CD8+ T cells support HSC self-renewal and contribute to their maintenance and/or recovery. This illustrates that CD8+ T cells act as important mediators in controlling the pool of HSCs in the BM.
Supplementary Material
Acknowledgments
We thank Carlijn Voermans for critical reading of the manuscript, the staff of the animal facilities of the AMC and the NKI for outstanding animal care, and the staff of Sanquin central facility for excellent cell sorting.
Footnotes
Funding: SG and MFP were financially supported by a Fellowship obtained by MAN from the Landsteiner Foundation for Blood Transfusion Research, grant n. #1014 www.lsbr.nl. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Geerman S, Hickson S, Brasser G, Pascutti MF, Nolte MA. Quantitative and Qualitative Analysis of Bone Marrow CD8(+) T Cells from Different Bones Uncovers a Major Contribution of the Bone Marrow in the Vertebrae. Front Immunol. 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herndler-Brandstetter D, Landgraf K, Jenewein B, et al. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol. 2011; 186(12):6965–6971. [DOI] [PubMed] [Google Scholar]
- 3.Nolte MA, Goedhart M, Geginat J. Maintenance of memory CD8 T cells: Divided over division. Eur J Immunol. 2017;47(11):1875–1879. [DOI] [PubMed] [Google Scholar]
- 4.Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003; 9(9):1151–1157. [DOI] [PubMed] [Google Scholar]
- 5.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11(10):685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libregts SF, Nolte MA. Parallels between immune driven-hematopoiesis and T cell activation: 3 signals that relay inflammatory stress to the bone marrow. Exp Cell Res. 2014;329(2):239–247. [DOI] [PubMed] [Google Scholar]
- 7.de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124(16):2479–2486. [DOI] [PubMed] [Google Scholar]
- 8.Geerman S, Nolte MA. Impact of T cells on hematopoietic stem and progenitor cell function: Good guys or bad guys? World J Stem Cells. 2017;9(2):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Žugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bruin AM, Demirel Ö, Hooibrink B, Brandts CH, Nolte MA. Interferon-γ impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121(18):3578–3585. [DOI] [PubMed] [Google Scholar]
- 11.Salerno F, Guislain A, Cansever D, Wolkers MC. TLR-Mediated Innate Production of IFN-γ by CD8+ T Cells Is Independent of Glycolysis. J Immunol. 2016;196(9):3695–3705. [DOI] [PubMed] [Google Scholar]
- 12.Pascutti MF, Erkelens MN, Nolte MA. Impact of Viral Infections on Hematopoiesis: From Beneficial to Detrimental Effects on Bone Marrow Output. Front Immunol. 2016;7:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185(3):517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13(5):376–389. [DOI] [PubMed] [Google Scholar]
- 15.Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015;43(7):498–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.